Primary amoebic meningoencephalitis (PAM) due to Naegleria fowleri is a fulminating infection that can result in death within days. The disease prognosis is poor, although early diagnosis and aggressive treatment might increase survival chances 1. Through contaminated water, N. fowleri enters nostrils and then migrates along the olfactory nerve, through the cribriform plate into the brain 2. PAM is characterized by parosmia, rapidly progressing to anosmia (with resultant ageusia) as the nerve cells of the olfactory bulbs are destroyed leading to seizures and finally death 3. Despite advances in antimicrobial chemotherapy and supportive case, the mortality rate associated with PAM has remained over 90% 1, 2, 3. A high mortality is attributed to (1) delayed diagnosis, (2) our incomplete understanding of the pathogenesis and pathophysiology of the disease, (3) lack of availability of safe and effective drugs, and (4) the difficulty in delivering anti‐N. fowleri drugs to the brain. So far, only three survivors of PAM have been documented 4. These patients were treated with amphotericin B, rifampicin, fluconazole, dexamethasone, and phenytoin intravenous and/or intrathecally within first week of the infection 4, 5. A successful prognosis was attributed to early diagnosis (limited deeper tissue involvement), followed by aggressive antimicrobial chemotherapy. Both amphotericin B 5 and miltefosine 6 have been suggested as useful agents against PAM 7. The aim of this letter is to debate whether an improved therapeutic outcome can be achieved by administering drugs through the nasal cavity (proposed here as “transcribrial” route) against PAM (Figure 1)?
Figure 1.
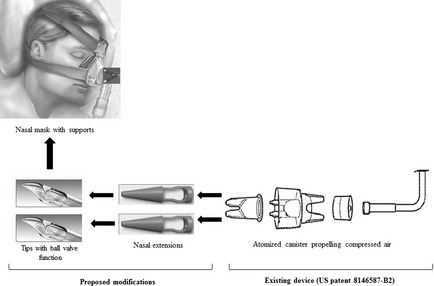
Proposed device to deliver anti‐Naegleria fowleri drugs to the brain via cribriform plate. Modifications to the existing device (US patent 8146587‐B2) will ensure that drugs are delivered at the site of infection, while ball‐valve action will prevent regurgitation back into the delivery system.
The proposed intranasal route will deliver drugs across the cribriform plate (an anatomically porous bone) that is located at the roof of the nasal cavity. Due to the sieve like nature of the cribriform plate, the tips at the terminals of the proposed device with ball‐valve function (one‐way drug delivery) are designed to reach and deliver drugs in vaporized form to area of the brain (the inferior surface of the frontal lobe), a site where N. fowleri reaches and concentrates as its natural course of the disease, before spreading to the rest of the central nervous system (CNS). The underlying rationale for proposing such a portal is that many therapeutically desired drugs like amphotericin B fail to attain minimum inhibitory concentration (MIC) in the CNS, when administered systemically 8. The proposed route will be advantageous in that it will (1) bypass selectivity of the blood–brain barrier that limits drug permeability to the brain tissue, (2) trail the natural route of entry of N. fowleri into the brain, (3) target the site of infection, (4) exert lethal effect of drugs by attaining MIC at a lower dose without venous drainage, (5) avoid adverse effects due to systemic administration. Olfactory targeting through intranasal delivery would provide access to the CNS without affecting the integrity of the blood–brain barrier 9. This can be achieved through the use of a modified intranasal instrument (US Patent‐ 8146587 B2). We propose that this instrument should be modified to extend its nasal terminals so it can reach porous cribriform plate (Figure 1). Additionally, ball‐valve effect should be added to the tip of the modified device so that to deliver the drug in vaporized form and prevent regurgitation to the flow system (Figure 1). The proposed route offers clear benefits over the conventional intravenous route, in that it would enable the use of water soluble drugs 10 to attain MIC at the epicenter of infection, overcome the BBB hindrance, minimal adverse effects by enjoying the benefits of topical administration, and dose adjustment at a faster pace.
Given that PAM patients are receiving adjuvant therapies including antiseizure, strong hypnotics, and even general anesthetics 10, patient compliance with the proposed route should not pose any concern. Further research will test the effectiveness of the proposed route to administer drugs in the management of PAM due to N. fowleri and possibly other pathogens causing brain infections.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We are thankful to Misbah Mannan for technical support and helpful discussions. This work is supported by the Aga Khan University.
References
- 1. Vargas‐Zepeda J, Gomez‐Alcala AV, Vasquez‐Morales JA, Licea‐Amaya L, De Jonckheere JF, Lores‐Villa F. Successful treatment of Naegleria PAM using IV amphotericin B, fluconazole, and rifampin. Arch Med Res 2005;36:83–86. [DOI] [PubMed] [Google Scholar]
- 2. Maclean RC, Richardson DJ, LePardo R, Marciano‐Cabral F. The identification of Naegleria fowleri from water and soil samples by nested PCR. Parasitol Res 2004;93:211–217. [DOI] [PubMed] [Google Scholar]
- 3. Marciano‐Cabral F, Cabral G. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol 2007;51:243–259. [DOI] [PubMed] [Google Scholar]
- 4. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free‐living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea . FEMS Immunol Med Microbiol 2007;50:1–26. [DOI] [PubMed] [Google Scholar]
- 5. Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med 1982;306:346. [DOI] [PubMed] [Google Scholar]
- 6. Schuster FL, Guglielmo BJ, Visvesvara GS. In vitro activity of miltefosine and voriconazole on clinical isolates of free‐living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri . J Eukaryot Microbiol 2006;53:121–126. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC) . Primary amebic meningoencephalitis – Arizona, Florida, and Texas, 2007. Morb Mortal Wkly Rep 2008;57:573–577. [PubMed] [Google Scholar]
- 8. Atkinson AJ Jr, Bennett JE. Amphotericin B pharmacokinetics in humans. Antimicrob Agents Chemother 1978;13:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen MM. Olfactory targeting through intranasal delivery. Discov Med 2011;11:497–503. [PubMed] [Google Scholar]
- 10. Baig AM, Iqbal J, Khan NA. In vitro efficacy of clinically available drugs against growth and viability of Acanthamoeba castellanii . Antimicrob Agents Chemother 2013;57:3561–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]


