Since humanin, a 24‐amino acid polypeptide, was first found in the surviving neurons of human patients with Alzheimer disease in 2001, it was reported that humanin protected neurons from beta‐amyloid‐induced apoptosis by binding to specific receptor and triggering a Jak/STAT prosurvival pathway 1. As we know, apoptosis and Jak/STAT prosurvival pathway were also mechanisms involved in cell responses induced by anoxia, ischemia, and so on 2. But the effects of humanin on neurons induced by anoxia or ischemia remain unknown.
In particular, anoxia responses of cells occurred through a number of mechanisms including electrophysiological mechanism. Voltage‐dependent potassium ion currents, such as transient outward potassium current (I to) and delayed rectifier potassium current (I K), are known to initiate apoptosis in Alzheimer disease 3. So, based on the hypothesis that humanin could protect neuronal cells from anoxia impairment as well as from beta‐amyloid impairment, the effect of humanin (Sigma, St. Louis, MO, USA) 2 mmol/L on activity and on potassium currents of hippocampal neurons in presence of dithionite (Sigma) was investigated.
The neuronal viability and potassium currents in neurons being cultured for 12 days were determined by MTT method and patch clamp technique, after dissociated from hippocampus of neonatal Wistar rats as reported 4. Voltage‐dependent potassium ion currents, both I K and I to (shown in Figure 1A–C), were recorded using a patch clamp amplifier (EPC‐10, HEKA, Pfalz, Lambrecht, Germany) and Pulse 8.5 software (HEKA) by whole‐cell manner. Tetrodotoxin 1 μmol/L was employed to abolish sodium ion current of neurons. Cultures treated by external solution containing dithionite 2 mmol/L for 5 min by a continuous perfusion pump were referred to as anoxia, as dithionite deprived oxygen out of the neurons 5.
Figure 1.
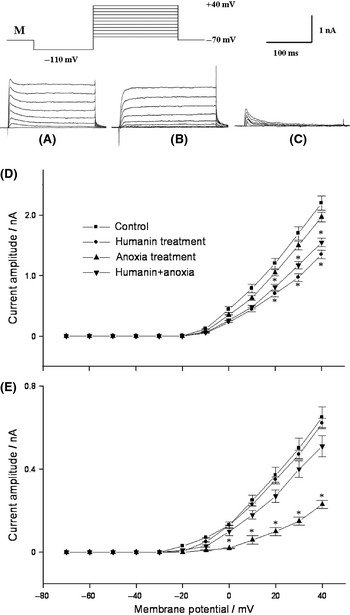
Voltage‐dependent potassium currents composed of delayed rectifier potassium current (I K) and transient outward potassium current (I to), and effects of humanin and anoxia on I‐V curves of I K and I to. Neurons were held at a holding potential of −70 mV, and potassium currents (A) were elicited by a series depolarizing pulses as follows: Hyperpolarizing prepulse to −110 mV was followed by depolarizing test pulses with a frequency of 0.1 Hz from −70 to 40 mV in 10 mV increment (M). To get I K (B), 4‐aminopyridine 1 mmol/L was added into external solution to abolish I to. I to (C) was isolated by subtracting current trace of I K from potassium currents utilizing the signal subtraction procedure in Pulse 8.5 software. Being normalized with respect to maximal value, currents amplitudes were expressed as mean ± SD and were analyzed by one‐way ANOVA Dunnett's test. *P < 0.05,versus controls. n = 10 neurons in each group. Amplitudes of I K (D) were decreased by humanin and humanin+anoxia significantly in membrane potentials of 20~40 mV, while amplitudes of I to (E) were decreased by anoxia significantly in membrane potentials of 0~40 mV.
Compared with that of controls, the viability of neurons treated by dithionite 2 mmol/L for 6 h decreased to 63 ± 10% (n = 10) significantly (P < 0.05),but not the neurons treated by mixture of humanin and dithionite 2 mmol/L for 6 h. Meanwhile, neuronal viability was not decreased significantly by anoxia, even if anoxia could change potassium current properties. Anoxia significantly decreased I to amplitudes (P < 0.05), but had no significant effect on I K amplitude. I K amplitudes of neurons were significantly decreased (P < 0.05) by humanin or by mixture of humanin and anoxia. Pretreatment with humanin for 5 min, anoxia did not decrease I to amplitudes significantly any more. While humanin and dithionite were washed out, I K and I to amplitudes recovered partly within several minutes. The effect of humanin on I to amplitudes was not found in the present experiment (shown in Figure 1D,E).
Steady‐state activation of I K and I to was fitted by Boltzmann function as follows: G/G max = [1 + exp(V ‐ V 1/2)/k]−1, in which G max is the maximal membrane conductance, V 1/2 is the potential at which the conductance is half steady activation, and k is a factor describing the slope of the activation curves. As shown in Table 1, after anoxia, V 1/2 of I K and I to was altered significantly (P < 0.05) toward depolarization. On the contrary, humanin shifted V 1/2 of I K significantly toward repolarization (P < 0.05), but not altered V 1/2 of I to. Meanwhile, mixture of humanin and anoxia had no effect on V 1/2 of I K or I to, and neither humanin nor anoxia altered k slope as well.
Table 1.
Effect of humanin on steady‐state activation of I K and I to in anoxia hippocampal neurons
| V 1/2 /mV | k / mV | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Anoxia | Humanin | Humanin + anoxia | Control | Anoxia | Humanin | Humanin + anoxia | |
| I K | −2.67 ± 0.516 | 6.33 ± 1.06a | −7.77 ± 1.65a | −3.69 ± 1.21 | 13.5 ± 2.05 | 12.0 ± 1.70 | 15.1 ± 2.46 | 14.5 ± 2.51 |
| I to | −19.1 ± 2.54 | −10.2 ± 1.32a | −17.2 ± 2.25 | −15.6 ± 3.37 | 17.2 ± 2.06 | 19.3 ± 2.64 | 15.7 ± 1.88 | 16.3 ± 2.62 |
Potential data were expressed as mean ± SD and were analyzed by one‐way ANOVA Dunnett test.
P < 0.05, versus controls.
n = 10 in each group.
Potassium efflux and intracellular potassium depletion contributed to hypoxia‐triggered apoptosis of central neurons 6. Decrease in I K by humanin in the present investigation, which was consistent with the evidence that potassium channel blockers attenuated hypoxia‐induced neuronal death 7, revealed humanin's neuroprotective effects. But on the other hand, a mild transient hypoxia, usually referred to as hypoxia precondition, decreased anoxia‐induced apoptosis in cultured hippocampal neurons 8. So, I K might not be influenced in the case of hypoxia precondition, which was supported by the present result that amplitudes of I K were not altered significantly by anoxia imitating hypoxia precondition.
The different effects of humanin and anoxia on Boltzmann function parameter V 1/2 hinted the dissimilarity of their effects on neuronal membrane conductance. Anoxia promoted excitability of neuron, while humanin antagonized this effect. Moreover, I to was significantly depressed under hypoxic conditions 9, and upregulation of I to protected neurons against cerebral ischemia 10. Present result of humanin protecting decrease in I to amplitude due to anoxia suggested that humanin supported voltage‐dependent potassium channels by inhibiting the effects of anoxia.
Conflict of Interest
The authors declare no conflict of interest.
The first two authors contributed equally to this work.
References
- 1. Arakawa T, Hirano A, Shiraki K, Niikura T, Kita Y. Advances in characterization of neuroprotective peptide, humanin. Curr Med Chem 2011;18:5554–5563. [DOI] [PubMed] [Google Scholar]
- 2. Raymond M, Li P, Mangin JM, Huntsman M, Gallo V. Chronic perinatal hypoxia reduces glutamate‐aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci 2011;31:17864–17871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu HB, Li ZB, Zhang HX, Wang XL. Role of potassium channels in Abeta(1‐40)‐activated apoptotic pathway in cultured cortical neurons. J Neurosci Res 2006;84:1475–1484. [DOI] [PubMed] [Google Scholar]
- 4. Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX. Interleukin‐1beta enhances NMDA receptor‐mediated current but inhibits excitatory synaptic transmission. Brain Res 2005;9:172–179. [DOI] [PubMed] [Google Scholar]
- 5. Gebhardt C, Heinemann U. Anoxic decrease in potassium outward currents of hippocampal cultured neurons in absence and presence of dithionite. Brain Res 1999;837:270–276. [DOI] [PubMed] [Google Scholar]
- 6. Shimoda LA, Polak J. Hypoxia and ion channel function. Am J Physiol Cell Physiol 2011;300:C951–C967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei L, Yu SP, Gottron F, Snider BJ, Zipfel GJ, Choi DW. Potassium channel blockers attenuate hypoxia‐ and ischemia‐induced neuronal death in vitro and in vivo. Stroke 2003;34:1281–1286. [DOI] [PubMed] [Google Scholar]
- 8. Wu LY, Ding AS, Zhao T, Ma ZM, Wang FZ, Fan M. Underlying mechanism of hypoxic preconditioning decreasing apoptosis induced by anoxia in cultured hippocampal neurons. Neuro signals 2005;14:109–116. [DOI] [PubMed] [Google Scholar]
- 9. Du Z, Chaoqian X, Shan H, Lu Y, Ren N. Functional impairment of cardiac transient outward K+ current as a result of abnormally altered cellular environment. Clin Exp Pharmacol Physiol 2007;34:148–152. [DOI] [PubMed] [Google Scholar]
- 10. Deng P, Pang ZP, Lei ZG, et al. Up‐regulation of A‐type potassium currents protects neurons against cerebral ischemia. J Cereb Blood Flow Metab 2011;31:1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]


