The balance between endothelial cell survival and death plays a pivotal role in brain remodeling and repair after stroke 1. Extensive changes in endothelial cell behavior have been implicated in angiogenesis and vasculogenesis 2. Angiogenesis is a prominent feature of ischemic stroke recovery, and increasing endothelial polarity might stimulate early angiogenesis and reflect a beneficial mechanism of revascularization and neuroprotection after stroke 3. Polarization is important for various aspects of the endothelial response to a variety of stressful stimuli, such as hypoxia and inflammation 4. However, the precise molecular cues that regulate endothelial polarity and function remain elusive.
In the current study, we set out to address the potential role of peroxiredoxin 1 in ischemia‐induced endothelial polarization. It is unknown whether peroxiredoxin 1 plays a role in angiogenesis, a process that involves the generation of new blood vessels and relies largely on vascular endothelial cell polarization and proliferation. We previously reported that peroxiredoxin 1 is a pivotal molecule for the protection of endothelial cells and microvessels from ischemia‐induced neurovascular damage both in vitro and in vivo 5. Earlier studies have shown that peroxiredoxin 1 carries out a wide range of cellular functions, including exerting a proliferative effect and playing an antioxidant protective role 5. It is also known that peroxiredoxin 1 is important for vascular endothelial growth factor (VEGF) expression 6, and it is therefore likely that peroxiredoxin 1 is involved in the induction of angiogenesis following a stroke.
Here, we first investigated the involvement of peroxiredoxin 1 in oxygen‐glucose deprivation (OGD)‐induced endothelial cell polarization. EA.hy926 endothelial cells were exposed to OGD for 6 and 12 h, and the expression of peroxiredoxin 1 was assayed using confocal immunocytochemistry. We defined polarized cells according to the proportion of the longest axis of the cell to that of the shortest axis of the cell 7. As shown in Figure 1A, peroxiredoxin 1‐positive endothelial cells in the OGD 6 h group exhibited polarized characteristics. After 12 h had elapsed, the cells respread, and their elongation became even more pronounced. They exhibited a polarized cell shape with a morphologically defined leading process and a uropod, whereas the control cells displayed oval‐shaped morphology. The results of quantitative analyses from three independent experiments are depicted in Figure 1B, indicating that cell elongation significantly increased after OGD, whereas cell ellipsoid decreased (Figure 1C). This elongation might involve directional spreading via a protrusion at the front of the cells mediated by various intracellular signaling mechanisms 7. These data raise the possibility that peroxiredoxin 1 plays a crucial role in the establishment of endothelial cell polarity in response to ischemic stimuli, which might be related to its proliferative effect and its contributions toward angiogenesis and brain repair.
Figure 1.
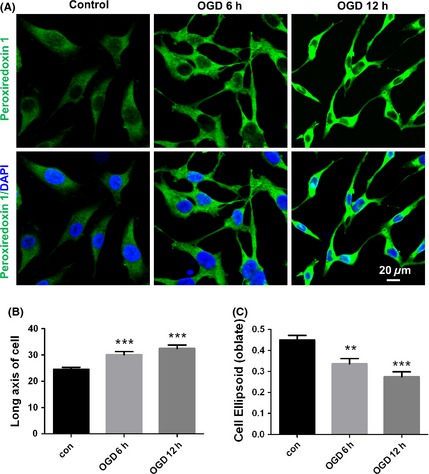
Peroxiredoxin 1 is related to OGD‐induced morphological polarization in EA.hy926 endothelial cells. (A) Changes in the immunostaining of peroxiredoxin 1 (green) 6 h and 12 h after OGD. Subcellular localization of polarized peroxiredoxin 1 expression was determined under laser confocal microscopy. Nuclei are counterstained with DAPI. Data represent three independent experiments. Scale bar = 20 μm. Quantitative analyses of polarized peroxiredoxin 1 cells are indicated as long axis (B) and cell ellipsoid (C). The error bars represent the SEM. **P < 0.01; ***P < 0.001 versus control.
To further ascertain the impact of the upregulation of peroxiredoxin 1 on endothelial polarization in vivo, a mouse transient middle cerebral artery occlusion (tMCAO) model was employed in the present study. Staining brain sections from tMCAO mice for peroxiredoxin 1 and the endothelial marker Tie2 revealed that peroxiredoxin 1 was localized to the endothelial cells of the brain (Figure 2). Peroxiredoxin 1 immunoreactivity was observed predominantly in ipsilateral brain microvessels 12 h after administering tMCAO (Figure 2A) and was accompanied by colocalization with Tie2 immunoreactivity (Figure 2B,C,D). Compared with sham‐operated animals, peroxiredoxin 1 expression was increased significantly in brain microvessels within the penumbra region after MCAO (Figure 2A,B,C), with polarized endothelial cells typically located toward the core region (Figure 2B,C). A representative Z‐stack image is shown in Figure 2D. It shows increased polarized peroxiredoxin 1 expression in the microvessels of the penumbra region. Therefore, both in vitro and in vivo, data imply that polarized peroxiredoxin 1 expression in endothelial/microvessels is not simply an event that occurs in parallel with ischemic injury but is perhaps related to the remodeling effect of peroxiredoxin 1 signaling. We previously reported the protective effects of peroxiredoxin 1 against ischemia‐induced neurological and functional deficits, which might be associated with the ability of peroxiredoxin 1 to block degradation. Because the role of peroxiredoxin 1 in preventing ischemia‐induced neurological deficits is associated with its protective effect on tight junction proteins 5, it is likely that peroxiredoxin 1‐mediated microvessel polarization mechanisms coordinate this effect in vivo.
Figure 2.
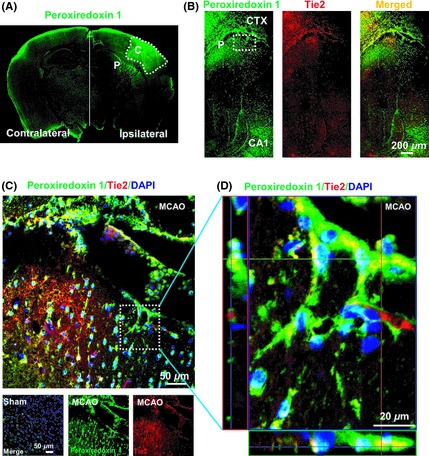
Peroxiredoxin 1 is associated with endothelial polarization in the brain microvessels of mice with cerebral ischemia. (A) The distribution of peroxiredoxin 1 staining on the contralateral and ipsilateral sides of the mouse brain 12 h after tMCAO. (B) Double‐immunohistochemical staining for peroxiredoxin 1 and Tie2 in the penumbra after tMCAO. Fluorescence staining for peroxiredoxin 1 (green) and Tie2 (red) was performed in ipsilateral brain regions of mice 12 h after ischemic brain injury. (C) Higher magnification images of endothelial staining from the insets are shown in (B) and sham. (D) Orthogonal projections onto the z–stack planes confirm the colocalization of peroxiredoxin 1 and Tie2 throughout the microvessels shown in (C). Nuclei are counterstained with DAPI. P, Penumbra; C, Core.
Considering these results and our previous findings 5, 8, it is tempting to speculate that peroxiredoxin 1 is important for angiogenesis because it modulates endothelial cell/vascular polarization under pathological conditions and retains its proangiogenic potential under physiological conditions. Although we cannot formally rule out this mechanism of peroxiredoxin 1‐regulated cell polarization, the conclusion that the upregulation of peroxiredoxin 1 may be secondary to the activation of NF‐E2‐related factor 2 (Nrf2) should also be considered 8. Nrf2 dysfunction may be a mechanism underlying impaired angiogenesis and microvascular rarefaction. In line with this possibility, the postinjury induction of Nrf2‐driven genes attenuates a loss of endothelial tight junction proteins and reduces blood–brain barrier permeability 9. The identities of the other cells and environmental cues that drive this process remain unclear and subject to investigation. In this respect, it is interesting that endothelial cells can provide a selective niche for the differentiation and functional polarization of macrophages 10. Nevertheless, this is the first report to demonstrate a potential role for peroxiredoxin 1 in orchestrating endothelial polarization and subsequently promoting neurovascular repair.
The current study has several limitations that need to be addressed in future. Additional investigations using pharmacological inhibition or genetic approaches are of great importance for elucidating the specific role of peroxiredoxin 1 on brain ischemia‐induced endothelial polarization. In addition, the balance/intracellular link between peroxiredoxin 1 signaling and other pathways regulating cell polarization is another unaddressed issue.
In summary, our studies identify an intracellular link between cell polarization and peroxiredoxin 1 signaling in endothelial cells following an ischemia‐like injury. Future studies of the peroxiredoxin 1 mechanisms driving polarization after stroke may reiterate that a potential therapeutic approach involves the beneficial remodeling processes of peroxiredoxin 1‐activating molecules.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by National Natural Science Foundations of China (81202534, 81120108023).
References
- 1. Rizzo MT, Leaver HA. Brain endothelial cell death: Modes, signaling pathways, and relevance to neural development, homeostasis, and disease. Mol Neurobiol 2010;42:52–63. [DOI] [PubMed] [Google Scholar]
- 2. Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: Therapeutic perspective for ischemic stroke. CNS Neurosci Ther 2013;19:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu RY, Luo DF, Xiao SH, et al. Kallikrein gene transfer induces angiogenesis and further improves regional cerebral blood flow in the early period after cerebral ischemia/reperfusion in rats. CNS Neurosci Ther 2012;18:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res 2007;100:782–794. [DOI] [PubMed] [Google Scholar]
- 5. Tao RR, Wang H, Hong LJ, et al. Nitrosative stress induces peroxiredoxin 1 ubiquitination during ischemic insult via E6AP activation in endothelial cells both in vitro and in vivo . Antioxid Redox Signal 2014; doi: 10.1089/ars.2013.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riddell JR, Maier P, Sass SN, Moser MT, Foster BA, Gollnick SO. Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF‐1α . PLoS ONE 2012;7:e50394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wojciak‐Stothard B, Ridley AJ. Shear stress‐induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3‐kinases. J Cell Biol 2003;161:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tao RR, Huang JY, Shao XJ, et al. Ischemic injury promotes Keap1 nitration and disturbance of antioxidative responses in endothelial cells: A potential vasoprotective effect of melatonin. J Pineal Res 2013;54:271–281. [DOI] [PubMed] [Google Scholar]
- 9. Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2‐driven genes protects the blood brain barrier after brain injury. J Neurosci 2007;27:10240–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He H, Xu J, Warren CM, et al. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2‐like macrophages. Blood 2012;120:3152–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]


