Summary
Aims
Deep‐vein thrombosis (DVT) represents a serious complication in acute stroke patients with pulmonary embolus (PE) as a potential outcome. Prediction of DVT may help with formulating a proper prevention strategy. To assess of the risk of deep venous thrombosis (DVT) in acute stroke patients, we developed and validated a clinical score in a cohort study.
Methods
Incidence of Deep Venous Thrombosis after Acute Stroke in China (INVENT‐China) is a multicenter prospective cohort study. The potential predictive variables for DVT at baseline were collected, and the presence of DVT was evaluated using ultrasonography on the 14 ± 3 days. Data were randomly assigned to either a training data set or a test data set. Multivariate logistic regression analysis was used to develop risk scores to predict DVT in the training data set and the area under the receiver operating characteristic curve to validate the score in the test data set.
Results
From 2006–2007, 862 hospital‐based acute stroke patients were enrolled in China. The overall incidence of DVT after acute stroke within two weeks was 12.4% (95%CI 10.3–14.7%). A seven‐point score derived in the training data set (age [≥65 years = 1], sex [female gender = 1]), obesity [BMI ≥ 25 kg/m2 = 1], active cancer [yes = 2], stroke subtype [cerebral hemorraghe = 1], muscle weakness [≥2 on Lower limb NIHSS score = 1] was highly predictive of 14‐day risk of DVT(c statistic = 0.70, 95% CI, 0.64–0.76, P < 0.001), in the overall study population(c statistic = 0.65, 95% CI 0.59–0.70, P < 0.001).
Conclusions
This clinical score may help identify acute stroke patients with high risk of DVT. In addition, it also serves as a platform to develop further models of DVT prediction in stroke patients based on clinical factors.
Keywords: Acute stroke, clinical prediction scale, deep venous thrombosis, prophylaxis
Introduction
Deep venous thrombosis (DVT) is a common complication in patients admitted to the hospital after stroke. Data arising from various studies have suggested that the actual direct effects of ischemic stroke do account for majority of morbidity within the first week of stroke; however, medical complications arising from the debilitating after effects of stroke predominately dictate mortality thereafter 1, 2. To elucidate on the medical complications poststroke, pulmonary embolism (PE) forms the most common cause of mortality in the second through fourth weeks of an ischemic and hemorrhagic stroke 3, 4. PE generally arise from venous thromboembolism that develops in a paralyzed lower extremity or pelvis. It has been reported in studies from Western societies that DVT occurred in up to 80% of patients with ischemic stroke who did not receive prophylactic therapy 1. In general, studies have estimated the overall prevalence of clinically evident DVT after acute stroke to be around 2–20% 5, 6, 7. Data from the CLOTS trials, the largest observational report, which evaluated 5632 immobile patients with acute stroke using duplex ultrasound showed detection of DVT within 10 days of enrollment in 11 percent and within 30 days in 15% 8. Although several studies have suggested that venous thromboembolism is less frequent among Asians than Caucasians, nevertheless, this notion has been challenged recently. For example, a recent study in Asian cohort found a high frequency of DVT following acute stroke to be similar to studies in Caucasian patients 9.
In general, DVT development may occur as early as the second day and has a peak incidence between two and seven days 7. DVTs can lead to postphlebitic leg and varicose ulcers in addition to delaying rehabilitation.
In addition, the morbidity and mortality from PE could be reduced either by more effective thromboprophylaxis or through earlier diagnosis and treatment of an established venous thrombosis embolism (VTE). Unfortunately, only a small percentage of patients with PE actually manifest objective symptoms, and hence, the signs and symptoms of PE remain notoriously nonspecific. As a result, PE is often both underdiagnosed and misdiagnosed, particularly in the elderly. Nevertheless, to make a definitive clinical diagnosis of DVT remains difficult, as there are no consistently reliable clinical signs or symptoms. Most cases of DVT that are detected with ancillary investigations are asymptomatic 10.
The options for lowering the risk of DVT has been documented quite extensively in the literature. Maneuvers used include early mobilization, administration of antithrombotic agents, and the use of external compression devices. The American Stroke Association (ASA) guidelines (2007) recommend subcutaneous administration of anticoagulants for treatment of immobilized patients to prevent deep‐vein thrombosis (Class I, Level of Evidence A). Aspirin is a potential intervention to prevent DVT but is less effective than anticoagulants (Class IIa, Level of Evidence A). The use of intermittent external compression devices is recommended for treatment of patients who cannot receive anticoagulants (Class IIa, Level of Evidence B) 11. However, in clinical medical practice, VTE prophylaxis in acutely stroke patients has been found to be suboptimal. Several studies have also validated this observation by showing a gap that exists between evidence‐based guidelines/recommendations and actual practice in the hospital setting 12, 13. Recently, in the “Get With The Guidelines”‐Stroke Acute Ischemic Stroke Population, the median site prophylaxis rate was estimated to be 95%, ranging from 17 (1 site) to 100% (101 sites) 14. In 2013, the ASA guidelines were revised for DVT prophylaxis, which validated aspirin use for treatment of patients who were ineligible for anticoagulants (Class IIa; Level of Evidence A). The use of intermittent external compression devices is reasonable for prevention of DVT in patients who cannot receive anticoagulants (Class IIa; Level of Evidence B) 15.
In contrast, the National Institute for Health and Clinical Excellence (NICE) guidelines recommend that routine prophylactic anticoagulation should not be used (grade A) for prevention of venous thromboembolism (VTE) after stroke. Anticoagulation with heparin/low‐molecular‐weight heparin (LMWH) is recommend only where the risk of DVT and PE is particularly high (e.g., due to immobilization, obesity, diabetes, previous stroke), and the risk of bleed complications (hemorrhagic transformation of stroke or bleeding into another site) is low 16. However, despite the aforementioned literature on the application of anticoagulation/DVT prevention strategies poststroke, there exists a paucity of an objective clinical prediction model to estimate risks of DVT formation in stroke patients. The statistical modeling that was based on the CLOTS trials cohorts (Clots in Legs or Thromboembolic Deterrent Stockings after Stroke trial) ultimately failed to stratify risk of DVT formation among individual stroke patients 17.
Hence, an evidence‐based method to assess a patient's risk of developing DVT after stroke becomes much needed in the healthcare setting. The purpose of this study was to establish and validate a multivariable model that could effectively predict DVT risk at 14 days for patients admitted with an acute stroke, and simultaneously create a practical clinical model that would enable the healthcare professional to weigh the risks and benefits of pharmacological prophylaxis.
Patients and Methods
Study Design and Participants
The Incidence of Deep Venous Thrombosis after Acute Stroke in China (INVENT‐China) study is a multicenter prospective cohort study 13. Assessment of DVT occurrence takes place at day 14 (±3) by compress ultrasound. Patients are enrolled if they meet the following criteria: older than 18 had acute stroke (ischemic or hemorrhagic) within 7 days; mRS ≥ 2 before enrollment; weakness in the lower limbs with NIH Stroke Scale score of ≥1 on item VI; able to obtain consent from the patient patient's legal representative. The exclusion criteria are as follows: TIAs, subarachnoid hemorrhage (SAH), brain tumor, cerebral venous thrombosis, history of VTE.
Data Collection
The following variables were prospectively recorded on separate case report forms: age, gender, body mass index (BMI), smoking habit, hypertension, diabetes, atrial fibrillation, TIA, ischemic heart disease, malignancy, history of VTE, and treatment methods (medical treatment, and the use of elastic stockings). The presence of clinical symptoms or signs of DVT/PE at any stage during the study period was noted. Ischemic stroke phenotypes were determined by the Oxfordshire Community Stroke Project classification. At each Doppler scan, the NIHSS score was assessed by a certified trial coordinator.
Endpoint Ascertainment
Complete compression duplex ultrasonography (CCUS) was used to examine the deep venous structures of both legs in all patients. CCUS was performed at day 14 from the stroke onset. All radiographic tests were carried out and interpreted by radiologists. The deep veins of thigh, popliteal region, and calf were screened carefully at approximately 2‐cm intervals in the transverse planes. At a more proximal level, patients were examined in a supine position from the level of the inguinal ligament to the adductor canal. The popliteal vein was examined at its trifurcation in the upper calf. The remainder of the calf veins were examined to the level of the malleolus. A diagnosis of DVT was arrived at if CCUS showed loss of vein compressibility by ultrasonic probe pressure, a clot, or an abnormal flow pattern (loss of phasic flow signal or loss of augmentation of flow) with distal compression. Lack of visualization/flow measurements were considered to have been inadequate for interpretation.
Ethical Aspects
Ethics approval was provided by the Medical Ethics Committee of the Capital Medical University affiliated Tiantan Hospital, and the local ethics committees of all contributing centers approved the protocol. Written informed consents have been obtained from all the participants.
Statistical Analysis
Statistical Analyses: Derivation of the Poststroke DVT Prediction System
With the 2:1 randomization, the total sample (n = 862) was divided into the assessment/score generation group (two‐thirds of total, n = 575) or validation group (one‐third of total, n = 287). Statistical analyses were performed using a statistical package (SAS, version 9.13; SAS Institute, Cary, NC, USA).
The poststroke DVT prediction system was developed using the data from the assessment cohort. First, bivariate analyses (χ2 test on discrete variables and t tests on continuous variables) were employed to evaluate the association between each independent variables and the outcome of DVT. Second, the variables that were associated with DVT in the bivariate analyses (P < 0.05) were entered into a multivariable logistic regression model. Backwards elimination was employed to select the final set of risk factors that were independently associated with DVT. Third, we assigned points for each variable based on the hazard ratios (HR) from the multivariable model. The HR's were rounded to the nearest whole number to determine the points. Fourth, a risk score was developed by summing the points for each risk factor present. Finally, we examined the outcome rate according to the risk score to identify three categories of risk: low, medium, and high risk of poststroke DVT.
Statistical Analyses: Validation of the Poststroke DVT Prediction System
The risk score and three category risk classification systems were tested in the validation cohort/study population. To assess the discriminatory ability of the DVT clinical scoring system, we calculated the c statistic from a logistic regression model predicting DVT and including the variables identified from the development process. The c statistic, which represents the area under the receiver operating characteristic (ROC) curve, ranges from 0.5 (which indicates no better discrimination than chance) to 1.0 (perfect discrimination). A c statistic value ≥0.70 is considered acceptable.
Results
Description of the Derivation and Validation Cohorts (Patient Characteristics)
The patients enrolled into this study, and the allocation to the derivation/validation group are shown in Figure 1. The assessment sample (n = 575 patients) and the validation sample (n = 287 patients) were well matched with respect to patient characteristics (Table 1). Incidence rates of DVT after acute stroke within 2 weeks from onset were comparable between both cohorts: 13.3% (76 DVTs) for the assessment cohort and 10.6% (30 DVTs) for the validation cohort.
Figure 1.
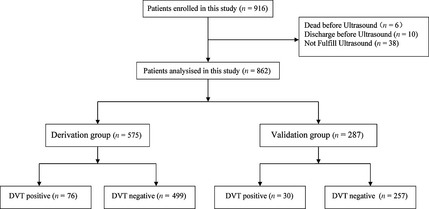
Patients included in the study and allocated to Derivation group or Validation group with 2:1 randomization.
Table 1.
Characteristics of the patients (n = 862)
| Characteristic | Derivation group (n = 575) | Validation group (n = 287) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Older age (≥65), y ,(n, %) | 342 (59.5) | 167 (58.2) | >0.05 |
| Female gender | 215 (37.4) | 107 (37.3) | >0.05 |
| Obesity (BMI ≥ 25 kg/m2) | 229 (40.2) | 116 (40.8) | >0.05 |
| Risk factors for stroke | |||
| Arterial hypertension | 373 (64.9) | 180 (62.7) | >0.05 |
| Diabetes mellitus | 151 (26.3) | 71 (24.7) | >0.05 |
| Atrial fibrillation | 55 (9.6) | 24 (8.4) | >0.05 |
| Currently smoking | 241 (41.9) | 107 (37.3) | >0.05 |
| Alcoholism | 176 (30.6) | 87 (30.3) | >0.05 |
| Metabolism syndrome | 210 (37.0) | 89 (32.0) | >0.05 |
| Laboratory results | |||
| TG (median,range) | 185 (32.2) | 76 (26.5) | >0.05 |
| TC | 113 (19.7) | 57 (19.9) | >0.05 |
| HDL | 98 (17.0) | 58 (20.2) | >0.05 |
| LDL | 165 (28.7) | 88 (30.7) | >0.05 |
| Fast glucose | 241 (42.5) | 110 (39.6) | >0.05 |
| eGFR | 448 (82.2) | 219 (80.8) | >0.05 |
| BUN | 65 (11.3) | 37 (12.9) | >0.05 |
| SCr | 47 (8.2) | 33 (11.5) | >0.05 |
| UA | 58 (10.1) | 37 (12.9) | >0.05 |
| Acute stroke characteristics | |||
| Acute ischemic stroke | 462 (80.3) | 218 (76.0) | >0.05 |
| NIHSS | 12 (9, 16) | 12 (9, 17) | >0.05 |
| Lower limb NIHSS score ≥ 2 | 315 (54.9) | 165 (58.9) | >0.05 |
| Risk factors for deep venous thrombosis | |||
| Active cancer | 12 (2.1) | 7 (2.4) | >0.05 |
| Vein puncture | 30 (5.2) | 11 (3.8) | >0.05 |
| DVT prophylaxis strategies | |||
| Anticoagulation in ischmeic stroke* | 105 (22.7) | 48 (22.0) | >0.05 |
| Antiplatelet agents in ischmeic stroke | 372 (80.5) | 177 (80.2) | >0.05 |
| Deambulation under the supervision of a physical therapist | 267 (46.4) | 129 (44.9) | >0.05 |
| Frequence of deep venous thrombosis | 76 (13.2) | 30 (10.5) | >0.05 |
BMI, body mass index; DVT, deep‐vein thrombosis. *Included anticoagulation in cardioembolsim etc.
Univariate Predictors of DVT After Acute Stroke
In the univariate variable analysis, older age, female gender, BMI (≥25 kg/m2), history of diabetes, atrial fibrillation, active cancer, metabolism syndrome, fasting glucose level, D‐dimer, a hemorrhagic stroke subtype, NIHSS ≥ 10 or lower limb NIHSS score ≥2, venous puncture history, and infections (pneumonia and urinary tract infection) were each associated with a greater risk of developing DVT after acute stroke in the assessment cohort (Table 2). Rehabilitation decreased the risk of DVT. Hypertension, smoking, alcoholism, hypercholesterolemia, the level of BUN/CRE/UA did not have significant association to the development of DVT.
Table 2.
Univariate analysis with respect to any deep‐vein thrombosis (DVT) in the derivation set
| Characteristics | Acute stroke patients (n = 575) | DVT (n = 76) | Unadjusted hazard ratio (95%CI) | P value |
|---|---|---|---|---|
| Older age (≥65 years) | 342 (59.5%) | 57 (75.0%) | 2.25 (1.30–3.90) | <0.01 |
| Female gender | 215 (37.4%) | 39 (51.3%) | 1.93 (1.19–3.14) | <0.01 |
| Obesity (BMI ≥ 25 kg/m2) | 229 (40.2%) | 42 (55.3%) | 2.03 (1.25–3.30) | <0.01 |
| Arterial hypertension | 373 (64.9%) | 55 (72.4%) | 1.49 (0.87–2.55) | >0.05 |
| Diabetes mellitus | 151 (26.3%) | 27 (35.5%) | 1.67 (1.00–2.78) | <0.05 |
| Atrial fibrillation | 55 (9.6%) | 13 (17.1%) | 2.25 (1.14–4.41) | <0.05 |
| Currently smoking | 241 (41.9%) | 29 (38.2%) | 0.84 (0.51–1.37) | >0.05 |
| Alcoholism | 176 (30.6%) | 22 (28.9%) | 0.91 (0.54–1.55) | >0.05 |
| Metabolism syndrome | 210 (37.0%) | 39 (51.3%) | 1.97 (1.21–3.21) | <0.05 |
| Hypercholesterolemia | 339 (59.0%) | 42 (55.3%) | 0.84 (0.52–1.37) | >0.05 |
| Fast glucose | 226 (39.3%) | 41 (53.9%) | 1.99 (1.22–3.23) | <0.05 |
| eGFR (≥60) | 448 (82.2%) | 60 (81.1%) | 0.92 (0.49–1.72) | >0.05 |
| BUN | 65 (11.3%) | 9 (11.8%) | 1.06 (0.50–2.25) | >0.05 |
| SCr | 47 (8.2%) | 9 (11.8%) | 1.63 (0.75–3.52) | >0.05 |
| UA | 58 (10.1%) | 5 (6.6%) | 0.59 (023–1.53) | >0.05 |
| Stroke subtype (ICH) | 113 (19.7%) | 24 (31.6%) | 2.13 (1.25–3.63) | <0.01 |
| NIHSS (≥10) | 397 (69.0%) | 59 (77.6%) | 1.65 (0.93–2.93) | >0.05 |
| Lower limb NIHSS score ≥2 | 315 (54.8%) | 53 (69.7%) | 2.08 (1.24–3.51) | <0.01 |
| Active cancer | 12 (2.1%) | 5 (6.6%) | 4.95 (1.53–16.0) | <0.01 |
| Vein puncture | 30 (5.2%) | 10 (13.2%) | 3.63 (1.63–8.09) | 0.001 |
| Infection | 97 (19.4%) | 25 (32.9%) | 2.03 (1.20–3.44) | <0.01 |
| Rehabilitation | 267 (46.4%) | 26 (34.2%) | 0.56 (0.34–0.92) | <0.05 |
BMI, body mass index.
Multivariate Predictors of DVT After Acute Stroke
The final multivariate model predicting DVT after acute stroke contained six variables which increased the risk of DVT: older age, female gender, BMI (≥25 kg/m2), active cancer, a hemorrhagic stroke subtype, and lower limb NIHSS score ≥2. Diabetes mellitus, metabolism syndrome, fast glucose, atrial fibrillation, and infections were not independent risk factors in the multivariable logistic regression analysis (Table 3).
Table 3.
Multivariate logistic regression model and allocation of points for the DVT risk scoring system in the derivation sample
| Characteristics | Adjusted hazard ratio (95%CI) | P value | Points |
|---|---|---|---|
| Older age (≥65 years) | 2.17 (1.21–3.90) | <0.01 | 1 |
| Female gender | 1.74 (1.04–2.92) | <0.05 | 1 |
| Obesity (BMI ≥ 25 kg/m2) | 2.00 (1.19–3.35) | <0.01 | 1 |
| Active cancer | 5.20 (1.47–18.5) | <0.05 | 2 |
| Stroke subtype(CH) | 2.03 (1.12–3.69) | <0.05 | 1 |
| Lower limb NIHSS score ≥2 | 1.88 (1.08–3.27) | <0.05 | 1 |
BMI, body mass index; DVT, deep‐vein thrombosis.
Clinical Risk Prediction Rule
The estimated HR from the multivariate logistic regression model were used to derive point scores that could be used to predict a patient's risk of developing DVT after acute stroke. The probability of poststroke DVT incidence can be estimated for an individual patient by summing points assigned to the value of each predictor to create a total point score that ranges from 0 to 7 (Table 3).
Model Validation
The overall incidence rate of DVT after acute stroke within two weeks was 12.4% (95%CI 10.3–14.7%) for the entire cohort. When individual DVT risk score was stratified, there was a steady increase in the rate of DVT incidences with increasing DVT risk scores (P < 0.001). The 14‐day risk of DVT was 3.5% (95%CI 3.2–3.8%) in patients with a score of 0, but 38.9% (95%CI 36.3–41.4%) in those with a score of more than 5 (Table 4).
Table 4.
DVT risk after acute stroke within 14 days from onset stratified according to DVT score in the study population
| DVT score | Patients (%) | Any DVT (%) | % Risk (95% CI) |
|---|---|---|---|
| 0 | 57 (6.6%) | 2 (1.9%) | 3.5 (3.2–3.8) |
| 1 | 182 (21.1%) | 12 (11.3%) | 6.6 (6.5–6.8) |
| 2 | 277 (32.1%) | 32 (30.2%) | 11.6 (11.5–11.8) |
| 3 | 240 (27.8%) | 33 (31.1%) | 13.8 (13.7–14.0) |
| 4 | 80 (9.3%) | 20 (18.9%) | 25.0 (24.5–25.6) |
| ≥5 | 18 (2.1%) | 7 (6.6%) | 38.9 (36.3–41.4) |
| Total | 854 (100%) | 106 (100%) | 12.4 (10.3–14.7) |
DVT, deep‐vein thrombosis.
Based on the DVT risk score, patients in the entire cohort with a score of 0–1 would be considered low risk (5.9%, 95%CI 5.8–6.0%), those with 2–3 points as moderate risk (12.6%, 95%CI 12.5–12.7%), and those with ≥4 points as high risk (27.6%, 95%CI 27.1–28.2%). On the basis of this DVT clinical risk score, 13.2% of patients in the entire cohort were at low risk, 61.3% at moderate risk, and 25.5% at high risk (Table 4).
The ROC curves for prediction of poststroke DVT are shown in Figure 2. The risk score demonstrated good discrimination in the derivation cohort. The corresponding AUC assessed by c statistic was 0.70 (95% CI, 0.64–0.76, P < 0.001). Similar good discrimination was seen in the prespecified subgroups in the entire cohort (c statistic 0.65, 95% CI, 0.59–0.70, P < 0.001).
Figure 2.
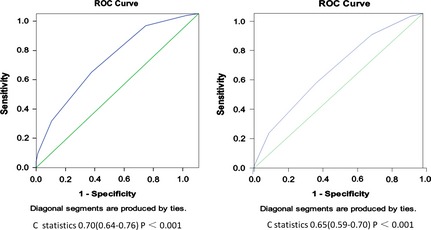
Receiver operating characteristic curves for DVT clinical model applied to the derivation set and the study population.
Discussion
We were able to generate and validate a simple scoring model to predict the occurrence of DVT in patients within 14 days of stroke onset. This model showed certain factors associated with a patients profile to have positive predictive value of DVT poststroke such as being female, older age, BMI (≥25 kg/m2), cancer, a hemorrhagic subtype stroke, and a lower limb NIHSS score ≥2.
In fact, these DVT predisposing variables have been elucidated previously in the literature 7.
For instance, female gender experienced a higher risk of DVT in our multivariate logistic model. A higher preponderance of females to develop DVT was also reported in cerebral hemorrhage patients in a Japanese study 18. Similar results were also seen in ischemic stroke patients both in Asian cohort and Caucasian cohort, although the tendence did not get a significant level 7, 9. In contrast to the studies in stroke patients, the male patient was more likely to develop DVT in general population, which indicated that the sex difference in the prevalence of deep‐vein thrombosis is contrary due to the heterogeneity in the study populations 19. Studies have shown oral contraceptives (OCPs) as the most important cause of thrombosis in young women, with the risk of thrombosis increasing within four months of OCP initiation 20. In addition, the HERS trial evaluated the association between hormone replace treatment (HRT) and VTE and arrived at the conclusion that HRT usage led to a 2‐fold increase in VTE risk, especially pronounced in the first year of treatment 21, 22. However, these phenomenons can hardly explain the higher risk of DVT among female stroke patients, because the usage of OCPs or HRT is scarce in our cohort due to elder age or culture difference. Furthermore, there are still some controversies on the relationship between OCPs or HRT and VTE. So, more studies are needed urgently to address this issue.
We have also validated and reinforced the association between obesity and DVT 23. Obesity may restrict venous return secondary to body fat acting as an impediment for efficient blood flow. Fatty tissue also had a proinflammatory, prothrombotic, and hypofibrinolytic role. The risk of DVT has been found to be directly proportional to body weight with an overall reduced risk of underweight subjects and a significantly higher risk in the obese subjects 24, 25. Furthermore, the Atherosclerosis Risk in Communities (ARIC) and the Cardiovascular Health Study (CHS) also demonstrated an increased risk of VTE formation with obesity with a hazard ratio of 2.7 for a BMI > 40 26. It is interesting to note that Pomp et al. 27 in their study showed obese women who used OCPs to have a 24‐fold higher thrombotic risk than women with a normal BMI who did not use these agents. Nevertheless, conditions such as ischemic heart disease, hypertension, and smoking have an unclear association with VTE predisposition/formation. Malignancy is also a well known to cause DVT 17, 28. Approximately 20% of patients with symptomatic deep venous thrombosis have a known active malignancy 29.
Deep‐vein thrombosis occurrence has also been found to be more commonly associated in patients with the hemorrhagic stroke subtype even after adjusting stroke severity 11. This increased occurrence may be associated with the overall lack of antithrombotic usage after a hemorrhagic stroke. While dehydration remains an established factor predisposing DVT in acute stroke patients, unfortunately, this aspect was never assessed as a potential risk variable in our study 17, 30. The severity of leg weakness also increased the risk of poststroke DVT as expected 7, 17. This observation has been supported by various studies including a meta‐analysis of asymptomatic DVT incidence in patients with immobilization of the lower extremities.
Our study involved determining a DVT assessment/risk scoring system with a high predictive accuracy that was ultimately derived and validated from a nationwide prospective observational study with a large sample size. Furthermore, our results were consistent with the recent report of the combined risk of DVT being 10.0% in the study group and 10.5% in the control group 31.
Our DVT prediction model was derived from a conglomeration of variables encompassing patient demographics, past medical history, stroke severity, and subtype, which demonstrated good differentiation in both the derivation cohort (c statistic 0.69, 95% CI 0.63–0.75) and the entire cohort as a whole (c statistic 0.64, 95% CI 0.59–0.70, P < 0.001). The risk algorithm provides a clinically useful method to predict and stratify DVT risk in poststroke patients. According to the risk algorithm, the risk of DVT after acute stroke can be divided into three categories ranging from low to high risk. This model can be readily incorporated into clinical practice. Furthermore, this risk score model can also be useful in determining sample size or stratifying patients in clinical trials for DVT prophylaxis and treatment. The options for lowering the risk of DVT have been documented extensively in the literature and include early mobilization, the use of external compression devices, and anticoagulation. Previous clinical practice guidelines recommend that subcutaneous administration of anticoagulants for treatment of acute ischemic patients to prevent DVT 31, 32.
It should be noted, however, that there have been no definitive data from randomized clinical trials that dictates treatment of DVT in patients with an acute ischemic or hemorrhagic stroke. Therefore, the choice of starting anticoagulation should be carefully reviewed with risks and benefits in consideration in light of a potential hemorrhagic transformation or a hematoma expansion. Conversely, the Cochrane review showed efficacy and safety of VTE prophylaxis with low‐ and medium‐dose LMWH or unfractionated heparin in patients with acute ischemic stroke 33. However, as with treating DVT after stroke, this benefit of VTE prophylaxis was offset by an increased risk of ICH for individual patients. Data showed occurrence of any bleeding to be 8% with the frequency of the intracranial hemorrhage being 2% for VTE prophylaxis 34. Therefore, to attain an optimal benefit/risk ratio, or objectively, less DVT and ICH, we hypothesized that VTE prophylaxis in stroke patients should be based on the following degrees of risks: early mobilization or external compression devices in the low risk group and anticoagulation in the moderate‐to‐high risk group. Whether this DVT prevention strategy is effective needs to be proven and tested in subsequent future clinical trials.
In is noteworthy to mention that there have been a few studies that used a simplified scoring systems to predict DVT occurrence after acute stroke. For instance, Kelly et al. used MRI to detect DVT thrombus 7. They found that the risk of having a DVT is elevated if a stroke patient is older than 70 (OR = 4.0, 95% CI 1.3–12), presents with an increased degree of dependency in the activities of daily living (ADL) (OR = 8.1, 95% CI 2.2–30.1), dehydrated by day 9 (OR = 4.7, 95% CI 1.1–16.3), urea >7.5 mmol/L (OR = 2.8, 95% CI 1–7.8), and with an urea: creatinine ratio >80 (OR = 3.4, 95% CI 1.2–9.6). However, Kelly et al. did not develop a working DVT risk‐predicting scoring system. More recently, Dennis et al. 8 created a clinical prediction model based on the Clots in Legs or Thromboembolic Deterrent Stockings after Stroke trial. They reported that three variables at baseline predicted risk of DVT after an acute stroke by multivariable logistic regression analysis: independence before stroke (OR 1.92 95% CI 1.18–3.11), unable to lift arms off bed (OR 2.22 95% CI 1.57–3.15), and a history of DVT/PE (OR 3.17 95% CI 1.82–5.52). The AUC in the score generating cohort was 0.61 but only 0.55 (95% CI 0.52–0.59) in the test cohort. This prediction model that was based on clinical factors alone was unable to predict and stratify the immobilized patients into high or low risk of DVT occurrence. The evolution of such a negative predictive model could be explained partially due to the exclusion of variables such as stroke severity and biomarkers like the plasma D‐dimer. On the other hand, CLOTS trial was designed primarily to evaluate the efficacy and safety of graduated compression stockings (GCS) and not to predict poststroke DVTs.
Our study has some limitations. Firstly, the selection of patients and the participating hospitals represent only those subset of people who participated in the trial, which is a very small proportion of stroke patients and healthcare institutions in China. Our cohort comprised of patients that were admitted to stroke centers in cities but not to community hospitals. Secondly, patients who were not independent on admission in terms of being able to fully perform the ADLs were not included in the study. The prevalence of poststroke DVT might thus be underestimated in this study because being bedridden increase the risk of DVT according to previous studies 7, 8, 9. Therefore, our prediction model is based on a national multicenter study of a relatively modest sample size that requires adjustment to represent a larger and more heterogeneous data sets 35. Thirdly, the primary outcome in the final multivariate model was the presence of DVTs in any location, including proximal and distal DVT. The application of ultrasound has been shown to offer a limited way of detecting small and distal DVTs (63.5 vs. 94.2%) 36. Given the fact there is a lack of consensus on the clinical significance of symptomatic isolated distal DVT, further studies should focus on both proximal and distal DVT detection using more advanced diagnostic technology 37, 38. Fourthly, the predictive discrimination of the clinical‐based model may have improved if D‐dimer values with an optimum cutoff value were incorporated to the DVT risk assessment scale. In this regard, further research is needed to determine whether the application of D‐dimer with optimum cutoff values in routine clinical practice would actually serve to augment DVT risk determination 39, 40, 41. In the end, we would encourage the initiation of multicentered prospective trials to validate and reinforce our DVT risk assessment scoring system to eliminate the aforementioned biases and to assess clinical utility in stroke patients.
To conclude, the risk of DVT after acute stroke during the first 14 days is highly predictable. Acute stroke patients at low, intermediate, and high risk of DVT can be identified by combining data on demographics, major comorbidities as well as the stroke severity on admission. The current score model can be used in routine clinical practice to identify patients at high risk of developing DVT and initiate the appropriate prevention or treatment strategies. Future research should therefore focus on identifying patients who fall at a high risk of DVT yet simultaneously ensuring low risk of bleeding complications when pharmacological prophylaxis is prescribed 15.
Conflict of Interest
These funding agencies did not participate in design or analysis, manuscript preparation, or approval of this study. The authors declare no conflict of interest.
Acknowledgments
This study was supported by Beijing Natural Science Foundation (Grant No. D0905004040231), the Ministry of Science and Technology and the Ministry of Health of the People's Republic of China (Grant No. 2006BA101A11 and 2009CB521905). This study was also supported by the GlaxoSmithkline (China) Ltd. We thank all our colleagues who collaborated on The Incidence of Deep Venous Thrombosis after Acute Stroke in China (INVENT‐China) study.
The first two authors contributed equally to this work.
References
- 1. Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke: Experience from the RANTTAS trial. RANTTAS Investigators. Stroke 1998;29:447–453. [DOI] [PubMed] [Google Scholar]
- 2. Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: A prospective review. Stroke 1984;15:492–496. [DOI] [PubMed] [Google Scholar]
- 3. Kelly J, Rudd A, Lewis R, Hunt BJ. Venous thromboembolism after acute stroke. Stroke 2001;32:262–267. [DOI] [PubMed] [Google Scholar]
- 4. Viitanen M, Winblad B, Asplund K. Autopsy‐verified causes of death after stroke. Acta Med Scand 1987;222:401–408. [DOI] [PubMed] [Google Scholar]
- 5. Kamran SI, Downey D, Ruff RL. Pneumatic sequential compression reduces the risk of deep vein thrombosis in stroke patients. Neurology 1998;50:1683–1688. [DOI] [PubMed] [Google Scholar]
- 6. Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke 1996;27:415–420. [DOI] [PubMed] [Google Scholar]
- 7. Kelly J, Rudd A, Lewis RR, et al. Venous thromboembolism after acute ischemic stroke: A prospective study using magnetic resonance direct thrombus imaging. Stroke 2004;35:2320–2325. [DOI] [PubMed] [Google Scholar]
- 8. Dennis M, Mordi N, Graham C, Sandercock P, CLOTS Trials Collaboration . The timing, extent, progression and regression of deep vein thrombosis in immobile stroke patients: Observational data from the CLOTS multicenter randomized trials. J Thromb Haemost 2011;9:2193–2200. [DOI] [PubMed] [Google Scholar]
- 9. De Silva DA, Pey HB, Wong MC, Chang HM, Chen CP. Deep vein thrombosis following ischemic stroke among Asians. Cerebrovasc Dis 2006;22:245–250. [DOI] [PubMed] [Google Scholar]
- 10. Kappelle LJ. Preventing deep vein thrombosis after stroke: Strategies and recommendations. Curr Treat Options Neurol 2011;13:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 12. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross‐sectional study. Lancet 2008;371:387–394. [DOI] [PubMed] [Google Scholar]
- 13. Zheng H, Liu L, Sun H, et al. Prophylaxis of deep venous thrombosis and adherence to guideline recommendations among in patients with acute stroke: Results from a multicenter observational longitudinal study in China. Neurol Res 2008;30:370–376. [DOI] [PubMed] [Google Scholar]
- 14. Douds GL, Hellkamp AS, Olson DM, et al. Venous Thromboembolism in the Get With The Guidelines‐Stroke Acute Ischemic Stroke Population: Incidence and Patterns of Prophylaxis. J Stroke Cerebrovasc Dis 2014;23:123–129. [DOI] [PubMed] [Google Scholar]
- 15. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 16. National Institute for Health and Clinical Excellence . NICE Clinical Guideline 92. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to Hospital. London: National Institute for Health and Clinical Excellence, 2010. [Google Scholar]
- 17. Dennis M, Sandercock P, Reid J, et al. Can clinical features distinguish between immobile patients with stroke at high and low risk of deep vein thrombosis? Statistical modelling based on the CLOTS trials cohorts. J Neurol Neurosurg Psychiatry 2011;82:1067–1073. [DOI] [PubMed] [Google Scholar]
- 18. Kawase K, Okazaki S, Toyoda K, et al. Sex difference in the prevalence of deep‐vein thrombosis in Japanese patients with acute intracerebral hemorrhage. Cerebrovasc Dis 2009;27:313–319. [DOI] [PubMed] [Google Scholar]
- 19. Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: Results from the Copenhagen City Heart Study. Circulation 2011;121:1896–1903. [DOI] [PubMed] [Google Scholar]
- 20. Kujovich JL. Hormones and pregnancy: Thromboembolic risks for women. Br J Haematol 2004;126:443–454. [DOI] [PubMed] [Google Scholar]
- 21. Miller J, Chan BK, Nelson HD. Postmenopausal estrogen replacement and risk for venous thromboembolism: A systematic review and meta‐analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:680–690. [DOI] [PubMed] [Google Scholar]
- 22. Grodstein F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet 1996;348:983–987. [DOI] [PubMed] [Google Scholar]
- 23. Severinsen MT, Kristensen SR, Johnsen SP, Dethlefsen C, Tjønneland A, Overvad K. Anthropometry, body fat, and venous thromboembolism: A Danish follow‐up study. Circulation 2009;120:1850–1857. [DOI] [PubMed] [Google Scholar]
- 24. Delluc A, Mottier D, Le Gal G, Oger E, Lacut K. Underweight is associated with a reduced risk of venous thromboembolism. Results from the EDITH case‐control study. J Thromb Haemost 2009;7:728–729. [DOI] [PubMed] [Google Scholar]
- 25. Kucher N, Tapson VF, Goldhaber SZ, DVT FREE Steering Committee . Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb Haemost 2005;93:494–498. [DOI] [PubMed] [Google Scholar]
- 26. Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: The longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002;162:1182–1189. [DOI] [PubMed] [Google Scholar]
- 27. Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: Obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol 2007;139:289–296. [DOI] [PubMed] [Google Scholar]
- 28. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: A meta‐analysis. Circulation 2008;117:93–102. [DOI] [PubMed] [Google Scholar]
- 29. Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population‐based study. Arch Intern Med 2002;162:1245–1248. [DOI] [PubMed] [Google Scholar]
- 30. Kelly J, Hunt BJ, Lewis RR, et al. Dehydration and venous thromboembolism after acute stroke. QJM 2004;97:293–296. [DOI] [PubMed] [Google Scholar]
- 31. CLOTS Trials Collaboration , Dennis M, Sandercock PA, et al. Effectiveness of thigh‐length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke next term (CLOTS trial 1): A multicentre, randomised controlled trial. Lancet 2009;373:1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141:e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandercock PA, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev 2008:CD000024. [DOI] [PubMed] [Google Scholar]
- 34. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): An open‐label randomised comparison. Lancet 2007;369:1347–1355. [DOI] [PubMed] [Google Scholar]
- 35. Kase CS, Albers GW, Bladin C, et al. Neurological outcomes in patients with ischemic stroke receiving enoxaparin or heparin for venous thromboembolism prophylaxis: Subanalysis of the Prevention of VTE after Acute Ischemic Stroke with LMWH (PREVAIL) study. Stroke 2009;40:3532–3540. [DOI] [PubMed] [Google Scholar]
- 36. Bembenek J, Karlinski M, Kobayashi A, Czlonkowska A. Early stroke‐related deep venous thrombosis: Risk factors and influence on outcome. J Thromb Thrombolysis 2011;32:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho VB, van Geertruyden PH, Yucel EK, et al. ACR Appropriateness Criteria(®) on suspected lower extremity deep vein thrombosis. J Am Coll Radiol 2011;8:383–387. [DOI] [PubMed] [Google Scholar]
- 38. Galanaud JP, Sevestre‐Pietri MA, Bosson JL, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: Results from the OPTIMEV study. Thromb Haemost 2009;102:493–500. [DOI] [PubMed] [Google Scholar]
- 39. Kelly J, Rudd A, Lewis RR, et al. Screening for proximal deep vein thrombosis after acute ischemic stroke: A prospective study using clinical factors and plasma D‐dimers. J Thromb Haemost 2004;2:1321–1326. [DOI] [PubMed] [Google Scholar]
- 40. Kuwashiro T, Toyoda K, Oyama N, et al. High plasma D‐dimer is a marker of deep vein thrombosis in acute stroke. J Stroke Cerebrovasc Dis 2012;21:205–209. [DOI] [PubMed] [Google Scholar]
- 41. Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Uses of different D‐dimer levels can reduce the need for venous duplex scanning to rule out deep vein thrombosis in patients with symptomatic pulmonary embolism. J Vasc Surg 2007;46:526–532. [DOI] [PubMed] [Google Scholar]


