Summary
Background
Sunitinib is an inhibitor of the multiple receptor tyrosine kinases (RTKs) for cancer therapy. Some sunitinib analogues could prevent neuronal death induced by various neurotoxins. However, the neuroprotective effects of sunitinib have not been reported.
Methods
Cerebellar granule neurons (CGNs) and SH‐SY5Y cells were exposed to low‐potassium and MPP + challenges, respectively. MTT assay, FDA/PI staining, Hoechst staining, DAF‐FM, colorimetric nitric oxide synthase (NOS) activity assay, and Western blotting were applied to detect cell viability, NO production, NOS activity, and neuronal NOS (nNOS) expression. Short hairpin RNA was used to decrease nNOS expression. In vitro NOS enzyme activity assay was used to determine the direct inhibition of nNOS by sunitinib.
Results
Sunitinib prevented low‐potassium‐induced neuronal apoptosis in CGNs and MPP +‐induced neuronal death in SH‐SY5Y cells. However, PTK787, another RTK inhibitor, failed to decrease neurotoxicity in the same models. Sunitinib reversed the increase in NO levels, NOS activity, and nNOS expression induced by low potassium or MPP +. Knockdown of nNOS expression partially abolished the neuroprotective effects of sunitinib. Moreover, sunitinib directly inhibited nNOS enzyme activity.
Conclusions
Sunitinib exerts its neuroprotective effects by inhibiting NO overproduction, possibly via the inhibition of nNOS activity and the decrease in nNOS expression.
Keywords: Cancer, Neuroprotection, Nitric oxide, Nitric oxide synthase, Receptor tyrosine kinase, Sunitinib
Introduction
Sunitinib (Sutent, SU11248) is an oral, small molecule approved by the U.S. Food and Drug Administration for the treatment of metastatic renal cell carcinoma and imatinib‐resistant gastrointestinal stromal tumors 1, 2. Sunitinib belongs to 3‐substituted indolin‐2‐ones, which were originally designed to compete with ATP for binding to multiple receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptor‐2 (VEGFR‐2), platelet‐derived growth factor receptor (PDGFR), and c‐KIT 3. Signaling transductions by RTKs play an important role in the angiogenesis, transformation, and proliferation of tumors. Therefore, inhibition of RTK signals by sunitinib leads to reduced tumor vascularization, cancer cell death, and eventually tumor shrinkage 4.
Clinical studies have shown that after oral administration, sunitinib could rapidly reach brain tissue 5, 6. Sunitinib treatment has been shown as safe and efficient in brain metastasis of renal cell carcinoma 7, suggesting that sunitinib is able to penetrate the blood–brain barrier and reach central nervous system (CNS) 8. Interestingly, it has been reported that accumulation of sunitinib in the brain could be restricted by P‐glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2), two ATP‐binding cassette drug efflux transporters in the blood–brain barrier (BBB) 9. And coadministration of elacridar and sunitinib resulted in markedly increased sunitinib accumulation in the brain 9, suggesting that the bioavailability of sunitinib in the brain could be increased by the pharmacological inhibition of the BBB transporters.
Many studies have shown that some anticancer 3‐substituted indolin‐2‐ones could produce neuroprotective effects. For example, SU5416, an analogue of sunitinib, prevented 1‐methyl‐4‐phenylpyridinium ion (MPP+)‐induced neuronal apoptosis in vitro and 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced neurotoxicity in vivo 10. SU4312, another analogue of sunitinib, decreased MPTP‐induced loss of dopaminergic neurons, reduced expression of mRNA for tyrosine hydroxylase, and impaired swimming behavior in zebrafish 11. The neuroprotective actions of these 3‐substituted indolin‐2‐ones are independent of their RTK inhibition, but are via the inhibition of nitric oxide (NO) overproduction 10, 11. Therefore, we speculated that sunitinib might also possess neuroprotective property. And this neuroprotective property of sunitinib might be independent of its anti‐RTK action.
Cerebellar granule neurons (CGNs) are among the most abundant neuronal types in the mammalian CNS. Many RTKs, such as VEGFR‐2, PDGFR, and c‐KIT, are present in CGNs 12, 13, 14. If cultured CGNs are subjected to certain stimuli/stress (e.g., low potassium), they would undergo apoptosis 15. Given the relative homogeneity of CGNs and the ease with which apoptosis can be reliably induced in them, cultured CGNs have been proved to be a facile in vitro model system for screening neuroprotectants 15. In addition, SH‐SY5Y cells are often used as in vitro models of neuronal function. They expressed many RTKs such as VEGFR‐2, PDGFR, and c‐KIT 16, 17, 18. Treatment of SH‐SY5Y cells with MPP+ represents one of the most widely used experimental neuronal models for screening neuroprotective drugs 19.
In this study, we discovered that sunitinib protected against low‐potassium‐induced neuronal apoptosis in CGNs and MPP+‐induced neuronal death in SH‐SY5Y cells. We further demonstrated that sunitinib exhibited its neuroprotective effects by inhibiting NO overproduction.
Materials and methods
Materials
Unless otherwise noted, all media and supplements used for cell cultures were purchased from Invitrogen (Carlsbad, CA, USA). Sunitinib and PTK787 were obtained from LC Laboratories (Woburn, MA, USA). Poly‐L‐lysine, fluorescein diacetate (FDA), propidium iodide (PI), Hoechst 33342, and MPP+ were obtained from Sigma Chemicals (St Louis, MO, USA). NG‐Monomethyl‐l‐arginine (L‐NMMA) and 7‐nitroindazole (7‐NI) were purchased from Calbiochem (San Diego, CA, USA). Sunitinib was dissolved in DMSO at a concentration of 4 mM. 7‐NI was dissolved in DMSO at a concentration of 100 mM. Sunitinib and 7‐NI were further diluted with Milli‐Q water before use. Other chemicals were prepared in Milli‐Q water.
Primary Cerebellar Granule Neuron Cultures
Cerebellar granule neurons were prepared from 8‐day‐old Sprague‐Dawley rats (The Animal Care Facility, The Hong Kong Polytechnic University) as described in our previous publication 15. Briefly, neurons were seeded at a density of 2.7 × 105 cells/mL in basal modified Eagle's (BME) medium containing 10% fetal bovine serum, 25 mM KCl, 2 mM glutamine, and penicillin (100 units/mL)/streptomycin (100 μg/mL). Cytosine arabinoside (10 μM) was added to the culture medium 24 h after plating to limit the growth of non‐neuronal cells.
SH‐SY5Y Cell Culture
The human neuroblastoma SH‐SY5Y cells were obtained from American Type Culture Collection (ATCC). The cells were maintained in supplemented Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 37°C, 5% CO2 incubator. All experiments were carried out for 48 h after the cells were seeded.
Measurement of Neurotoxicity
The percentage of surviving neurons was determined by the activity of mitochondrial dehydrogenases with 3(4,5‐dimethylthiazol‐2‐yl)‐2.5‐diphenyltetrazolium bromide (MTT) assay 20. The assay was performed according to the specifications of the manufacturer (MTT kit I; Roche Applied Science, Mannheim, Germany). Briefly, the neurons were cultured in 96‐well plates, and 10 μL of 5 mg/mL MTT labeling reagent was added to each well containing cells in 100 μL of medium. Plates were incubated at 37°C for 4 h in a humidified incubator. After the incubation, 100 μL of the solvating solution (0.01 N HCl in 10% SDS solution) was added to each well for 16–20 h. The absorbance of the samples was measured at a wavelength of 570 nm with 655 nm as a reference wavelength. Unless otherwise indicated, the extent of MTT conversion in CGNs placed at 25K medium and SH‐SY5Y cells exposed to MPP+ is expressed as a percentage of the control.
FDA/PI Double‐Staining Assay
Viable neurons were stained with fluorescein formed from FDA, which is de‐esterified only by living cells. PI can penetrate cell membranes of dead cells to intercalate into double‐stranded nucleic acids. Briefly, neurons were washed twice with ice‐cold PBS. After incubation with 10 μg/mL FDA and 5 μg/mL PI for 15 min, the neurons were examined and photographed using a fluorescence microscope (Nikon Instruments Inc. Melville, NY, USA).
Hoechst Staining
Chromatin condensation was detected by staining the cell nucleus with Hoechst 33342 as described in our previous publication 15. CGNs were grown in a 35‐mm dish. After treatment, cells were washed with ice‐cold PBS, fixed with 4% formaldehyde in PBS for 15 min, membrane‐permeabilized in 0.1% Triton X‐100 for 15 min, and blocked in 1% BSA. Cells were then stained with Hoechst 33342 (5 μg/mL) at 4°C for 5 min. Images were acquired using a fluorescence microscope (Nikon Instruments Inc.) at ×100 magnification. Ultraviolet excitation and emission wavelengths were used to obtain images of nuclei labeled with Hoechst 33342. To quantify the percentage of apoptotic nuclei in each group, photographs of each dish (n = 3 dishes in each group for three independent experiments) were taken at five random fields and the number of pyknotic nuclei and total nuclei (n = 300) were counted, and the percentage of pyknotic nuclei was averaged.
Measurement of Intracellular NO
Intracellular NO was monitored with 4‐amino‐5‐methylamino‐2′,7′‐difluorofluorescein (DAF‐FM) diacetate, a pH‐insensitive fluorescent dye that emits increased fluorescence after reaction with an active intermediate of NO formed during the spontaneous oxidation of NO to NO2 21. DAF‐FM (Invitrogen) was dissolved in DMSO at a concentration of 1 mM and stored frozen at −20°C. DAF‐FM was further diluted with Milli‐Q water before use and added to CGNs at 4 h after low‐potassium challenge or SH‐SY5Y cells at 24 h after MPP+ challenge (final concentration: 5 μM). After incubation for 30 min in a CO2 incubator, cells were washed twice with ice‐cold PBS and incubated for another 30 min to allow complete de‐esterification of the intracellular diacetate for strong fluorescence. The DAF‐FM fluorescence in cells was quantified by a multidetection microplate reader using excitation and emission wavelengths of 495 and 515 nm, respectively. The measured fluorescence values were expressed as a percentage of the fluorescence in the control cells.
shRNA Design
ShRNA against rat nNOS was designed according to a previous publication 22. Briefly, the siRNA sequence GCACUGGUGGAGAUCAACA, which corresponds to exon 10 of the rat nNOS (GenBank Accession No. NM_052799), was used to generate shRNA. Oligonucleotides that contained the sense and antisense sequences of the siRNA target of interest flanking a standard hairpin loop sequence (TTCAAGAGA) were synthesized. Sense and antisense oligonucleotides were then annealed and cloned into pG418‐GFP vector to express shRNA directed against nNOS under the control of the U6 promoter (GenePharma, Shanghai, China). A negative control shRNA (ShNC) with the same nucleotide composition, but lacking significant sequence homology to the genome, was also used in the experiments.
Cell Transfection
2.0 × 105 cells were transfected with 3 μg of indicated plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Selection media that contained 100 μg/mL G418 (Sigma Chemicals) were added to the cells 24 h after transfection. The expression of nNOS in transfected cells was detected by Western blotting at 48 h after transfection.
Western Blotting Analysis
Briefly, 24 h after MPP+ treatment, SH‐SY5Y cells were harvested in a cell lysis buffer. Protein was separated on SDS–polyacrylamide gel and transferred onto polyvinyl difluoride membranes. After membrane blocking, proteins were detected using primary antibodies. Antibody against nNOS was obtained from Cell Signaling Technology (Beverly, MA, USA). Antibody against β‐actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). After incubation at 4°C overnight, signals were obtained after binding to chemiluminescent secondary antibodies (Santa Cruz, CA, USA). Blots were developed using an ECL plus kit (Amersham Bioscience, Aylesbury, UK) and exposed to Kodak autoradiographic films.
Colorimetric NOS Activity Assay
For the NOS activity assay in cells, the activity of NOS was measured in SH‐SY5Y cells by a colorimetric assay kit (Calbiochem, Darmstadt, Germany) 20. The total levels of nitrate and nitrite were detected for indirect reflection of NOS activity by following the kit's instructions. 30 μL of supernatant of SH‐SY5Y cells from 96‐well plates exposed to 1 mM MPP+ for 24 h was transferred into a fluorescent plate and incubated with 10 μL of nitrate reductase and enzyme cofactors for 40 min at room temperature. Then 10 μL of 2,3‐diaminonaphthalene was used as the substrate to react with the nitrite for 20 min. The absorbance of the samples was measured at a wavelength of 540 nm.
In vitro NOS Activity Assay
In vitro NOS activity was determined by monitoring the conversion of L‐[3H]arginine to [3H]citrulline following the instructions provided by the kit (Calbiochem, San Diego, CA, USA). The reaction mixture contained a final volume of 40 μL with 25 mM Tris‐Cl at pH 7.4, 3 μM tetrahydrobiopterin, 1 μM FAD, 1 μM FMN, 1 mM NADPH, 0.6 mM CaCl2, 0.1 μM calmodulin, 2.5 μg of pure nNOS enzyme, 5 μl L‐[3H]arginine (Perkin Elmer, Waltham, MA, USA), and 1‐100 μM of sunitinib. The reaction mixture was incubated at 22°C for 45 min. The reaction was quenched by adding 400 μL of stopping buffer (50 mM HEPES, pH 5.5, and 5 mM EDTA). Unreacted L‐[3H]arginine was then trapped by 100 μL of equilibrated resin in a spin cup followed by centrifugation at 13,200 rpm for 30 s. The filtrate was quantified by liquid scintillation counting.
Data Analysis and Statistics
Data were expressed as the mean ± SEM, and statistical significance was determined by analysis of variance with Dunnett's test in the case of multiple comparisons with control or Tukey's test. Differences were accepted as significant at P < 0.05.
Results
Sunitinib, but Not PTK787, Partially Prevents Low‐Potassium‐Induced Neuronal Apoptosis in CGNs
We previously reported that low potassium could induce typical apoptosis in CGNs 23. At 8 days in vitro, CGNs were switched to the 5 mM KCl BME medium (5K medium, low‐potassium challenge) containing gradually increased concentrations of sunitinib (0.1–2 μM). CGNs placed at 25 mM KCl BME medium (25K medium) were regarded as a control. We measured the cell viability with an MTT assay 24 h after the low‐potassium treatment. We found that sunitinib partially prevented low‐potassium‐induced cell death in CGNs in a concentration‐dependent manner (Figure 1A). Treatments with 2 μM sunitinib in 25K medium for 26 h did not show any cell‐proliferative or cytotoxic effects in CGNs (Data not shown). PTK787 (another RTK inhibitor), L‐NMMA (an NOS inhibitor, IC50 value of 0.65 μM for nNOS), and 7‐NI (an NOS inhibitor, IC50 value of 0.7 μM for nNOS) were also tested in the same model. Interestingly, PTK787 at both 3 and 10 μM failed to block neuronal death in vitro (Figure 1A). L‐NMMA at 10–30 μM and 7‐NI at 5–10 μM prevented low‐potassium‐induced neuronal death in CGNs (Figure 1A).
Figure 1.
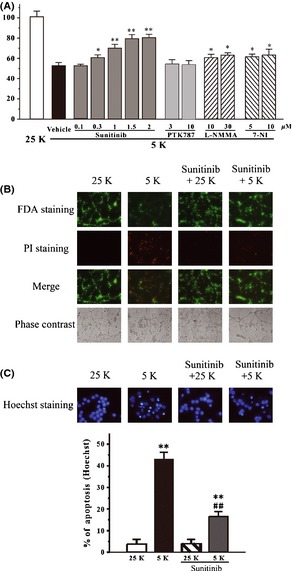
Sunitinib blocks low‐potassium‐induced neuronal apoptosis in CGNs. (A) Sunitinib, but not PTK787, prevented low‐potassium‐induced neuronal death in a concentration‐dependent manner. At 8 days in vitro, CGNs were switched to 5K medium containing sunitinib, PTK787, L‐NMMA, 7‐NI, or DMSO (0.05%; vehicle control; black bar) at indicated concentrations. CGNs placed at 25K medium (white bar) were regarded as a control. Cell viability was measured by MTT assay at 24 h after low‐potassium challenge. (B) Sunitinib blocked neuronal death induced by low potassium. CGNs were switched to 5K or 25K medium with or without 1.5 μM sunitinib. After 24 h of low‐potassium challenge, CGNs were assayed with FDA/PI double staining. (C) Sunitinib reversed the morphological alteration induced by low potassium. CGNs were switched to 5K or 25K medium with or without 1.5 μM sunitinib. After 24 h of low‐potassium challenge, CGNs were assayed with Hoechst staining. The number of pyknotic nuclei with condensed chromatin were counted from representative Hoechst staining photomicrographs and represented as a percentage of the total number of nuclei counted. Data, expressed as percentage of control (CGNs were cultured in 25K medium), were the mean ± SEM of three separate experiments; *P < 0.05 and **P < 0.01 versus 5K group in (A) or versus control in (C); ## P < 0.01 versus 5K group in (C) (Tukey's test). CGNs, Cerebellar granule neurons.
To further characterize the protective effects of sunitinib against neurotoxicity induced by low potassium, CGNs were switched to the 5K medium containing 1.5 μM sunitinib for 24 h. The neurons were examined by FDA/PI double staining. It was found that sunitinib significantly blocked the loss of neurons and reversed the morphological alteration induced by low potassium, including unhealthy bodies and broken extensive neuritic network (Figure 1B and C). According to the number of pyknotic bodies stained by Hoechst 33342, sunitinib significantly prevented neuronal apoptosis induced by low potassium in CGNs (Figure 1C).
Sunitinib Prevents Low‐Potassium‐Induced Increase of Intracellular NO Release in CGNs
To investigate whether sunitinib protected against low‐potassium‐induced neurotoxicity by acting on intracellular NO production, an intracellular NO measurement was used in our study. Low potassium induced an increase in intracellular NO level in a time‐dependent manner with a peak at 4 h after low‐potassium treatment in CGNs (Figure 2A). When CGNs were treated with sunitinib and low potassium simultaneously, sunitinib antagonized the stimulatory effect of low potassium on intracellular NO production (Figure 2B).
Figure 2.
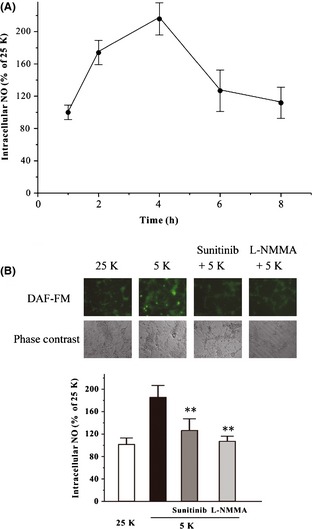
Sunitinib blocks low‐potassium‐induced NO overproduction in CGNs. (A) Low‐potassium challenge increases the level of intracellular NO in CGNs in a time‐dependent manner. CGNs were exposed to 5K medium for various durations as indicated. The intracellular NO level was measured using DAF‐FM diacetate after the low‐potassium challenge. (B) Sunitinib reversed the increased intracellular NO induced by low potassium. CGNs were switched to the 5K medium containing 1.5 μM sunitinib, 10 μM L‐NMMA, or DMSO (0.05%; vehicle control; black bar). CGNs placed at 25K medium (white bar) were regarded as a control (white bar). Intracellular NO level was measured at 4 h after low‐potassium challenge. Data, expressed as percentage of control (CGNs were cultured in 25K medium), were the mean ± SEM of three separate experiments; **P < 0.01 versus low‐potassium group (anova and Dunnett's test). CGNs, Cerebellar granule neurons; NO, nitric oxide.
Sunitinib Partially Prevents MPP+‐Induced Neurotoxicity and NO Overproduction in SH‐SY5Y Cells
We further determined whether sunitinib could exhibit neuroprotective effects against NO‐related neurotoxicity induced by other neurotoxins. Previous studies have shown that NO is implicated in the neurotoxicity of MPP+ 24, 25. SH‐SY5Y cells were pretreated with gradually increased concentrations of sunitinib for 2 h and then treated with 1 mM MPP+ for another 24 h. Cell viability was measured using the MTT assay. Sunitinib partially prevented MPP+‐induced neuronal death at 0.1–0.3 μM 26. Treatment with 0.3 μM sunitinib alone for 26 h did not show any cell‐proliferative or cytotoxic effects. However, sunitinib at a higher concentration (1 μM) showed toxicity to SH‐SY5Y cells (data not shown). PTK787, L‐NMMA and 7‐NI were also tested in this model. PTK787 at 1 or 3 μM failed to block neuronal loss induced by MPP+ in SH‐SY5Y cells (Figure 3A). L‐NMMA at 30 μM and 7‐NI at 5–10 μM prevented MPP+‐induced neuronal death in SH‐SY5Y cells (Figure 3A).
Figure 3.
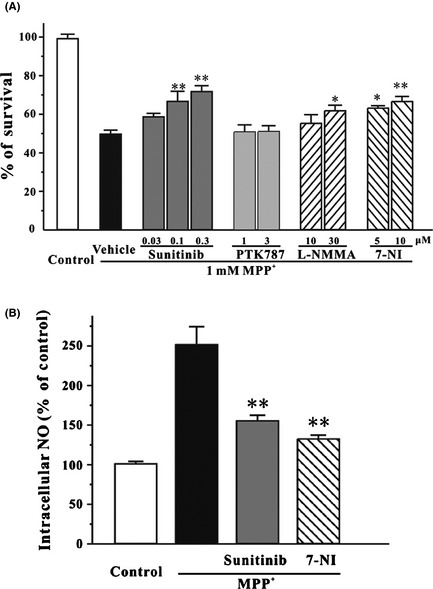
Sunitinib reverses MPP +‐induced neurotoxicity and NO overproduction in SH‐SY5Y cells. (A) Sunitinib, but not PTK787, prevented MPP +‐induced neuronal death in a concentration‐dependent manner. SH‐SY5Y cells were treated with sunitinib, PTK787, L‐NMMA, 7‐NI, or DMSO (0.05%; vehicle control; black bar) at the indicated concentrations for 2 h and then exposed to 1 mM MPP +. SH‐SY5Y cells placed at normal medium (white bar) were regarded as a control. Cell viability was measured by the MTT assay at 24 h after MPP + challenge. (B) Sunitinib reverses the increased intracellular NO induced by MPP +. SH‐SY5Y cells were pre‐incubated with 0.3 μM sunitinib, 5 μM 7‐NI, or 0.05% DMSO (vehicle control, black bar) for 2 h and exposed to 1 mM MPP +. SH‐SY5Y cells placed at normal medium (white bar) were regarded as a control. Intracellular NO level was measured at 24 h after MPP + challenge. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments; *P < 0.05 and **P < 0.01 versus MPP + group (anova and Dunnett's test). NO, nitric oxide.
DAF‐FM diacetate was also used to evaluate the intracellular NO level in this model. It was found that 0.3 μM sunitinib attenuated the MPP+‐triggered elevation of intracellular NO level, suggesting that sunitinib prevented MPP+‐induced neuronal death through inhibiting NO overproduction (Figure 3B).
Sunitinib Decreases Endogenous NOS Activity Elevated by MPP+ in SH‐SY5Y Cells
To examine whether sunitinib could inhibit endogenous NOS activity in SH‐SY5Y cells, a colorimetric NOS activity assay was used in our study. SH‐SY5Y cells were pretreated with sunitinib or 7‐NI for 2 h and then treated with 1 mM MPP+ for another 24 h. The total levels of nitrate and nitrite were detected for indirect reflection of endogenous NOS activity. Sunitinib at 0.3 μM significantly decreased endogenous NOS activity increased by MPP+ in SH‐SY5Y cells (Figure 4).
Figure 4.
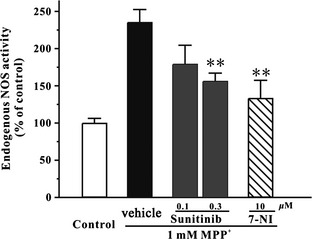
Sunitinib prevents MPP +‐induced enhancement of endogenous NOS activity in SH‐SY5Y cells. SH‐SY5Y cells were pre‐incubated with sunitinib, 7‐NI, or DMSO (0.05%; vehicle control; black bar) at the indicated concentrations for 2 h and exposed to 1 mM MPP +. SH‐SY5Y cells placed at normal medium (white bar) were regarded as a control. Endogenous NOS activity was measured at 24 h after MPP + challenge using colorimetric NOS activity assay. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments; **P < 0.01 versus MPP + group (anova and Dunnett's test). NOS, nitric oxide synthase.
Sunitinib Reduces the Expression of nNOS Increased by MPP+ in SH‐SY5Y Cells
To determine whether sunitinib affected the expression of nNOS in SH‐SY5Y cells, Western blotting analysis was used in our study. As shown in Figure 5, sunitinib at 0.3 μM significantly reversed the increased expression of nNOS by MPP+.
Figure 5.
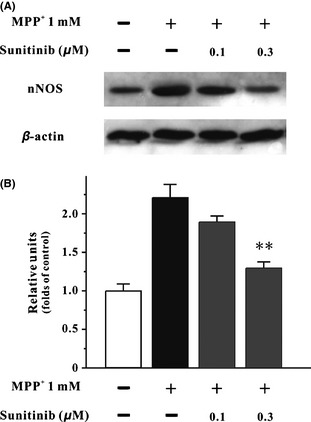
Sunitinib reduces the expression of nNOS increased by MPP + in SH‐SY5Y cells. (A) SH‐SY‐5Y cells were pretreated with sunitinib or DMSO (0.05%, vehicle control) at the indicated concentrations for 2 h and then treated with 1 mM MPP + for 24 h. The total proteins were extracted for Western blot analysis with specific nNOS and β‐actin antibodies. (B) Statistic analysis of nNOS expression in each treatment group. Data were expressed as the ratio of OD values to the corresponding controls. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments; **P < 0.01 versus MPP + group (anova and Dunnett's test). nNOS, neuronal nitric oxide synthase.
nNOS Reduction Abolishes the Neuroprotective Effects of Sunitinib in SH‐SY5Y Cells
To further determine whether the neuroprotective effects of sunitinib were through the inhibition of NO overproduction, we investigated the effects of sunitinib on MPP+‐induced neurotoxicity in ShRNA‐mediated nNOS knockdown in SH‐SY5Y cells. Western blot analysis showed that nNOS ShRNA (ShnNOS) caused a reduction in nNOS protein level (Figure 6A). Analyses of cell viability revealed that nNOS reduction resulted in a significant decrease in MPP+‐induced cell death (Figure 6B). We also found that in contrast to the neuroprotective effects of sunitinib observed in the vector or in the ShNC‐treated SH‐SY5Y cells, sunitinib in ShRNA‐mediated nNOS knockdown in SH‐SY5Y cells did not significantly inhibit MPP+‐induced cell death (Figure 6B). These results provided direct evidence for the mechanism of the neuroprotective effects of sunitinib as acting through the inhibition of NO overproduction.
Figure 6.
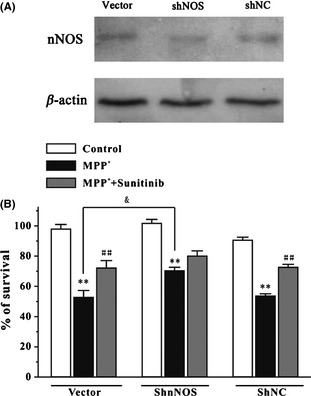
nNOS reduction abolishes the neuroprotective effects of sunitinib against MPP +‐induced neuronal death in SH‐SY5Y cells. (A) SH‐SY5Y cells were transfected with pG418‐GFP plasmid (vector), pG418‐GFP plasmid encoding nNOS ShRNA (ShnNOS), and pG418‐GFP plasmid encoding negative control ShRNA (ShNC). Forty‐eight hours after transfection, the level of nNOS and β‐actin in the cell lysates was analyzed by Western blotting assay. (B) nNOS reduction abolished the neuroprotective effects of sunitinib against MPP +‐induced neuronal death. SH‐SY5Y cells transfected with vector, ShnNOS, or ShNC were treated with or without 0.3 μM sunitinib for 2 h and then exposed to 1 mM MPP +. Cell viability was measured at 24 h after MPP + challenge by MTT assays. Data were the mean ± SEM of three separate experiments; **P < 0.01 versus control; ## P < 0.01 versus MPP + group; & P < 0.05 versus MPP + vector group (Tukey's test). nNOS, neuronal nitric oxide synthase.
Sunitinib Directly Inhibits nNOS Enzyme Activity in a Concentration‐Dependent Manner
Furthermore, an in vitro NOS activity assay was used to investigate whether sunitinib directly affected the enzyme activity of NOS. L‐[3H]arginine, pure nNOS enzyme, and sunitinib were mixed in a test tube. nNOS activity was determined by monitoring the conversion of l‐[3H]arginine to [3H]citruline by nNOS. It was found that sunitinib directly inhibited nNOS in a concentration‐dependent manner with an IC50 value of 9.6 μM (Figure 7). However, sunitinib at 100 μM showed no inhibitory activity against iNOS or eNOS (Data now shown).
Figure 7.
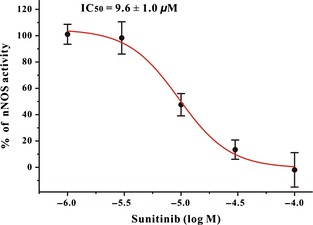
Sunitinib directly inhibits nNOS enzyme activity in a concentration‐dependent manner. The inhibitory effects of sunitinib on rat cerebellum nNOS were shown in the graph. The IC 50 value was also indicated in the graph. Each point was an average of three independent experiments. nNOS, neuronal nitric oxide synthase.
Discussion
Sunitinib is a multiple RTK inhibitor approved by the U.S. Food and Drug Administration for the treatment of cancer 1, 2. Many studies have shown that several sunitinib analogues could produce potent neuroprotective effects via the inhibition of NO overproduction 10, 11, suggesting that sunitinib might also possess neuroprotective effects. In this study, we have for the first time demonstrated that sunitinib protects against low‐potassium‐induced neuronal apoptosis in CGNs and MPP+‐induced neuronal death in SH‐SY5Y cells. Our study further revealed that these neuroprotective effects of sunitinib are mediated by inhibition of NO overproduction.
Sunitinib is a multiple RTK inhibitor that mainly targets VEGFR‐2 (IC50 values of 0.01 μM for VEGFR‐2) 27. Most RTKs, including VEGFR‐2, PDGFR, and c‐KIT, are present in CGNs and SH‐SY5Y cells 12, 13, 14, 16, 17, 18. In our previous study, we have measured the activity of VEGFR‐2, a main target of sunitinib, in low‐potassium‐ and MPP+‐challenged CGNs 12. Either low potassium or MPP+ decreased the activity of VEGFR‐2; and chemicals that act on VEGFR‐2 could prevent neuronal death via the activation of VEGFR‐2 12. Therefore, the inhibition of VEGFR‐2 should worsen rather than prevent low‐potassium‐ or MPP+‐induced neuronal death. However, sunitinib produced neuroprotective effects in our present study, suggesting that the neuroprotective effects of sunitinib might be independent of its anti‐VEGFR‐2 action. To further clarify that the neuroprotective effects of sunitinib were independent of its inhibition of VEGFR‐2, PTK787 (Vatalanib IC50 values of 0.037 μM for VEGFR‐2), another RTK inhibitor, was assessed in parallel 28. Although PTK787 inhibits many RTKs, it is most selective for VEGFR‐2 28. Interestingly, PTK787 at 1–10 μM could not prevent neuronal death, suggesting that the neuroprotective effects of sunitinib were not closely correlated with its anti‐VEGFR‐2 property.
Although NO is not the principal mediator of neuronal death in Parkinson's disease or Alzheimer's disease, it serves as a pro‐apoptotic factor to mediate the neurotoxicity of low potassium in CGNs and that of MPP+ in SH‐SY5Y cells 24, 25, 29. We found that: (1) nNOS inhibitors reduced neuronal death induced by low potassium or MPP+; and (2) sunitinib decreased the increased level of intracellular NO induced by low potassium or MPP+, suggesting that sunitinib might exert its neuroprotective effects by inhibition of NO overproduction.
To further explore whether sunitinib reduced NO overproduction by inhibition endogenous NOS, Western blotting and colorimetric NOS activity assays were used in our study. We have shown that sunitinib significantly decreased both NOS activity and nNOS protein expression elevated by MPP+. Moreover, ShRNA‐mediated nNOS reduction abolished the neuroprotective effects of sunitinib. These results suggest that sunitinib decreases NO overproduction by reducing nNOS expression and inhibiting NOS activity.
However, sunitinib and other NOS inhibitors could not produce full neuroprotection in our study. We speculated that although NO was implicated in the neurotoxicity induced by low potassium in CGNs and by MPP+ in SH‐SY5Y cells, the contribution of NO in the neurotoxicity in these models is partial. Other factors such as impairment of cellular energy metabolism and dysregulation of mitogen‐activated protein kinase signaling pathways are also involved in the neurotoxicity in these models 30, 31, 32. Therefore, the neuroprotective effects of sunitinib and NOS inhibitors are not completed.
Previous studies have shown that SU4312 and SU5416, two sunitinib analogues, could directly inhibit nNOS in vitro 10, 11. Moreover, a favorable interaction between indolin‐2‐one structure of SU4312 and the heme group inside nNOS protein has been identified 11. As sunitinib has a chemical structure similar to that of SU4312 and also possesses indolin‐2‐one structure, we speculated that sunitinib might also act on nNOS. An in vitro NOS activity assay was performed by mixing l‐[3H]arginine, pure nNOS enzyme, and sunitinib in a test tube. nNOS activity was determined by monitoring the conversion of l‐[3H]arginine to [3H]citruline by nNOS. By using this assay, we demonstrated that sunitinib directly inhibited the enzyme activity of nNOS. However, the large difference between the concentrations of sunitinib required to prevent neuronal death and to directly inhibit nNOS, suggests that the neuroprotective effects of sunitinib may not mainly come from the inhibition of nNOS enzyme activity. We speculated that sunitinib might act on other targets to produce neuroprotective effects. For example, sunitinib might downregulate PI3‐K/Akt signaling pathway, a critical mediator in the activation of nNOS gene transcription, to reduce nNOS expression, or block AMP‐activated protein kinase to inhibit NOS activity 33. To investigate the precise mechanisms underlying the neuroprotective effects of sunitinib, further experiments are being carried out in our laboratory.
Sunitinib has additional targets besides VEGFR‐2 and nNOS. For example, sunitinib could also inhibit Ret, an RTK coreceptor for glial‐cell‐line‐derived neurotrophic factor (GDNF). Previous studies have shown that GDNF is a potent neurotrophic factor for the development and survival of dopaminergic neurons 34, 35. Ret ablation could cause progressive loss and adult onset of dopaminergic neurons 36, suggesting that sunitinib might play dual role in neuroprotection and neurodegeneration when applied in the brain.
In conclusion, our findings demonstrated that sunitinib protected against low‐potassium‐induced neuronal apoptosis in CGNs and MPP+‐induced neuronal death in SH‐SY5Y cells. Although further studies are still needed to elucidate the precise neuroprotective mechanisms of sunitinib, our results suggest that sunitinib exerts its neuroprotective effects by inhibiting NO overproduction, possibly via the inhibition of nNOS activity and the decrease in nNOS expression.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the Research Grants Council of Hong Kong (PolyU5609/09M, PolyU5610/11M), The Hong Kong Polytechnic University (G‐U952, G‐YM32 and G‐YZ15), The University of Hong Kong (HKU 775812M), the Science and Technology Development Fund (FDCT) of Macao SAR (078/2011/A3 and 045/2007/A3), the Research Committee of the University of Macau (MYRG139(Y1‐L4)‐ICMS12‐LMY and UL017/09‐Y1), the National Natural Science Foundation of China (81202510), Medical Scientific Research Foundation of Guangdong Province (A2013353), and the Hong Kong Scholar Program jointly funded by the Hong Kong Polytechnic University and the Chinese Government (122870). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We sincerely thank Ms. Josephine Leung for proofreading our manuscript.
The first two authors contributed equally to this work.
References
- 1. Rock EP, Goodman V, Jiang JX, et al. Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 2007;12:107–113. [DOI] [PubMed] [Google Scholar]
- 2. Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther 2007;29:1338–1353. [DOI] [PubMed] [Google Scholar]
- 3. Sun L, Tran N, Tang F, et al. Synthesis and biological evaluations of 3‐substituted indolin‐2‐ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem 1998;41:2588–2603. [DOI] [PubMed] [Google Scholar]
- 4. Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov 2007;6:734–745. [DOI] [PubMed] [Google Scholar]
- 5. Patyna S, Peng G. Distribution of sunitinib and its active metabolite in brain and spinal cord tissue following oral or intravenous administration in rodents and monkeys. Eur J Cancer Suppl 2006;12:21. [Google Scholar]
- 6. van der Veldt AA, van den Eertwegh AJ, Hoekman K, Barkhof F, Boven E. Reversible cognitive disorders after sunitinib for advanced renal cell cancer in patients with preexisting arteriosclerotic leukoencephalopathy. Ann Oncol 2007;18:1747–1750. [DOI] [PubMed] [Google Scholar]
- 7. Medioni J, Cojocarasu O, Belcaceres JL, Halimi P, Oudard S. Complete cerebral response with sunitinib for metastatic renal cell carcinoma. Ann Oncol 2007;18:1282–1283. [DOI] [PubMed] [Google Scholar]
- 8. Addeo R, Caraglia M. The oral tyrosine kinase inhibitors lapatinib and sunitinib: new opportunities for the treatment of brain metastases from breast cancer? Expert Rev Anticancer Ther 2011;11:139–142. [DOI] [PubMed] [Google Scholar]
- 9. Tang SC, Lagas JS, Lankheet NA, et al. Brain accumulation of sunitinib is restricted by P‐glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer 2012;130:223–233. [DOI] [PubMed] [Google Scholar]
- 10. Cui W, Zhang Z, Li W, et al. Unexpected neuronal protection of SU5416 against 1‐Methyl‐4‐phenylpyridinium ion‐induced toxicity via inhibiting neuronal nitric oxide synthase. PLoS One 2012;7:e46253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui W, Zhang Z, Li W, et al. The anti‐cancer agent SU4312 unexpectedly protects against MPP(+) ‐induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br J Pharmacol 2013;168:1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui W, Li W, Han R, et al. PI3‐K/Akt and ERK pathways activated by VEGF play opposite roles in MPP+‐induced neuronal apoptosis. Neurochem Int 2011;59:945–953. [DOI] [PubMed] [Google Scholar]
- 13. Nait Oumesmar B, Vignais L, Baron‐Van Evercooren A. Developmental expression of platelet‐derived growth factor alpha‐receptor in neurons and glial cells of the mouse CNS. J Neurosci 1997;17:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manova K, Bachvarova RF, Huang EJ, et al. c‐kit receptor and ligand expression in postnatal development of the mouse cerebellum suggests a function for c‐kit in inhibitory interneurons. J Neurosci 1992;12:4663–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, Pi R, Chan HH, et al. Novel dimeric acetylcholinesterase inhibitor bis7‐tacrine, but not donepezil, prevents glutamate‐induced neuronal apoptosis by blocking N‐methyl‐D‐aspartate receptors. J Biol Chem 2005;280:18179–18188. [DOI] [PubMed] [Google Scholar]
- 16. Cohen PS, Chan JP, Lipkunskaya M, Biedler JL, Seeger RC. Expression of stem cell factor and c‐kit in human neuroblastoma. The Children's Cancer Group. Blood 1994;84:3465–3472. [PubMed] [Google Scholar]
- 17. Mohan N, Karmakar S, Banik NL, Ray SK. SU5416 and EGCG work synergistically and inhibit angiogenic and survival factors and induce cell cycle arrest to promote apoptosis in human malignant neuroblastoma SH‐SY5Y and SK‐N‐BE2 cells. Neurochem Res 2011;36:1383–1396. [DOI] [PubMed] [Google Scholar]
- 18. Hynds DL, Summers M, Van Brocklyn J, O'Dorisio MS, Yates AJ. Gangliosides inhibit platelet‐derived growth factor‐stimulated growth, receptor phosphorylation, and dimerization in neuroblastoma SH‐SY5Y cells. J Neurochem 1995;65:2251–2258. [DOI] [PubMed] [Google Scholar]
- 19. Wu F, Poon WS, Lu G, et al. Alpha‐synuclein knockdown attenuates MPP+ induced mitochondrial dysfunction of SH‐SY5Y cells. Brain Res 2009;1292:173–179. [DOI] [PubMed] [Google Scholar]
- 20. Li W, Xue J, Niu C, et al. Synergistic neuroprotection by bis(7)‐tacrine via concurrent blockade of N‐methyl‐D‐aspartate receptors and neuronal nitric‐oxide synthase. Mol Pharmacol 2007;71:1258–1267. [DOI] [PubMed] [Google Scholar]
- 21. Sheng JZ, Wang D, Braun AP. DAF‐FM (4‐amino‐5‐methylamino‐2′,7′‐difluorofluorescein) diacetate detects impairment of agonist‐stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: reversal by vitamin C and L‐sepiapterin. J Pharmacol Exp Ther 2005;315:931–940. [DOI] [PubMed] [Google Scholar]
- 22. Mahairaki V, Xu L, Farah MH, et al. Targeted knock‐down of neuronal nitric oxide synthase expression in basal forebrain with RNA interference. J Neurosci Methods 2009;179:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu H, Dou J, Li W, et al. Mecamylamine prevents neuronal apoptosis induced by glutamate and low potassium via differential anticholinergic‐independent mechanisms. Neuropharmacology 2008;54:755–765. [DOI] [PubMed] [Google Scholar]
- 24. Przedborski S, Jackson‐Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A 1996;93:4565–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hantraye P, Brouillet E, Ferrante R, et al. Inhibition of neuronal nitric oxide synthase prevents MPTP‐induced parkinsonism in baboons. Nat Med 1996;2:1017–1021. [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Smith KM, Chong AL, et al. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia 2009;11:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun 2007;356:323–328. [DOI] [PubMed] [Google Scholar]
- 28. Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor‐induced responses and tumor growth after oral administration. Cancer Res 2000;60:2178–2189. [PubMed] [Google Scholar]
- 29. Bobba A, Atlante A, Moro L, Calissano P, Marra E. Nitric oxide has dual opposite roles during early and late phases of apoptosis in cerebellar granule neurons. Apoptosis 2007;12:1597–1610. [DOI] [PubMed] [Google Scholar]
- 30. Yeste‐Velasco M, Folch J, Trullas R, et al. Glycogen synthase kinase‐3 is involved in the regulation of the cell cycle in cerebellar granule cells. Neuropharmacology 2007;53:295–307. [DOI] [PubMed] [Google Scholar]
- 31. Ma C, Ying C, Yuan Z, et al. dp5/HRK is a c‐Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J Biol Chem 2007;282:30901–30909. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez‐Polo RA, Soler G, Fuentes JM. MPP+: mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol 2004;30:253–264. [DOI] [PubMed] [Google Scholar]
- 33. Aparicio LM, Pulido EG, Gallego GA. Sunitinib‐induced asthenia: from molecular basis to clinical relief. Cancer Biol Ther 2011;12:765–771. [DOI] [PubMed] [Google Scholar]
- 34. Oo TF, Kholodilov N, Burke RE. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line‐derived neurotrophic factor in vivo. J Neurosci 2003;23:5141–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci 2003;116:3855–3862. [DOI] [PubMed] [Google Scholar]
- 36. Kramer ER, Aron L, Ramakers GM, et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol 2007;5:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]


