Summary
Aims
Intraplaque neovascularization and foam cell infiltration contribute to the development of unstable plaque, leading to thromboembolism and stroke. Cell adhesion molecules (CAMs) have been reported to be involved in the progression of atherosclerosis and plaque vulnerability. The aim of this study was to assess the association of adhesion molecule CD146 with carotid plaque instability.
Methods
We collected forty atherosclerotic plaques from 40 patients undergoing carotid endarterectomy. The clinical information of each patient was obtained, and the plaque morphology and characteristics were examined by the ultrasound. The CD146 expressions of the plaques were graded by using semiquantitative scales. The serum level of soluble form of CD146 was detected by enzyme‐linked immunosorbent assay (ELISA).
Results
CD146 expression was mainly on the intraplaque blood vessels and infiltrated macrophages. The CD146 expression was strongly correlated with the matrix metalloproteinase‐9(MMP‐9)expressions (P < 0.001) in the plaques. Soluble CD146 (sCD146) was also elevated in patients with atherosclerotic plaques. There was significant correlation between the increased CD146 expression and sCD146 level (P = 0.0057). sCD146 correlated well with serum MMP‐9 (P < 0.0044), IL‐6 (P = 0.0044) and high sensitivity C‐reactive protein (hsCRP) (P = 0.005).
Conclusions
Adhesion molecules CD146 and its soluble form strongly correlated with the development of inflammation of atherosclerosis and plaque instability. CD146 may be a promising biomarker for monitoring the development and instability of atherosclerotic plaque in patients with carotid diseases.
Keywords: Adhesion molecule CD146, Atherosclerosis, Plaque instability
Introduction
Atherosclerosis (AS) mainly occurred on the large arteries and is characterized by the chronic inflammation in the lesions 1, 2, 3. Changes within the vessel wall of the internal carotid artery (ICA) can lead to plaque vulnerability, which is the main reason for carotid‐related cerebrovascular ischemic events 4, 5, 6, 7. It has been reported that inflammatory factors, protease and so on play an important role on the process of plaque vulnerability, such as the matrix metalloproteinases (MMPs), especially the matrix metalloproteinase‐9(MMP‐9) 8. The increased level of serum MMP‐9 was detected in the atherosclerotic patients with instable plaques compared with those with stable plaques, suggesting the correlation of MMP‐9 and the instability of plaques. The adhesion molecules are also reported to account for the process of plaque vulnerability. The initiation of AS is triggered by endothelial dysfunction and induction of inflammation, accompanied by the increased expression of adhesion molecules that stimulates the adhesion and transmigration of leukocyte into the vascular subendothelial space 9, 10. The increased expression of cell adhesion molecules(CAMs) on the vascular endothelial cells, such as intercellular adhesion molecule‐1(ICAM‐1), vascular cell adhesion molecule‐1(VCAM‐1), P‐selectin, etc., has been reported to play a critical role on the pathogenesis of atherogenesis 11, 12, 13. Monitoring changes of CAMs at the inflammatory sites can help predicting major vascular events.
Although it is difficult to quantify the expression of these molecules on the cell surface in vivo, their soluble forms can be detected in serum by specific antibodies using ELISA. Circulating soluble CAMs are the results of cleavages of membrane‐bound CAMs. Their concentration in serum/plasma has been associated with the state of cardiovascular disease 14, 15. Detecting the serum soluble CAMs may provide evidence for early intervention and secondary prevention of stroke related to carotid diseases, and monitoring effectiveness of treatment.
CD146, an adhesion molecule that was originally identified as a melanoma marker 16, has been studied as angiogenesis marker and reported to be able to upregulate neovascular endothelial cells 17, 18, and mediate many chronic inflammatory processes 19, 20, 21. Its soluble form, sCD146, originated from the membrane‐bound CD146, has also been well studied in many inflammatory diseases 22, 23, 24, 25. As a chronic inflammatory disease, AS is characterized by neovascularization and inflammatory cell infiltration in lesion plaques. However, there has still little known about CD146 and its sCD146 in AS. We hypothesize that CD146 and sCD146 may also be related with the progression of atherosclerotic lesions.
Patients and Methods
Patients
This study was approved by the Ethics Committee at Beijing Anzhen Hospital, Capital Medical University and the written informed consent was obtained from each patients. Between March 2011 and September 2012, 40 patients (26 men, 14 women; mean age, 65.6 ± 8.25 years) from Beijing Anzhen Hospital with high‐grade (≥70%) extracranial carotid artery stenosis were selected for carotid endarterectomy. Forty age‐matched and sex‐matched healthy individuals were selected as controls. Detailed information on the patient and control groups was shown in Table 1. The subjects had any of the following conditions were excluded: acute stroke within 3 months, liver and kidney dysfunction, tumors, systematic inflammatory diseases and autoimmune diseases. Risk factors for atherosclerotic disease, including history of diabetes metabolism, hypercholesterolemia, and levels of high sensitivity C‐reactive protein (hsCRP) were recorded in all patients.
Table 1.
Clinical information of controls and patients with AS
| Control | AS with stable plaque | AS with vulnerable plaque | |
|---|---|---|---|
| No. of patients | 40 | 16 | 24 |
| Sex (F:M) | 16:24 | 9:7 | 5:19 |
| Mean age (years) | 63.1 ± 10.8 | 66.3 ± 7.8 | 64.7 ± 8.2 |
| History of hypertension n (%) | 0 | 14 (87.5%) | 19 (79%) |
| History of DM n (%.) | 0 | 2 (12.5%) | 10 (41.6%) |
| History of coronary artery disease n (%) | 0 | 8 (50%) | 7 (29.1%) |
| History of stroke n (%) | 0 | 4 (25%) | 12 (50%) |
| TG (mm) | 1.56 ± 1.11 | 1.70 ± 0.99 | 1.58 ± 1.31 |
| TC (mm) | 4.12 ± 1.09 | 4.68 ± 0.77 | 3.73 ± 0.95 |
| LDL‐C (mm) | 2.23 ± 0.86 | 2.83 ± 0.88 | 2.36 ± 0.67 |
| HDL‐C (mm) | 1.05 ± 0.25 | 1.11 ± 0.25 | 0.97 ± 0.25 |
| hsCRP (mg/L) | 0.58 ± 0.82 | 2.22 ± 1.86 | 3.82 ± 4.89 |
DM, diabetes metabolism; TG, triglyceride; TC, total cholesterol; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; hsCRP, high sensitivity C‐reactive protein.
The Ultrasound Examination of Carotid Plaques
We performed the carotid ultrasound (US) examinations in the following predefined areas: bilateral distal, bulb, and proximal ICAs. We defined the atherosclerotic plaques as a local thickening of the intima of ≥1.5 mm. To evaluate the vulnerability of carotid plaque, we performed high resolution B‐mode US, using 7.5 MHz probe (Vivid 7, GE‐Vingmed, Horten, Norway). Two independent observers (WJ and ZQ), who were blinded to all of the clinical information and results, rated plaque morphology according to the standard protocol. The characteristics of the plaques were described according to the modified Gray Weale classification 26. Patients with uniformly hyperechoic or predominantly (>50%) hyperechoic were classified into the stable plaque group. Patients with uniformly hypoechoic or predominantly (>50%) hypoechoic were classified into the vulnerable plaque group.
Blood Samples
Fasting blood samples from an antecubital vein were collected and placed at 4°C for half an hour, then centrifuged at 2000 rpm for 5 min. To avoid the loss of biological activity, serum samples were collected as soon as possible, stored at −70°C and thawed just before testing. The total cholesterol (TC) and triglyceride (TG) concentrations in the serum were assayed by routine enzymatic methods. The concentration of low density lipoprotein cholesterol (LDL‐C) and high density lipoprotein cholesterol (HDL‐C) were determined by a direct homogeneous assay (Au5421, BECKMAN, COULTER, America). The serum hsCRP level was measured with immunoturbidimetric assay (Diagnostica kit, DiaSys Diagnostic Systems GmbH, Holzheim, Germany).
Tissue Sampling
Forty entire carotid plaques of the human carotid AS lesions were obtained from carotid endarterectomy, then were fixed in neutral buffered formalin and embedded in paraffin. Normal carotid arterial specimens (n = 5) served as the control were from the donor of heart transplant operations, fixed in neutral buffered formalin, and then embedded in paraffin.
Antibodies and Reagents
The following proteins and antibodies were used in this study: recombinant human soluble CD146 (Sinobiological Co., Ltd. Beijing, China); mouse originated anti‐CD146 monoclonal antibodies, such as AA1, AA4, and AA98 (generated in Yan's laboratory 18, 27). We used AA1 and AA98 for ELISA 28, and AA4 for immunohistochemistry (paraffin‐embedded). We labeled AA98 with biotin in Tianjin Sungene Biotech Co., Ltd.
The following antibodies were also used in this study: antihuman CD31, antihuman CD68, antihuman α‐SMA (Abcam); and horseradish peroxidase‐conjugated antimouse and antirabbit secondary antibodies (GE Healthcare, Uppsala, Sweden). The serum cytokine,such as IL‐6, from patients with AS was detected by the BD Cytometric Bead Array (CBA) human Th1/Th2/Th17 cytokine kit.
Immunohistochemical Staining
For immunohistochemistry staining, deparaffinized paraffin‐embedded tissue sections were incubated first with CD146 antibody (AA4), and then with biotin‐conjugated antimouse antibody (1:1000), followed by HRP‐conjugated streptavidin (Dianova, Rodeo, CA, USA). A ready‐to‐use solution of 3,3'‐Diaminobenzidine was used to observe the expression of CD146. The sections were finally counterstained with hematoxylin. Sections without primary antibody were served as negative controls. For immunofluorescence, deparaffinized sections were stained with antibodies specific for α‐SMA, CD68, CD31 or CD146 (AA4) followed by fluorescence‐labeled secondary antibodies. The nuclei were counterstained with 4', 6‐diamidino‐2‐phenylindole (DAPI). The sections were visualized using a confocal laser‐scanning microscope (Olympus).
Detection of sCD146 and MMP‐9 by ELISA
Detection of serum sCD146 was performed as described previously 23. In brief, the serum sCD146 level was determined by ELISA using the capture antibody, AA1 (2 μg/mL) and the detection antibody, biotin‐conjugated AA98 (1.5 μg/mL); the detection enzyme was HRP‐conjugated streptavidin (Dianova). The standard curve was determined using the recombinant sCD146 in phosphate‐buffered saline (PBS) buffer (from 80 to 1.25 ng/mL). Serum samples from patients and controls were diluted 1:9 in PBS before measurement. A commercialized solution of 3, 30, 5, 50‐tetramethylbenzidine (TMB) was used as a HRP enzyme substrate. A wavelength of 450 nm sample absorption was measured using a BioRad ELISA reader (Richmond, CA, USA). The level of MMP‐9 in serum samples was determined using commercialized enzyme‐linked immunosorbent assay kit according to manufacturer's instructions.
Statistical Analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) software was used to perform the statistical analyses. All the measurement data was shown as the mean ± standard deviation (SD). For comparison of different groups, the nonparametric Mann–Whitney U test was employed. For evaluation of the correlations between the sCD146 level and clinical indices, the multiple linear regression and Spearman's Rank correlation coefficient were employed. We defined the criterion of statistical significance as P < 0.05.
Results
CD146 was Upregulated in Human Atherosclerotic Plaques
Using the immunohistochemistry, CD146 expression in atherosclerotic plaques from 40 patients with carotid endarterectomy and five healthy normal carotid arteries was detected. In the atherosclerotic plaques, CD146 was expressed mainly on the smooth muscle cells (α‐SMA+) (Figure 1A), infiltrated macrophages (CD68+) (Figure 1B), and endothelial cells of neovascular blood vessels (CD31+) (Figure 1C). While in the health arteries, CD146 was expressed on the smooth muscle cells of the media and perivascular small blood vessels (Figure 1D). Furthermore, the density of CD146 expression was stronger in plaque of neovascular blood vessels than that in the perivascular vessels of healthy arteries (Figure 1E), implying that the overexpression of CD146 was associated with the atherosclerotic lesions.
Figure 1.
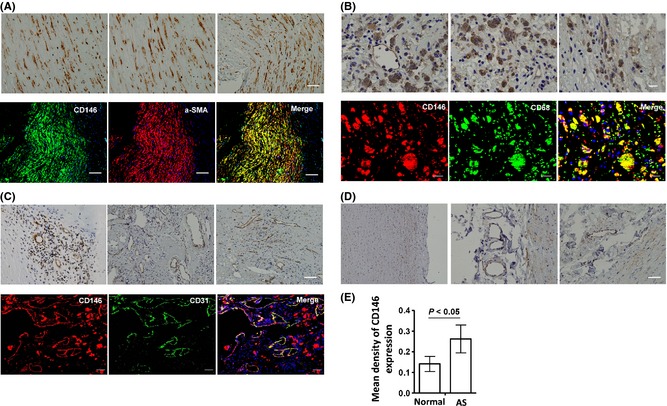
CD146 expression on AS plaques and normal artery. (A) CD146 expression on the smooth muscle cells in the plaques. (B) CD146 expression on the macrophages in the plaques. (C) CD146 expression on the endothelial cells in the plaques. (D) CD146 expression in the normal artery. (E) The expression of CD146 is increased in the blood endothelial cells of plaques (n = 40) compared with that in the normal artery (n = 5).
CD146 Expression was Associated with the Development of Vulnerable Atherosclerotic Plaques
To test whether CD146 expression was related to the development of vulnerable plaque, the relationship of CD146 expression and necrotic core area in atherosclerotic plaques was analyzed. A positive correlation between the mean density of CD146 expression and the necrotic core area (Figure 2A) was found, indicating the overexpression of CD146 in the plaques. Furthermore, by analyzing the CD146 expression between stable plaques and instable plaques, we found that the mean density of CD146 expression in the instable plaques was significantly stronger than that in the stable plaques (Figure 2B), confirming the fact that overexpression of CD146 was associated with the development of vulnerable atherosclerotic plaques.
Figure 2.
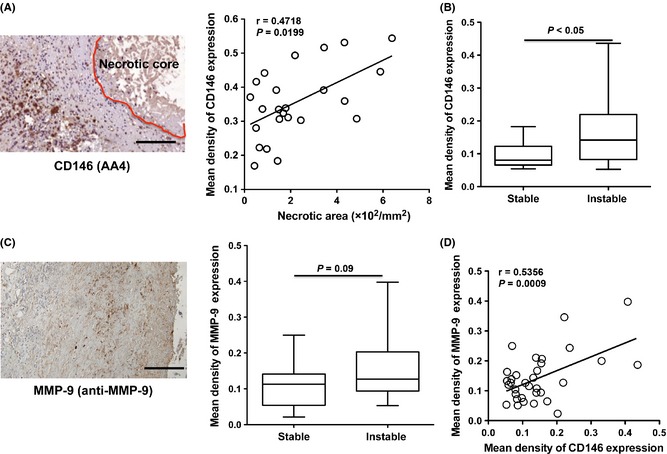
CD146 overexpression is associated with the vulnerablility of plaque. (A) CD146 expression is correlated with the necrotic area of plaque (n = 24). (B) CD146 expression is increased in the patients with vulnerable atherosclerotic plaques (n = 24). (C) MMP‐9 expression is increased in the patients with vulnerable atherosclerotic plaques (n = 24). (D) The correlation analysis of the CD146 expression and MMP‐9 expression in the plaques (n = 40).
It has been reported that the MMPs, especially MMP‐9, contributes to the development of vulnerable plaque 29. To further confirm this association of CD146 expression and plaque progression, we have detected MMP‐9 expression in human carotid atherosclerotic plaques. These findings provided the proof of upregulation of MMP‐9 in the vulnerable plaques (Figure 2C). In addition, there was a significantly positive correlation between the mean density of CD146 expression and the MMP‐9 expression (Figure 2D). These data suggested that the overexpression of CD146 in the plaques might predict the development of vulnerable atherosclerotic plaques.
Soluble CD146 was Increased in the Patients with Atherosclerosis
To test the relationship of CD146 expression and AS inflammation, detecting serum soluble CD146 in 40 health donors and 40 patients with AS by using the sandwich ELISA (Figure 3A) was conducted. Tests showed that level of serum sCD146 was significantly elevated in patients with AS (Figure 3B). Because the soluble CD146 has been reported to originate from the membrane‐bound CD146, the correlation of the level of serum sCD146 and expression density of CD146 in the AS plaques (n = 40) was further analyzed. We found that there was a significant positive correlation between sCD146 and CD146 expression density (Figure 3C). These data showed that there was a significant positive correlation of CD146 and sCD146 in patients with AS indicating that sCD146 originated from the membrane‐bound CD146,might correlate with the inflammation of AS.
Figure 3.
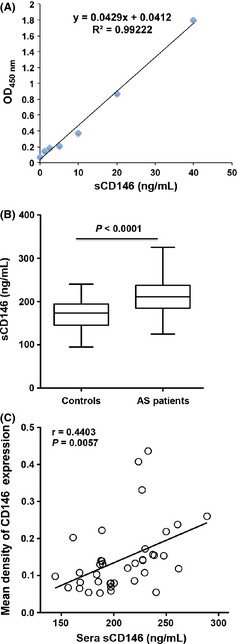
Serum sCD146 is elevated in the patients with AS. (A) Standard curve for the detection of sera sCD146 by sandwich ELISA. (B) sCD146 is increased in the patients with AS (n = 40) compared with normal donors (n = 40). (C) sCD146 has a positive correlation with the mean density of CD146 expression in the plaques (n = 40).
Analysis of the Correlation of sCD146 and Clinical Parameters
To further analyze the correlation of sCD146 and inflammatory factors, serum levels of IL‐6 and hsCRP, reflecting the activity of inflammation by both, were tested. Both IL‐6 and hsCRP were increased in patients with AS compared with that in the normal donors (Figure 4 A,B). In addition, sCD146 had a positive correlation with IL‐6 and hsCRP (P = 0.0044; P = 0.005) (Figure 4C,D), indicating that sCD146 maybe a promising biomarker for monitoring active inflammatory process in AS.
Figure 4.
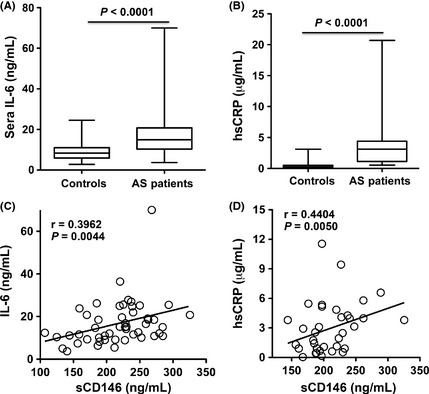
Serum sCD146 in the patients with AS is correlated with the inflammation of disease. (A) IL‐6 is increased in patients with AS (n = 40). (B) hsCRP is increased in patients with AS (n = 40). (C) sCD146 has a positive correlation with IL‐6 (n = 40). (D) sCD146 has a positive correlation with hsCRP (n = 40).
It has been reported that many soluble forms of adhesion molecules, such as sICAM‐1 and sVCAM‐1, are associated with the risk factors of AS, e.g. age, homocysteine, etc. 30, 31. To investigate whether the level of sCD146 was associated with the risk of developing AS, the correlation of sCD146 and clinical risk factors were analyzed. No such correlation between these factors was found (Table 2), indicating that sCD146 level alone might be an independent risk factor for the development of atherosclerotic inflammation.
Table 2.
Correlation analysis between sera sCD146 and various clinical parameters in patients with AS
| Sera sCD146 | Correlation coefficient | P value |
|---|---|---|
| Age (years) | −0.0533 | 0.7576 |
| Hypertension (years) | −0.0025 | 0.9886 |
| Diabetes (years) | 0.0339 | 0.8445 |
| TG (mm) | −0.1758 | 0.3050 |
| TC (mm) | 0.0815 | 0.6367 |
| LDL‐C (mm) | −0.0492 | 0.7755 |
| HDL‐C (mm) | 0.1156 | 0.5022 |
TG, triglyceride; TC, total cholesterol; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol.
sCD146 Level Correlated with the Vulnerability of Plaque
Active inflammation promotes the instability of plaques. To test the association of sCD146 and the plaque instability, sCD146 levels of patients with or without instable atherosclerotic plaques were analyzed. The analysis showed that sCD146 was elevated in patients with instable plaques compared with those with stable plaques, consistent with the finding of increased serum MMP‐9 in patients with instable plaques (Figure 5A,B). In addition, sCD146 had a positive correlation with the MMP‐9 levels in patients with AS (Figure 5C). These data suggested that sCD146 might be a new biomarker for instability of plaques.
Figure 5.
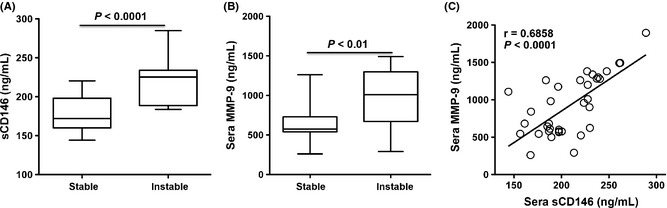
Serum sCD146 is correlated with the vulnerablility of atherosclerotic plaques. (A) Serum sCD146 is elevated in atherosclerotic patients with vulnerable plaques (n = 24). (B) Serum MMP‐9 is elevated in atherosclerotic patients with vulnerable plaques (n = 24). (C) sCD146 has a positive correlation with serum MMP‐9 (n = 40).
Discussion
Many studies have shown that serum soluble CAMs, such as sICAM‐1, sVCAM‐1, sP‐selectin and sE‐selectin, are associated with cardiovascular events 32, 33, 34. Monitoring the levels of these sCAMs can predict the seriousness of vascular events and assist in diagnoses of AS. CD146 has been recognized as an angiogenesis marker and reported to upregulate neovascular endothelial cell 17, 18. In this study, we first found CD146 was upregulated in human atherosclerotic plaques. Its overexpression might predict the intraplaque microvascular angiogenesis within the atherosclerotic lesions. It is considered that the intraplaque neovascularization is an important feature in plaque development and vulnerability, which increases the risk of rupture and cerebral emboli event. In addition, several pathological studies have confirmed that the presence and degree of neovascularization within the plaque is strongly associated with plaque rupture risk 35, 36, 37. Because sCD146 has been reported to come from the membrane‐bound CD146, using the sandwich ELISA, we found that sCD146 was elevated in the atherosclerotic patients compared with that in normal donors. Furthermore, sCD146 level is higher in patients with vulnerable plaques than in those with stable plaques. sCD146 also correlated with the expression of serum MMP‐9, which was associated with plaque vulnerability. Furthermore, sCD146 correlated with the levels of hsCRP and IL‐6 in patients with AS, which was indicative of an acute inflammatory process. Our study indicated rather strongly that sCD146 might be a promising biomarker for monitoring the active inflammatory process in AS. Detecting sCD146 levels in patients with AS can be helpful in predicting the seriousness of vascular events and planning for early intervention.
In the process of developing AS, the vulnerability of plaques is the leading cause of disease progression 38, 39, 40, 41. These plaques may rupture, forming thrombus, blocking blood vessel, sending emboli, and causing stroke, tissue ischemia and necrosis. Preventing plaque from becoming vulnerable can reduce the occurrence of serious vascular events. The well‐known pathological factors that affect the development of plaque vulnerability are angiogenesis, macrophage infiltration and thinning of the fibrous cap of plaques 42, 43, 44, 45. It has been reported that MMPs, especially MMP‐9, play an important role in the plaque fibrous cap thinning process 29, 46. Many studies showed that serum MMP‐9 levels were significantly higher in patients with vulnerable plaques than those with stable plaques, indicating the association of MMP‐9 and plaque vulnerability. In our study, sCD146 was found to be generated in a matrix metalloproteinase‐dependent way 23, 47. In patients with AS, the membrane‐bound CD146 on the neovascular endothelial cells in the plaques may be an important source of sCD146. Therefore, serum levels of sCD146 may not only reflect the activity of MMPs, but also activity of neovascular endothelial cells. Furthermore, sCD146 correlated with the level of inflammation, such as in the elevation of levels of hsCRP and IL‐6. sCD146 may be more sensitive in monitoring the instability of plaque.
Others and we have found that sCD146 is associated with the inflammation activity of various vascular diseases, such as inflammatory bowel disease, multiple sclerosis, diabetic nephropathy, multiple vasculitis, etc. Many studies suggest that sCD146 may have multiple roles in the development of vascular inflammation. A recent report showed that sCD146 has pro‐angiogenic property and induces angiogenesis in animal models of ischemic limb necrosis 48. In our recent study, we found that sCD146 may be an inflammatory factor inducing cell adhesion molecules such as ICAM‐1, VCAM‐1 expression in endothelial cells and facilitating the transendothelial migration of inflammatory cells, thereby promoting the development of inflammation 23.
What is then the exact role of sCD146 in AS development? Some studies have been suggested that serum sCD146 may be associated with the migration of monocytes into the plaques 49, 50. sCD146 may bind with an unknown ligand on the monocytes, and promote the interaction of monocytes and the activated endothelial cells, thereby facilitating the transmigration of monocytes to the plaques, and promoting the plaque instability and causing AS progression. In addition, sCD146 may be associated with plaque neovascularization and indirectly or directly promote the plaque instability due to its pro‐angiogenic and pro‐inflammatory properties. Further studies of the role and mechanism of sCD146 during the process of the development of AS are needed.
Conclusion
Our study has provided the evidence for the first time that CD146, a new adhesion molecule, is associated with the progression of atherosclerotic lesions in the diseased vessel wall. Testing for serum sCD146 might predict the degree of inflammatory activity and development of vulnerable atherosclerotic plaque.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
We thank for David Z. Wang careful reading and editing of our manuscript. This work was partially supported by grants from the National Natural Science Foundation of China (31300729, 81272409).
The first two authors contributed equally to this work.
References
- 1. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 2010;30:1282–1292. [DOI] [PubMed] [Google Scholar]
- 4. Rothwell PM. Carotid artery disease and the risk of ischaemic stroke and coronary vascular events. Cerebrovasc Dis 2000;10(Suppl 5):21–33. [DOI] [PubMed] [Google Scholar]
- 5. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 6. Pelisek J, Rudelius M, Zepper P, et al. Multiple biological predictors for vulnerable carotid lesions. Cerebrovasc Dis 2009;28:601–610. [DOI] [PubMed] [Google Scholar]
- 7. Heider P, Pelisek J, Poppert H, Eckstein HH. Evaluation of serum matrix metalloproteinases as biomarkers for detection of neurological symptoms in carotid artery disease. Vasc Endovascular Surg 2009;43:551–560. [DOI] [PubMed] [Google Scholar]
- 8. Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 2003;59:812–823. [DOI] [PubMed] [Google Scholar]
- 9. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 1994;8:504–512. [PubMed] [Google Scholar]
- 10. Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Res Cardiol 2005;100:93–101. [DOI] [PubMed] [Google Scholar]
- 11. Carlos TM, Harlan JM. Leukocyte‐endothelial adhesion molecules. Blood 1994;84:2068–2101. [PubMed] [Google Scholar]
- 12. Inoue M, Ishida T, Yasuda T, et al. Endothelial cell‐selective adhesion molecule modulates atherosclerosis through plaque angiogenesis and monocyte‐endothelial interaction. Microvasc Res 2010;80:179–187. [DOI] [PubMed] [Google Scholar]
- 13. McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol 2002;14:581–586. [DOI] [PubMed] [Google Scholar]
- 14. Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM‐1, ICAM‐1, and E‐selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997;96:4219–4225. [DOI] [PubMed] [Google Scholar]
- 15. Malik I, Danesh J, Whincup P, et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta‐analysis. Lancet 2001;358:971–976. [DOI] [PubMed] [Google Scholar]
- 16. Schlagbauer‐Wadl H, Jansen B, Muller M, et al. Influence of MUC18/MCAM/CD146 expression on human melanoma growth and metastasis in SCID mice. Int J Cancer 1999;81:951–955. [DOI] [PubMed] [Google Scholar]
- 17. Jiang T, Zhuang J, Duan H, et al. CD146 is a coreceptor for VEGFR‐2 in tumor angiogenesis. Blood 2012;120:2330–2339. [DOI] [PubMed] [Google Scholar]
- 18. Yan X, Lin Y, Yang D, et al. A novel anti‐CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood 2003;102:184–191. [DOI] [PubMed] [Google Scholar]
- 19. Bardin N, Reumaux D, Geboes K, et al. Increased expression of CD146, a new marker of the endothelial junction in active inflammatory bowel disease. Inflamm Bowel Dis 2006;12:16–21. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Zhang BR, Zeng XF, Wang X. A pilot study on the significance of leucocyte CD146 expression in vasculitis Zhonghua Nei Ke Za Zhi 2006;45:748–751. [PubMed] [Google Scholar]
- 21. Duan H, Xing S, Luo Y, et al. Targeting endothelial CD146 attenuates neuroinflammation by limiting lymphocyte extravasation to the CNS. Sci Rep 2013;3:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bardin N, Moal V, Anfosso F, et al. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb Haemost 2003;90:915–920. [DOI] [PubMed] [Google Scholar]
- 23. Duan H, Luo Y, Hao H, et al. Soluble CD146 in cerebrospinal fluid of active multiple sclerosis. Neuroscience 2013;235:16–26. [DOI] [PubMed] [Google Scholar]
- 24. Figarella‐Branger D, Schleinitz N, Boutiere‐Albanese B, et al. Platelet‐endothelial cell adhesion molecule‐1 and CD146: soluble levels and in situ expression of cellular adhesion molecules implicated in the cohesion of endothelial cells in idiopathic inflammatory myopathies. J Rheumatol 2006;33:1623–1630. [PubMed] [Google Scholar]
- 25. Reumaux D, Bardin N, Colombel JF, Dignat‐George F, Duthilleul P, Vermeire S. Restoration of soluble CD146 in patients with Crohn's disease treated with the TNF‐alpha antagonist infliximab. Inflamm Bowel Dis 2007;13:1315–1317. [DOI] [PubMed] [Google Scholar]
- 26. Geroulakos G, Ramaswami G, Nicolaides A, et al. Characterization of symptomatic and asymptomatic carotid plaques using high‐resolution real‐time ultrasonography. Br J Surg 1993;80:1274–1277. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Zheng C, Zhang J, et al. Generation and characterization of a panel of monoclonal antibodies against distinct epitopes of human CD146. Hybridoma (Larchmt) 2008;27:345–352. [DOI] [PubMed] [Google Scholar]
- 28. Zheng C, Qiu Y, Zeng Q, et al. Endothelial CD146 is required for in vitro tumor‐induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Int J Biochem Cell Biol 2009;41:2163–2172. [DOI] [PubMed] [Google Scholar]
- 29. George SJ. Therapeutic potential of matrix metalloproteinase inhibitors in atherosclerosis. Expert Opin Investig Drugs 2000;9:993–1007. [DOI] [PubMed] [Google Scholar]
- 30. Vora DK, Fang ZT, Liva SM, et al. Induction of P‐selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circ Res 1997;80:810–818. [DOI] [PubMed] [Google Scholar]
- 31. Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine‐activated vascular cell adhesion molecule‐1 gene expression in human vascular endothelial cells. J Clin Invest 1995;95:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballantyne CM, Entman ML. Soluble adhesion molecules and the search for biomarkers for atherosclerosis. Circulation 2002;106:766–767. [DOI] [PubMed] [Google Scholar]
- 33. Guray U, Erbay AR, Guray Y, et al. Levels of soluble adhesion molecules in various clinical presentations of coronary atherosclerosis. Int J Cardiol 2004;96:235–240. [DOI] [PubMed] [Google Scholar]
- 34. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C‐reactive protein, interleukin‐6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005;112:976–983. [DOI] [PubMed] [Google Scholar]
- 35. Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004;110:2843–2850. [DOI] [PubMed] [Google Scholar]
- 36. McCarthy MJ, Loftus IM, Thompson MM, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg 1999;30:261–268. [DOI] [PubMed] [Google Scholar]
- 37. Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation 2004;110:2032–2038. [DOI] [PubMed] [Google Scholar]
- 38. Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996;94:2013–2020. [DOI] [PubMed] [Google Scholar]
- 39. Kolodgie F D, Narula J, Haider N, Virmani R. Apoptosis in atherosclerosis. Does it contribute to plaque instability? Cardiol Clin 2001;19:127–139, ix. [DOI] [PubMed] [Google Scholar]
- 40. Kuge Y, Takai N, Ishino S, Temma T, Shiomi M, Saji H. Distribution profiles of membrane Type‐1 matrix metalloproteinase (MT1‐MMP), matrix metalloproteinase‐2 (MMP‐2) and cyclooxygenase‐2 (COX‐2) in rabbit atherosclerosis: comparison with plaque instability analysis. Biol Pharm Bull 2007;30:1634–1640. [DOI] [PubMed] [Google Scholar]
- 41. Newby AC, Libby P, van der Wal AC. Plaque instability–the real challenge for atherosclerosis research in the next decade? Cardiovasc Res 1999;41:321–322. [PubMed] [Google Scholar]
- 42. Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol 2009;218:7–29. [DOI] [PubMed] [Google Scholar]
- 43. Krupinski J, Font A, Luque A, Turu M, Slevin M. Angiogenesis and inflammation in carotid atherosclerosis. Front Biosci 2008;13:6472–6482. [DOI] [PubMed] [Google Scholar]
- 44. Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 2005;3:63–68. [DOI] [PubMed] [Google Scholar]
- 45. Briley‐Saebo KC, Cho YS, Tsimikas S. Imaging of oxidation‐specific epitopes in atherosclerosis and macrophage‐rich vulnerable plaques. Curr Cardiovasc Imaging Rep 2011;4:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wagsater D, Zhu C, Bjorkegren J, Skogsberg J, Eriksson P. MMP‐2 and MMP‐9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(‐/‐)Apob(100/100) mouse. Int J Mol Med 2011;28:247–253. [DOI] [PubMed] [Google Scholar]
- 47. Boneberg EM, Illges H, Legler DF, Furstenberger G. Soluble CD146 is generated by ectodomain shedding of membrane CD146 in a calcium‐induced, matrix metalloprotease‐dependent process. Microvasc Res 2009;78:325–331. [DOI] [PubMed] [Google Scholar]
- 48. Harhouri K, Kebir A, Guillet B, et al. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind‐limb ischemia. Blood 2010;115:3843–3851. [DOI] [PubMed] [Google Scholar]
- 49. Garibaldi S, Barisione C, Ghigliotti G, et al. Soluble form of the endothelial adhesion molecule CD146 binds preferentially CD16 + monocytes. Mol Biol Rep 2012;39:6745–6752. [DOI] [PubMed] [Google Scholar]
- 50. Bardin N, Blot‐Chabaud M, Despoix N, et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 2009;29:746–753. [DOI] [PubMed] [Google Scholar]


