Alzheimer's disease (AD), the most common form of dementia in the elderly, is still a matter of concern, because there is not an efficient therapeutic treatment. Recently, our group has synthesized and reported N‐((5‐(3‐(1‐benzylpiperidin‐4‐yl)propoxy)‐1‐meth‐yl‐1H‐indol‐2‐yl)methyl)‐N‐methylprop‐2‐yn‐1‐amine (ASS234) (Figure 1A), as a new multitarget compound for the potential treatment and prevention of AD, able to act simultaneously as a reversible inhibitor of both human AChE/BuChE, and as an irreversible inhibitor of human MAO A/B 1. In addition, ASS234 inhibits both Αβ 1–42 and Αβ 1–40 self‐aggregation, limiting fibrillar and oligomeric species formation, showing a significant reduction in Αβ 1–42‐mediated toxicity in SH‐SY5Y human neuroblastoma cells. Moreover, ASS234 completely blocks the aggregation of both Αβ 1–42 and Αβ 1–40 mediated by AChE 2 and crosses the blood–brain barrier 3.
Figure 1.
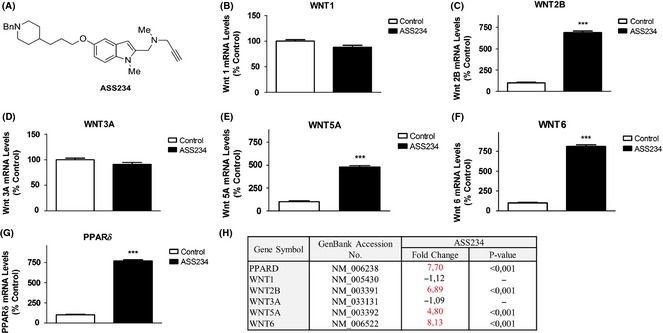
(A) Represents chemical structure of ASS234. (B–G) Shows results from real‐time PCR arrays targeting select genes after ASS234 (5 μM) treatment. (H) Representative table of data, in which specific gene expression was compared with controls [cells treated with DMSO (0.1%) were the negative control]. Each bar represents mean ± SEM of six independent experiments. ACTB was used as an internal control. ***P < 0.001, significantly different from controls.
However, the mechanism by which ASS234 plays a neuroprotective role in AD pathology remains unclear. Recent evidence suggests that the wingless‐type MMTV integration site family (Wnt) signaling pathway is involved in neuroprotective activities related to AD 4, 5, 6, 7, 8. Thus, the aim of this study was to determine whether ASS234 is able to activate Wnt signaling pathway.
For this purpose, we used SH‐SY5Y cells incubated with ASS234 (5 μM) for 24 h. Total RNA was extracted using the Trizol reagent method (Invitrogen, Madrid, Spain). We evaluated the gene expression of some members of Wnt1 class signal (Wnt1, Wnt2b, Wnt3a), which represent the “canonical” Wnt/β‐catenin pathway (Figure 2A), and some members of the Wnt5a class signal (Wnt6, Wnt5a), which represent the “noncanonical” Wnt/PCP and Wnt/Ca2+ pathways (Figure 2B). Relative changes in gene expression were calculated using the Ct (cycle threshold) method. Bioinformatic analysis was carried out with the Ingenuity Pathway Analysis (IPA) software (Ingenuity H Systems, Redwood City, CA, USA; http://www.ingenuity.com).
Figure 2.
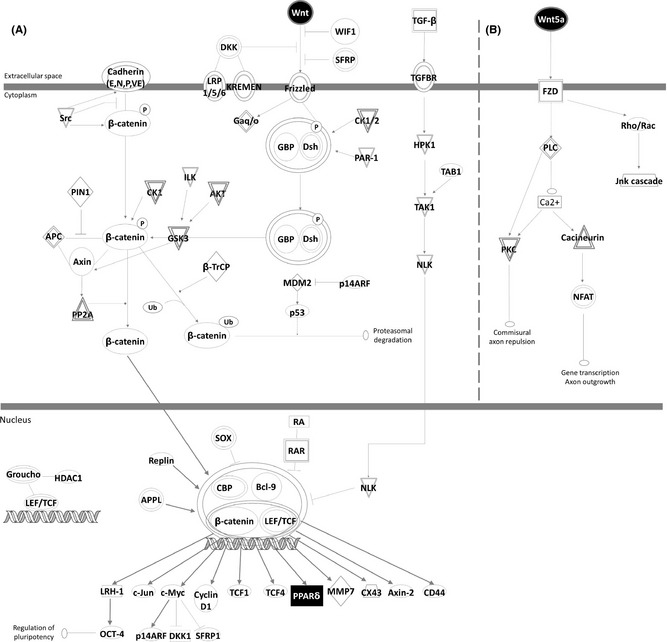
Ingenuity Pathway Analysis (IPA) of ASS234 treatment. (A) The canonical Wnt/β‐catenin pathway. (B) The noncanonical Wnt/PCP and Wnt/Ca2+ pathways. The genes that are shaded were determined to be significant from the statistical analysis. A solid line represents a direct interaction between the two gene products, and a dotted line means there is an indirect interaction. Black represents up‐regulated genes, and white represents not studied genes.
Herein, in ASS234‐treated cells, Wnt2b, Wnt5a, and Wnt6 gene expression was significantly increased (Figure 1C,E,F). IPA provided a number of downstream genes regulated by Wnt canonical pathway (Figure 2A). Furthermore, one of these genes, PPARδ, a key gene related to neuroprotective effects against AD 9, was significantly increased by ASS234 treatment (Figure 1G).
From these results, we concluded that ASS234 is able to induce canonical and noncanonical Wnt pathways, which represent another possible mechanism through which this compound mediates its protective actions. Knowing that the activation of Wnt signaling rescues memory loss and improves synaptic dysfunction in transgenic mice model of AD amyloid pathology 10, these findings indicate that ASS234 could be a novel promising drug for AD therapy, although further studies are needed to explore the relevance of this mechanism in animal models of AD. These studies are in progress and will be reported in due course.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
JMC thanks MINECO (Grant SAF2012‐33304) and UCJC (Grant 2013‐20) for support. OMB‐A thanks MINECO (Spain) for a FPI fellowship.
References
- 1. Bolea I, Juárez‐Jiménez J, de los Ríos C, et al. Synthesis, biological evaluation, and molecular modeling of donepezil and N‐[(5‐(benzyloxy)‐1‐methyl‐1H‐indol‐2‐yl)methyl]‐N‐methylprop‐2‐yn‐1‐amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer's disease. J Med Chem 2011;54:8251–8270. [DOI] [PubMed] [Google Scholar]
- 2. Bolea I, Gella A, Monjas L, et al. The multipotent, permeable drug ASS234 inhibits Aβ aggregation, possesses antioxidant properties and protects from Aβ‐induced apoptosis. Curr Alzheimer Res 2013;9:797–808. [DOI] [PubMed] [Google Scholar]
- 3. Stasiak A, Mussur M, Unzeta M, Samadi A, Marco‐Contelles JL, Fogel WA. Effects of novel monoamine oxidases and cholinesterases targeting compounds on brain neurotransmitters and behavior in rat model of vascular dementia. Curr Pharm Des 2014;20:161–171. [DOI] [PubMed] [Google Scholar]
- 4. Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol 2008;86:281–296. [DOI] [PubMed] [Google Scholar]
- 5. Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther 2008;118:58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reyes AE, Chacon MA, Dinamarca MC, Cerpa W, Morgan C, Inestrosa NC. Acetylcholinesterase‐Ab complexes are more toxic than Ab fibrils in rat hippocampus: Effect on rat b‐amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol 2004;164:2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inestrosa NC, Montecinos‐Oliva C, Fuenzalida M. Wnt signaling: Role in Alzheimer disease and schizophrenia. J Neuroimmune Pharmacol 2012;7:788–807. [DOI] [PubMed] [Google Scholar]
- 8. Inestrosa NC, Urra S, Colombres M. Acetylcholinesterase (AChE)–amyloid‐beta‐peptide complexes in Alzheimer's disease. The Wnt signaling pathway. Curr Alzheimer Res 2004;1:249–254. [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Hama SA, Paek KS, et al. Transcriptional up‐regulation of antioxidant genes by PPARd inhibits angiotensin II‐induced premature senescence in vascular smooth muscle cells. Biochem Biophys Res Commun 2011;406:564–569. [DOI] [PubMed] [Google Scholar]
- 10. Vargas JY, Fuenzalida M, Inestrosa NC. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer's disease model. J Neurosci 2014;34:2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]


