Summary
Aims
This study investigated whether anticerebral ischemia new drug, l‐3‐n‐butylphthalide (l‐NBP), improved behavioral recovery and enhanced hippocampal neurogenesis after cerebral ischemia in rats.
Methods and Results
The middle cerebral artery of rats was blocked for 2 h. The daily oral administrations of 30 mg/kg l‐NBP or vehicle were begun from the second day until the rats were sacrificed. L‐NBP treatment markedly increased 5‐bromo‐2′‐deoxyuridine (BrdU)‐positive cells in the hippocampal dentate gyrus (DG) of injured hemisphere on day 28 after ischemia. The amount of newborn cells and newly mature neurons was also increased. The expressions of growth‐associated protein‐43 and synaptophysin were significantly elevated in l‐NBP‐treated rats. However, l‐NBP markedly reduced the percentage of BrdU+/GFAP + cells. Additionally, the levels of catalytical subunit of protein kinase A (PKA), protein kinase B (Akt), and cAMP response element‐binding protein (CREB) were significantly increased, and the activation of the signal transducer and activation of transcription 3 (STAT3) and the expressions of cleaved caspase‐3 and Bax were obviously inhibited by l‐NBP. Consequently, l‐NBP attenuated the behavioral dysfunction.
Conclusions
It first demonstrates that l‐NBP may improve the behavioral outcome of cerebral ischemia by promoting neurogenesis and neuroplasticity. Activation of CREB and Akt and inhibition of STAT3 signaling might be involved in.
Keywords: Cerebral ischemia, CREB, L‐3‐n‐butylphthalide, Neurogenesis, Neuroplasticity
Introduction
Stroke is the most fatal reason for permanent disability all over the world 1. The strategy of recovering brain function after ischemic insult is attracting more and more attentions. A number of evidence suggests that ischemic significantly increases neurogenesis in the rodent subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus and subventricular zone (SVZ) 2. The ischemia‐induced newborn precursors might migrate into the damaged stratum and become mature neurons 3. But, neuronal stem cells (NSCs) proliferation in the DG reached peak after 7 days. Then, the majority died within several weeks after cerebral ischemic. The survival rate of newly generated cells is very low 4. It indicates that the therapeutics of increasing the survival of NSCs might be beneficial.
It has been reported that traditional Chinese medicine or herbal extracts might enhance neuronal neurogenesis and contribute to functional rebuilt of cerebral ischemia, such as Ginkgo biloba 5, Ginsenosides 6, puerarin 7, and tetramethylpyrazine 8. However, these traditional Chinese medicines have been used only as adjuvant treatment for stroke patients in clinic. The approved antistroke drugs had not been reported to promote neurogenesis.
L‐3‐n‐butylphthalide (l‐NBP) was originally extracted from the seeds of Apium graveolens Linn. Soon, the racemic 3‐n‐butylphthalide (dl‐NBP) was chemically synthesized. Stroke patients in China have used dl‐NBP from 2002, and it has shown good effective effect. Dl‐NBP not only improved acute syndrome, but also contributed to recover the stroke‐related disability, such as walking, limb motor, language, sensor, thinking, and memory 9. Our previous studies found that the neuroprotective function of l‐NBP was similar to or excel than that of dl‐NBP. It has been reported that long‐term functional recovery and hippocampus‐dependent memory after cerebral ischemia were closely associated with hippocampal neurogenesis 10, 11. L‐NBP could reverse cognitive impairments in animal model with chronic cerebral ischemia 12, 13. It is little known whether the effect of NBP on improving functional recovery is related to neurogenesis after ischemic insult. L‐NBP, the origin of NBP, is a natural product and shows more potential in stroke therapeutic. Thus, we are planning to examine whether l‐NBP could enhance hippocampal neurogenesis after focal cerebral ischemia with rat model.
Materials and Methods
Cerebral Ischemia and Reperfusion in Rats
The male Sprague Dawley rats of 8 weeks old were obtained from the Experimental Animal Center, Chinese Academy of Medical Science (Beijing, China). We used 400 mg/kg chloral hydrate to anaesthetize rats. Then, a nylon monofilament suture of 4‐0 was inserted by the external carotid stump into the internal carotid artery of rats. After 2 h, the suture was withdrawn and the circulation was recovered (MCAO). The sham rats underwent the same surgery except the filament insertion. The experimental protocols were approved by institutional guidelines of the Experimental Animal Center of the Chinese Academy of Medical Sciences, Beijing, China.
Drug Administration and Experimental Design
L‐NBP (purity >99.5%) was obtained from Institute of Materia Medica and diluted in vegetable oil. The experiment included three groups: the sham group, the ischemic group, and the l‐NBP group. The daily oral administrations of 30 mg/kg l‐NBP or vehicle were begun from the day after surgery until animals were sacrificed. Sham rats were given an equivalent volume of vegetable oil. To examine whether l‐NBP increases neurogenesis, 50 mg/kg 5‐bromo‐2′‐deoxyuridine (BrdU) was administered twice every day at 8‐h intervals by i.p. injection. The details about the experimental schedule are described in Figure 1.
Figure 1.
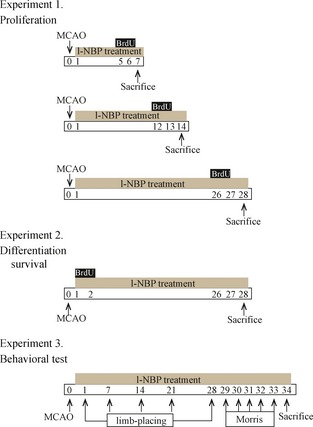
Experiment design. Time points represent days after MCAO induction.
Behavioral Test
Limb‐placing Test
The limb‐placing test was carried out before surgery and on postoperative days 1, 7, 14, 21, and 28 according to the reported method with minor modifications 14. The experiment included 7 limb‐placing tests to check the sensorimotor function of forelimb and hindlimb to sensitivity and proprioceptive stimulation. The detail is shown in Data S1.
Morris Water Maze Task
Morris water maze task 15 was begun from day 29 to day 33 after cerebral ischemia. The protocol followed the previous report 12. Every animal was trained twice each day for 5 days. The intertrial interval was 1 min. Rats were slightly placed into the water from the wall of the pool. We recoded the swimming paths of rats by a video camera with a computer through an image analyzer. The escape latency of finding the hidden platform was determined. The whole time was 60 second. If the rat cannot find the hidden platform during 60 second, the rat was guided to the hidden platform by the experimenter. And the escape latency was considered as 60 second. Each rat was permitted to stand on the platform for 30 second before the experimenter took it from the water.
Immunofluorescence Staining
The rats were transcardially perfused with saline, and followed by 4% paraformaldehyde on days 7, 14, or 28 after MCAO. We took the rats’ brains out and put them in the paraformaldehyde solution. The cryostat sections (40 μm) were cut coronally using a Leica microtome. Five coronal brain sections per DG, spaced 400 μm apart, were selected. For BrdU staining, the method was simply modified according to the previous description 16. The amount of BrdU+/NeuN+ and BrdU+/GFAP+ cells was determined by fluorescence confocal microscopy (BX61; Olympus, Tokyo, Japan).
Western Blots
We homogenized the brain tissues in Tris buffer. Then, the homogenate was centrifuged at 15000 g at 4°C for 15 min. Fifty‐microgram protein samples were loaded and performed electrophoresis and transferred to polyvinylidene fluoride membranes. The membrane was blocked with blocking solution and incubated with primary antibodies overnight. We have listed the primary antibodies in Table 1. On the second day, the membranes were reacted with HRP‐conjugated secondary antibodies. Then, an enhanced chemiluminescence kit was used to detect the signals.
Table 1.
Primary antibodies used in this study
| Antibody | Host | Dilution | Application | Source |
|---|---|---|---|---|
| PKA‐γ (catalytic subunit) | Rabbit | 1:1000 | WB | Novus |
| Total Akt | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Phosphor‐Akt (Ser473) | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Total CREB | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Phosphor‐CREB (Ser133) | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| BDNF | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Bax | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Cleaved –caspase ‐3 | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| STAT3 | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| Phosphor‐STAT3 (Tyr705) | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| GAP‐43 | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| SYN | Rabbit | 1:1000 | WB | Cell Signaling Technology |
| β‐actin | Mouse | 1:10000 | WB | Sigma |
| BrdU | Rat | 1:400 | IF | Millipore |
| NeuN | Mouse | 1:100 | IF | Millipore |
| GFAP | Rabbit | 1:100 | IF | Sigma |
WB, Western blot; IF, immunofluorescent staining.
Statistical Analysis
The values were shown as mean ± SEM. We performed the statistical assay using two‐way analysis of variance (ANOVA) and one‐way ANOVA. The differences between the groups were analyzed by Bonferroni's post hoc test. P < 0.05 was considered to be statistically significant.
Results
L‐NBP Promoted the Neural Precursor Cells Proliferation
As shown in Figure 2, the amount of BrdU+ cells in DG region was unchangeable in the sham group. In the vehicle group, BrdU+ cells were markedly elevated and reached a peak at postischemic day 7 and then decreased at postischemic day 14, but the amount was still high compared to sham group. The cell birth returned to the original level in the DG of injured hemisphere 28 days after MCAO. In contrast, BrdU+ cell in the DG was robustly raised by l‐NBP treatment on days 7, 14, and 28 after MCAO. Particulary on day 28 after surgery, the BrdU+ cells were about onefold higher, respectively, in the l‐NBP treatment group, compared to the vehicle group.
Figure 2.
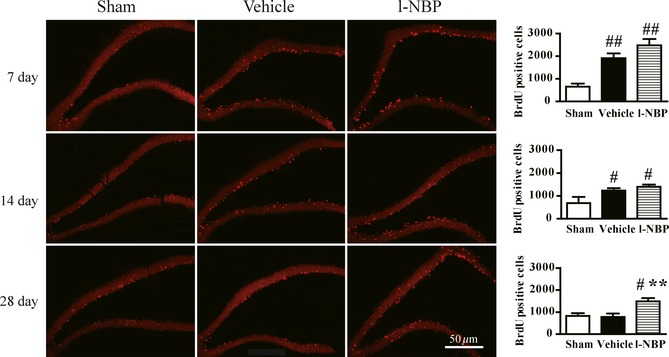
L‐NBP promoted proliferation of neural precursor cells in the DG of ischemic hemisphere on day 28 after focal cerebral ischemia in rats. Representative fluorescence images and quantitative analysis of BrdU immunoreactive cells on days 7, 14, and 28 after reperfusion. Data were expressed as mean ± SEM. n = 12–15 rats per group. # P < 0.05, ## P < 0.01 versus sham group; **P < 0.01 versus vehicle group.
L‐NBP Enhanced the Newborn Cells Survival
We treated rats with l‐NBP for 28 days and BrdU for 2 days (days 1–2 after surgery), to observe the influence of l‐NBP on the cell fate. The BrdU+ cells in the vehicle group were a little bit more than that in the sham group. Interesting, the number of BrdU+ cells was markedly raised in the l‐NBP group compared to the vehicle or sham rats (Figure 3). Moreover, most of BrdU+ cells were shown in the granular cell layer. The data suggested that l‐NBP treatment increased newly born cells survival in the DG region after ischemia.
Figure 3.
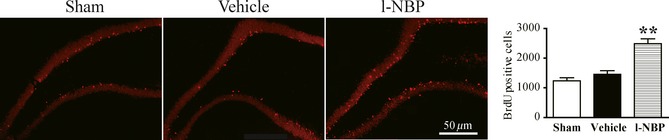
L‐NBP enhanced the survival of newborn cells. Representative fluorescence images and quantitative analysis of BrdU positive cells. Data were expressed as mean ± SEM. n = 12–13 rats per group. **P < 0.01 versus vehicle group.
L‐NBP Regulated the Differentiation of Newborn Neurons and Synapse Functions
In this study, we found that approximately 80% of the BrdU+ cells in the DG of injured hemisphere were colocated with NeuN. However, only 10% of the BrdU+ cells were colocated with GFAP 28 days after MCAO in vehicle and sham groups. BrdU+ cells in the vehicle‐treated rats were similar to that in the sham‐operated rats. Furthermore, the effect of l‐NBP on the differentiation of newborn cells towards neuron and astrocyte was evaluated in the DG of injured hemisphere. A markedly rise of BrdU+/NeuN+ cells and a robust reduction in the percentage of BrdU+/GFAP+ cells were determined in l‐NBP‐treated rats. Moreover, there was no difference in the ratio of BrdU+/NeuN+ cells and the number of BrdU+/GFAP+ cells in vehicle‐ and l‐NBP‐treated rats (Figure 4A and B). It indicated that l‐NBP promoted neurogenesis but not astrogliogenesis after cerebral ischemia of rat model.
Figure 4.
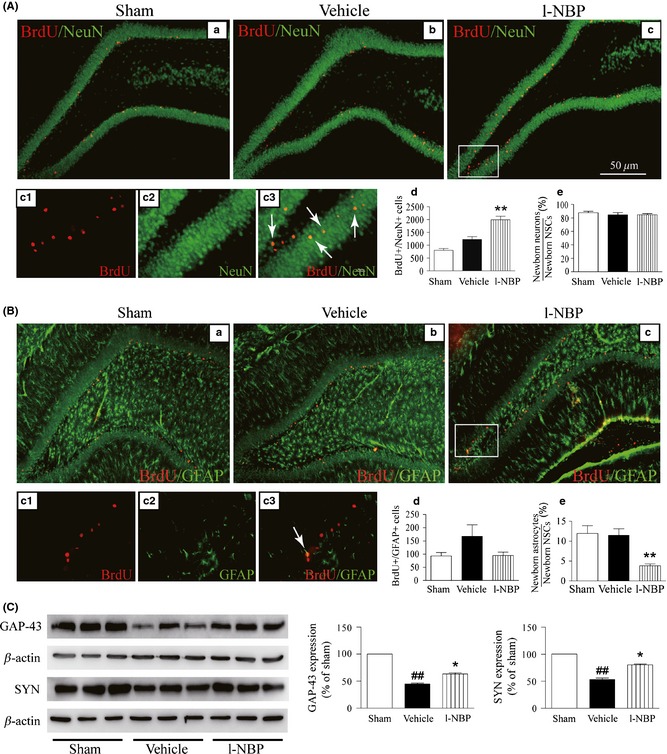
L‐NBP increased the number of newborn neurons and the expressions of GAP‐43 and SYN but decreased the percentage of newborn GFAP‐positive cells. Representative confocal fluorescence images immunolabeled with BrdU (red) and NeuN (green) or GFAP (green) antibodies, BrdU+/NeuN+ cells shown yellow color (arrow) (A), and BrdU+/GFAP + cells shown yellow color (arrow) (B). Quantitative analysis of BrdU+/NeuN+ (A‐d) and BrdU+/GFAP + cells (B‐d), the percentage of BrdU+/NeuN+ (A‐e) and BrdU+/GFAP + (B‐e). Western blots of GAP‐43 and SYN in the hippocampus of injured hemisphere treated with vehicle or l‐NBP, respectively (C). Quantified results were normalized to β‐actin expression. Values were expressed as percentages compared to sham‐operated rats (set to 100%) and represented as group mean ± SEM. n = 12–13 rats per group. ## P < 0.01 versus sham group, *P < 0.05, **P < 0.01 versus vehicle‐treated group.
GAP‐43 and SYN are the most established and widely used synaptic proteins and take part in the development of nerve synapses and adjust plasticity of nerve synapses 17. Here, the expressions of GAP‐43 and SYN were markedly downregulated in the vehicle‐treated rats at postischemic day 28. However, l‐NBP obviously increased GAP‐43 and SYN expressions (Figure 4C). It indicated that l‐NBP treatment promoted neurogenesis and neuroplasticity as well as suppressed astrogliogenesis in the DG of injured hemisphere.
L‐NBP Promoted Sensorimotor and Cognitive Functions Recovery
All cerebral ischemia rats showed initial severe sensorimotor dysfunction by checking the limb‐placing test, and accompanied by gradual recovery. The difference of the scores of limb‐placing test between sham and vehicle groups was obvious at all time points. The cerebral ischemia rats treated with l‐NBP immediately showed an improved score after the first treatment until day 7 after operation (Figure 5A). The Morris water maze task showed that vehicle rats spent more time to look for the hidden platform compared to sham group. However, l‐NBP‐treated rats attained a significantly lower time to search the platform compared to vehicle rats (Figure 5B). It indicated that l‐NBP treatment could induce the beneficial effect for improvement of sensorimotor and cognitive function in a focal cerebral ischemia model.
Figure 5.
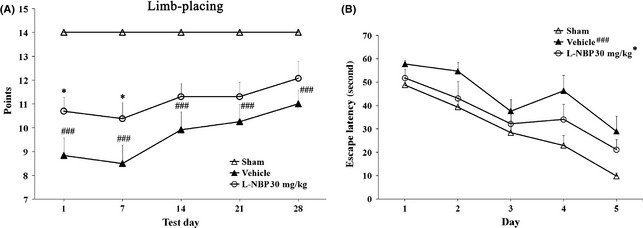
L‐NBP promoted behavioral function recovery after focal cerebral ischemia in rats. Limb‐placing test was performed to assess the recovery of the forelimb and hindlimb functions (A). Morris water maze test was performed to evaluate cognitive function (B). Values were represented as group mean ± SEM. n = 8–12 rats per group. ### P < 0.001 versus sham group, *P < 0.05 versus vehicle‐treated group.
L‐NBP Promoted Neurogenesis by Regulating PKA/BDNF/CREB and STAT3 Signaling Pathway
The expression of the catalytical subunit of PKA was nonsignificantly changed in the vehicle rats. But, l‐NBP increased the level of the catalytic subunit of PKA compared to the vehicle group (Figure 6A). The level of phosphor‐CREB (Ser133) was stable between the vehicle‐treated and sham‐operated rats. However, l‐NBP treatment increased the levels of phosphor‐CREB (Ser133) compared to vehicle group. The total CREB levels were not shown difference among the groups at postischemic day 28 (Figure 6B). Moreover, a marked decrease of BDNF was shown in vehicle‐treated group compared to sham group. However, l‐NBP increased BDNF levels by 25.60% at postischemic day 28 compared to the vehicle treatment (Figure 6C). The important feature of STAT3 during the neural development is promoting astrogliogenesis 18. During 12 h after cerebral ischemia, the level of phosphorylated STAT3 at Tyr705 was enhanced 19. We found that the phosphor‐STAT3 (Tyr705) level was markedly increased in the vehicle‐treated rats at postischemic day 28. At the meantime, the expression of phosphor‐STAT3 (Tyr705) was almost not detected in the l‐NBP treatment rats. The expressions of total STAT3 were similar among the groups (Figure 6D). These results showed that l‐NBP inhibited STAT3 activity after ischemia.
Figure 6.
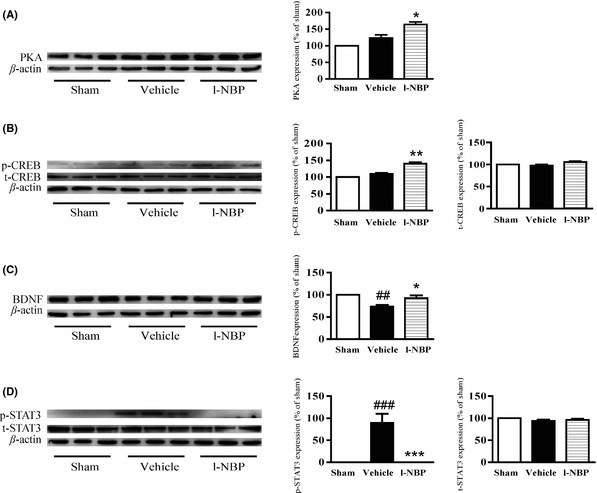
L‐NBP increased the expressions of the catalytical subunit of PKA and BDNF and the activity of CREB and inhibited STAT3 activity at postischemic day 28 in the hippocampus of ischemic hemisphere in rats. Western blots of the catalytical subunit of PKA (A), phosphor‐CREB (Ser133) (B), total CREB (B), BDNF (C), phosphor‐STAT3 (Tyr705) (D), and total STAT3 (D) in the hippocampus of rats treated with vehicle or l‐NBP, respectively. Quantified results were normalized to β‐actin expression. Values were expressed as percentages compared to sham‐operated rats (set to 100%) and represented as group mean ± SEM. n = 6 rats per group. ## P < 0.01, ### P < 0.001 versus sham group, *P < 0.05, **P < 0.01, *** P < 0.001 versus vehicle‐treated group.
L‐NBP Inhibited Neuronal Apoptotic by Regulating Akt Signaling Pathway
The level of phosphor‐Akt was similar in the hippocampus between vehicle group and sham group. L‐NBP markedly increased the phosphor‐Akt (Ser473) at postischemic day 28. The total Akt levels were not shown difference among the groups (Figure 7A). It has been demonstrated that Akt can suppress the conformational variety of Bax and the following translocation to mitochondria; therefore, the apoptosis‐related signaling pathway was inhibited 20. We found that the activity of cleaved caspase‐3 was markedly increased, but the levels of Bax and Bcl‐2 had no obvious changes after ischemia between the vehicle rats and the sham rats. However, l‐NBP treatment significantly reduced cleaved caspase‐3 and Bax levels. Meanwhile, l‐NBP treatment did not affect the Bcl‐2 expression (Figure 7B). It suggested that l‐NBP treatment might inhibit neuronal apoptosis by regulating Akt signaling pathway.
Figure 7.
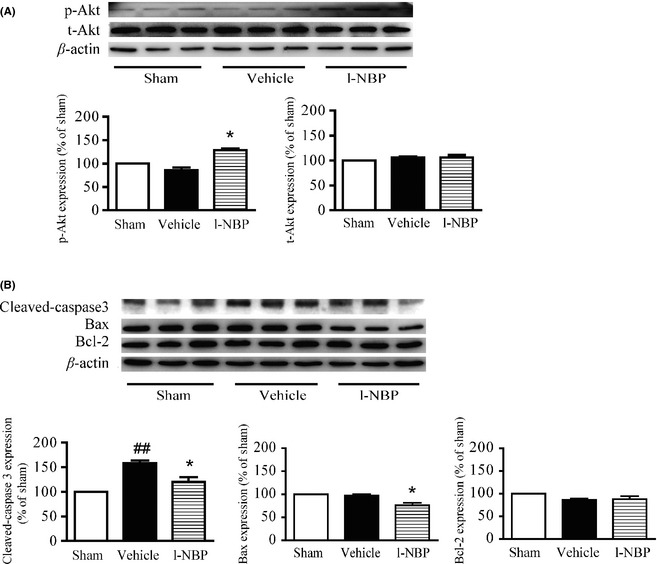
L‐NBP increased Akt activity and decreased the levels of cleaved caspase‐3 and Bax but had no effect on the expression of Bcl‐2 at postischemic day 28 in the hippocampus of ischemic hemisphere after focal cerebral ischemia in rats. Western blots of phosphor‐Akt (Ser473) (A), total Akt (A), cleaved caspase‐3 (B), Bax (B), and Bcl‐2 (B) in the hippocampus of rats treated with vehicle or l‐NBP, respectively. Quantified results were normalized to β‐actin expression. Values were expressed as percentages compared to the sham‐operated rats (set to 100%) and represented as group mean ± SEM. n = 6 rats per group. ## P < 0.01 versus sham group, *P < 0.05 versus vehicle‐treated group.
Discussion
Cerebral ischemic is the most important reason of high mortality and morbidity. In recent twenty years, the neuroprotective agents have been considered potential in stroke treatment but without successful example. Therefore, finding new neuroprotectants that activate endogenous NSC to substitute neurons loss after ischemia might be a new strategy for the stroke therapeutics. The positive effect of l‐NBP on cerebral ischemia by decreasing oxidative damage, inhibiting inflammatory response, and reducing neuronal apoptosis had been demonstrated in multiple animal models 21, 22, 23. Here, we reported that l‐NBP promoted the proliferation, survival, and differentiation of newborn neural cells and increased the neuroplasticity.
BrdU can bind to cellular DNA in S phase, and it had become a widely accepted tool to check cell proliferation 24, 25. Our results showed that BrdU+ cells were markedly raised and reached a peak at postischemic day 7, then most of newborn cells underwent death, and cells birth reduced to the original level in the DG of injured hemisphere 28 days after ischemia, consistently with previous study 26. Our data showed that l‐NBP treatment for 4 weeks by oral administration obviously upregulated the amount of BrdU+ cells in the DG of injured hemisphere, suggesting that l‐NBP could promote basal neurogenesis after ischemia in rats. At the meantime, l‐NBP significantly promoted sensorimotor and cognitive functional recovery, indicating that l‐NBP might contribute to restore the permanent disability in stroke therapeutics. Recent research showed that conditional depletion of neurogenesis could inhibit the functional recovery in the cerebral ischemic animal model, suggesting that the neurogenesis in the early stage of cerebral ischemia regulated the process of sensorimotor recovery 27. Thus, the activity of l‐NBP on functional recovery might be attributed to l‐NBP‐induced newborn neurons survival in the hippocampus. In addition, our results exhibited that l‐NBP treatment markedly upregulated the expressions of both GAP‐43 and SYN compared to the vehicle group. It indicated that l‐NBP might improve the neuroplasticity in the hippocampus of injured hemisphere.
Cyclic AMP activates PKA signaling and then leads to the release of catalytical subunit of PKA. This subunit transfers to the nucleus where it results in CREB phosphorylation at Ser133 28. It is well known that hippocampal cAMP/PKA/CREB signaling plays an important role in memory formation 29. The previous study demonstrated that restrain of the CREB by a CREB‐dominant negative mutant could block hippocampal neurogenesis in the DG region after focal cerebral ischemia. It indicates that CREB may contribute to the newborn neurons survival in the hippocampus. It is well known that BNDF supplies a helpful condition to the newborn cells of the hippocampal DG. Thus, BDNF might involve in CREB‐regulated newborn neurons survival 30. In our results, l‐NBP‐mediated CREB activation resulted in BDNF release and then facilitated sensorimotor function reestablishment by promoting hippocampal newborn neurons survival after focal cerebral ischemia. At the meantime, BDNF can enhance the expressions of GAP‐43 and SYN 31. Therefore, l‐NBP treatment might increase the newborn neurons survival and neuroplasticity through PKA signaling pathway by increasing CREB activity and upregulating BDNF expression. The inhibition of STAT3 had been demonstrated to promote the neurogenesis and restrained the astrogliogenesis in NSC 32. Our results exhibited that l‐NBP markedly inhibited STAT3 activity in the hippocampus of injured hemisphere. Consequently, the STAT3 pathway might contribute to the l‐NBP‐stimulated increase of newborn neurons and no effect on newborn GFAP cells of the DG of injured hemisphere in rats.
Akt signaling pathway has been shown to closely associate with the cell survival by suppressing the activation of some proapoptotic proteins. At the meantime, Akt signaling pathway also contributes to postconditioning's protection against 33. L‐NBP had been demonstrated to protect Aβ‐ and H2O2‐induced cellular damage by regulating Bcl‐2/Bax expressions and inhibiting caspase‐3 activation 34, 35. In the present experiment, l‐NBP markedly raised Akt activity and downregulated caspase‐3 activity and the level of Bax in the hippocampus of injured hemisphere. In addition, PKA/CREB signaling pathway was reported to inhibit neuronal apoptosis by enhancing Bcl‐2 expression 29. It indicated that l‐NBP treatment might rely on functional activation of PKA and Akt prosurvival pathways via inactivation of proapoptotic proteins to promote the survival of newborn neuronal cells.
This work represents the first preclinical study demonstrating the effect of l‐NBP on neurogenesis and neuroplasticity after cerebral ischemic. The finding provides a further evidence that l‐NBP promotes functional recovery after cerebral ischemia and proposes the hypothesis that the long‐term application of l‐NBP may contribute to alleviate the disability after stroke.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Data S1. The score of Limb‐placing Test
Acknowledgments
The authors thank Li Zhang for her help in the immunofluorescence staining. This study was supported by the grants from National Natural Sciences Foundation of China (No. 81373387), National Science and Technology Major Special Project on Major New Drug Innovation of China (2012ZX09301002‐004).
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics‐2014 update a report from the American Heart Association. Circulation 2014;129:E28–E292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral‐artery occlusion ‐ evaluation of the model and development of a neurologic examination. Stroke 1986;17:472–476. [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Liu PP, Ying GY, et al. Effects of thioredoxin‐1 on neurogenesis after brain ischemia/reperfusion injury. CNS Neurosci Ther 2013;19:204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doeppner TR, Dietz GPH, El Aanbouri M, et al. TAT‐Bcl‐X‐L improves survival of neuronal precursor cells in the lesioned striatum after focal cerebral ischemia. Neurobiol Dis 2009;34:87–94. [DOI] [PubMed] [Google Scholar]
- 5. Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1‐mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761A (R)) after permanent ischemic stroke in mice. Mol Neurobiol 2014;49:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng GQ, Cheng W, Wang Y, et al. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol 2011;133:724–728. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Xue Q, Li X, et al. Amelioration of stroke‐induced neurological deficiency by lyophilized powder of Catapol and Puerarin. Int J Biol Sci 2014;10:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao XL, Liu Y, Qi CF, et al. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol Res 2010;32:547–555. [DOI] [PubMed] [Google Scholar]
- 9. Cui LY, Zhu YC, Gao S, et al. Ninety‐day administration of dl‐3‐n‐butylphthalide for acute ischemic stroke: A randomized, double‐blind trial. Chin Med J 2013;126:3405–3410. [PubMed] [Google Scholar]
- 10. Sharp FR, Liu JL, Bernabeu R. Neurogenesis following brain ischemia. Brain Res Dev Brain Res 2002;134:23–30. [DOI] [PubMed] [Google Scholar]
- 11. Ortega FJ, Jolkkonen J, Mahy N, Rodriguez MJ. Glibenclamide enhances neurogenesis and improves long‐term functional recovery after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2013;33:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng Y, Xu SF, Chen GQ, Wang L, Feng YP, Wang XL. l‐3‐n‐butylphthalide improves cognitive impairment induced by chronic cerebral hypoperfusion in rats. J Pharmacol Exp Ther 2007;321:902–910. [DOI] [PubMed] [Google Scholar]
- 13. Xu J, Wang YY, Li N, Xu LJ, Yang HY, Yang Z. L‐3‐n‐butylphthalide improves cognitive deficits in rats with chronic cerebral ischemia. Neuropharmacology 2012;62:2424–2429. [DOI] [PubMed] [Google Scholar]
- 14. Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J. An alpha(2)‐adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology 2001;40:597–606. [DOI] [PubMed] [Google Scholar]
- 15. Morris R. Developments of a water‐maze procedure for studying spatial‐learning in the rat. J Neurosci Methods 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- 16. Li WL, Cai HH, Wang B, et al. Chronic fluoxetine treatment improves ischemia‐induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res 2009;87:112–122. [DOI] [PubMed] [Google Scholar]
- 17. Tarsa L, Goda Y. Synaptophysin regulates activity‐dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 2002;99:1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molné M, Studer L, Tabar V, Ting YT, Eiden MV, McKay RDG. Early cortical precursors do not undergo LIF‐mediated astrocytic differentiation. J Neurosci Res 2000;59:301–311. [DOI] [PubMed] [Google Scholar]
- 19. Choi JS, Kim SY, Cha JH, et al. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia 2003;41:237–246. [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 2001;20:7779–7786. [DOI] [PubMed] [Google Scholar]
- 21. Xu HL, Feng YP. Effects of 3‐n‐butylphthalide on production of vasoactive substances by cerebral and aortic endothelial cells. Acta Pharmacol Sin 1999;20:929–933. [PubMed] [Google Scholar]
- 22. Peng Y, Zeng XK, Feng YP, Wang XL. Antiplatelet and antithrombotic activity of L‐3‐n‐butylphthalide in rats. J Cardiovasc Pharmacol 2004;43:876–881. [DOI] [PubMed] [Google Scholar]
- 23. Chang Q, Wang XL. Effects of chiral 3‐n‐butylphthalide on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol Sin 2003;24:796–804. [PubMed] [Google Scholar]
- 24. Liu JL, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 1998;18:7768–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doeppner TR, Nagel F, Dietz GPH, et al. TAT‐Hsp70‐mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2009;29:1187–1196. [DOI] [PubMed] [Google Scholar]
- 26. Leconte C, Bihel E, Lepelletier FX, et al. Comparison of the effects of erythropoietin and its carbamylated derivative on behaviour and hippocampal neurogenesis in mice. Neuropharmacology 2011;60:354–364. [DOI] [PubMed] [Google Scholar]
- 27. Wang XM, Mao XO, Xie L, Sun F, Greenberg DA, Jin KL. Conditional depletion of neurogenesis inhibits long‐term recovery after experimental stroke in mice. PLoS ONE 2012;7:e38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez GA, Montminy MR. Cyclic‐Amp stimulates Somatostatin Gene‐transcription by phosphorylation of Creb at Serine‐133. Cell 1989;59:675–680. [DOI] [PubMed] [Google Scholar]
- 29. Lee JH, Park SY, Shin HK, et al. Protective effects of cilostazol against transient focal cerebral ischemia and chronic cerebral hypoperfusion injury. CNS Neurosci Ther 2008;14:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP‐response‐element‐binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 2004;101:9453–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang T, Xie KW, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci 1995;15:4796–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu F, Hata R, Ma YJ, et al. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res 2005;81:163–171. [DOI] [PubMed] [Google Scholar]
- 33. Qi ZF, Luo YM, Liu XR, et al. AKT/GSK3β‐dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci Ther 2012;18:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng Y, Xing CH, Xu SF, et al. L‐3‐n‐butylphthalide improves cognitive impairment induced by intracerebroventricular infusion of amyloid‐beta peptide in rats. Eur J Pharmacol 2009;621:38–45. [DOI] [PubMed] [Google Scholar]
- 35. Peng Y, Hu Y, Feng N, Wang L, Wang X. L‐3‐n‐butylphthalide alleviates hydrogen peroxide‐induced apoptosis by PKC pathway in human neuroblastoma SK‐N‐SH cells. Naunyn Schmiedebergs Arch Pharmacol 2011;383:91–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. The score of Limb‐placing Test


