Abstract
Background
Diffuse idiopathic skeletal hyperostosis (DISH) is a condition characterized by the formation of new bone along the anterolateral spinal column at four adjacent vertebral bodies.
Purpose
To propose and validate criteria for the early phase of DISH by using CT data from two large-scale retrospective cohorts, each with 5-year follow-up.
Materials and Methods
For this retrospective study, CT data at baseline and follow-up in 1367 patients (cohort I) from 2004 to 2011 were evaluated by two observers to define no DISH, early-stage DISH, and definite DISH on the basis of interval development of consecutive complete or incomplete bone bridges. An independent group of 2267 participants from the COPDGene cohort from 2008 to 2016 was used to validate the early DISH criteria (cohort II). The sensitivity and specificity of early DISH criteria were based on findings in the last CT study as the reference standard by using a nested case-control design. κ Values were calculated between seven readers and with a 3-month interval for one reader.
Results
Cohort I consisted of 100% men, with a mean age of 60.0 years ± 5.6 (standard deviation) and a mean time between baseline and follow-up CT of 5.0 years ± 1.1. Cohort II consisted of 51% men, with a mean age of 59.9 years ± 8.6 and a mean time between baseline and follow-up CT of 5.4 years ± 0.5. In the derivation cohort, 55 patients comprised the early DISH group. Early DISH was defined as the presence of a spinal segment with a complete bone bridge with an adjacent segment of at least a near-complete bone bridge and another adjacent segment with at least the presence of newly formed bone or when three or more adjacent segments were recorded as showing a near-complete bone bridge. In the validation cohort, sensitivity for early DISH (vs no DISH) was 96% (99 of 103 participants; 95% confidence interval [CI]: 90%, 99%). The corresponding specificity was 83% (1695 of 2034 participants; 95% CI: 82%, 85%). The Fleiss κ for interrater reliability was 0.78 (95% CI: 0.77, 0.78), and the κ for intrarater reliability was 0.89 (95% CI: 0.82, 0.96).
Conclusion
Early diffuse idiopathic skeletal hyperostosis (DISH) criteria had high sensitivity and specificity for predicting the development of DISH.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Block in this issue.
Summary
Early diffuse idiopathic skeletal hyperostosis was defined as the presence of a spinal segment with a complete bone bridge with an adjacent segment of at least a near-complete bone bridge and another adjacent segment with at least the presence of newly formed bone or when at least three adjacent segments were recorded as showing a near-complete bone bridge.
Key Points
■ The sensitivity of new criteria for early diffuse idiopathic skeletal hyperostosis (DISH) (vs no DISH) was 96%; the specificity was 83%.
■ For new criteria for early DISH, the interrater agreement was 0.78; intrarater agreement was 0.89.
Introduction
Diffuse idiopathic skeletal hyperostosis (DISH) is a condition characterized by the formation of new bone along the anterolateral spinal column (1). The lower thoracic spine is most frequently affected, and ossifications of peripheral entheses are also frequently present in DISH (1,2). The prevalence of DISH varies between 2.9% and 42.0%, depending on the criteria used, demographic background, and presence of associated factors (3–6). Risk factors for developing DISH are older age, metabolic derangement (hypertension, obesity, diabetes mellitus), and cardiovascular disease (1,4). The pathogenesis of DISH is unknown (1).
The three criteria established by Resnick and Niwayama are the criteria most frequently used for the diagnosis of DISH and include bridging of four adjacent vertebral bodies by newly formed bone, without severe loss of the intervertebral disk height and without degeneration of the apophyseal and sacroiliac joints (3,7). The Resnick and Niwayama criteria were designed so as to include only “definite” cases of DISH in their study, excluding other spinal pathologies such as ankylosing spondylitis (7). As a consequence, the threshold criteria for DISH are high and therefore possibly reflect a late or even end stage of DISH (3). Longitudinal research on the natural course of DISH has exposed a process of slow, ongoing formation of new bone (8–10). Over time, the number of affected vertebral body segments increases and pointy incomplete bone bridges become flowing complete bone bridges (8–10). The results of longitudinal studies stress the need for criteria describing earlier phases of DISH, to allow us to investigate the pathogenesis of DISH in its earlier immature developmental phase and potentially identify genetic factors (3,11). Understanding the pathogenesis of DISH could contribute not only to the development of treatment options for DISH but also, for example, to a better understanding of (ectopic) bone formation and regeneration.
The purpose of this study was to develop criteria for diagnosis and classification of the early phase of DISH on chest CT scans from two large-scale research populations. Intra- and interrater reliability of early DISH criteria were evaluated.
Materials and Methods
For cohort I (described below), no written informed consent was needed according to the regulations of this institution (medical ethical board correspondence number #17/164, University Medical Center Utrecht, Utrecht, the Netherlands). For cohort II (also described below), written informed consent was obtained from all participating participants.
Cohort I
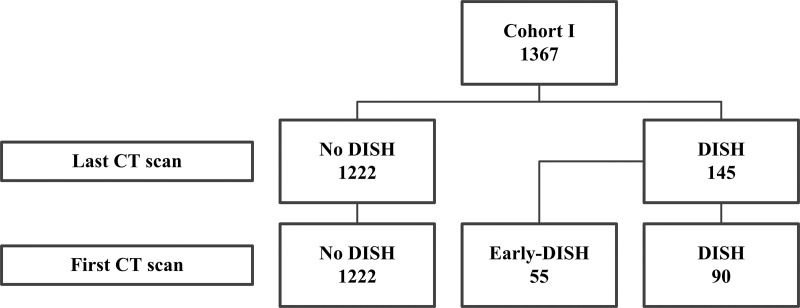
Chest CT studies in the patients in cohort I were used to develop criteria for DISH. For cohort I, CT studies were anonymized after they were extracted from the picture archiving and communication system (PACS) of a single medical center (University Medical Center Utrecht, Utrecht, the Netherlands). Patients were selected for this cohort if they were male, they were more than 50 years old, and they had two unenhanced chest CT studies available in the PACS that were performed at an interval of at least 2.5 years apart (obtained between 2004 and 2011; Fig E1 [online]) (10). The CT studies in the 1367 patients matching the inclusion criteria were performed at 120 kVp and a range of tube currents during an inspiratory breath hold by using 16–64-section detector CT scanners (Philips Medical Systems, Cleveland, Ohio). For all CT scans, thin-section axial data were stored (maximum section thickness, 1 mm). Two independent observers (J.S.K., a PhD candidate with 3 years of experience in research regarding the imaging of DISH, and J.J.V., an orthopedic surgeon with 12 years of experience in research regarding the imaging of DISH) reviewed the CT studies in the bone setting for the presence of DISH by using the Resnick and Niwayama criteria, and disagreement on the diagnosis was solved by consensus (7,10). The sacroiliac joints could not be evaluated (because only chest CT scans were available), and, to avoid the inclusion of patients with an alternative spinal condition such as ankylosing spondylitis, the apophyseal joints of the thoracic spine were carefully assessed for signs of fusion. In accordance with the Resnick and Niwayama criteria, patients with loss of intervertebral disk height or ankylosis of the facet joints were regarded as not having DISH in this cohort (7). In 90 patients, DISH was present in both the first and the last CT study (definite DISH group; mean follow-up, 4.8 years; Fig 1). In 55 patients, DISH was present only in the last CT study and not in the first CT study (early DISH group; mean follow-up, 5.0 years). From among the 1222 patients without DISH in the first and last CT studies, a random sample was drawn of 55 patients (no-DISH group; mean follow-up, 4.9 years). The groups with early DISH (n = 55) and no DISH (n = 55) were used to develop criteria for the early phase of DISH.
Figure 1:
Patient flowchart for the presence of early diffuse idiopathic skeletal hyperostosis (DISH) in cohort I. Patients were given a diagnosis of DISH on both the first and the last noncontrast chest CT studies according to the criteria by Resnick and Niwayama (7) (new bony bridges over at least four adjacent vertebral bodies, without severe loss of intervertebral disk height and no ankylosis of the apophyseal joints). Patients without DISH at the first CT study but with DISH at the last CT study (n = 55) were regarded as having had early DISH at the first CT study. In 90 patients, DISH was present in both the first and the last CT study.
Development of Early DISH Criteria
The first and last CT studies of the patients with no or early DISH were assessed for the presence and location of bone bridges and degree of flow of the newly formed bone (on sagittal and coronal images of the spinal column) and the relative location of new bone formation (on transverse images of the spinal column) by using a scoring system with acceptable interrater reliability scores (0.6–0.9) (10). The presence and completeness of a bone bridge was assessed for each segment, which consisted of the intervertebral disk and the vertebral bodies above and below it.
Figure 2 shows the scoring system. Each segment of the spine was scored from 0 to 3 as follows: A score of 0 indicated normal vertebral bodies without formation of new bone; a score of 1, anterior new bone formation—connected or unconnected to the vertebral body above or below (eg, nodular soft-tissue calcifications)—without a solid bony bridge (osteophytes with ≥ 2 mm distance between two bone structures) OR a connection between two adjacent vertebral bodies without abundantly formed bone; a score of 2, near-complete bridging by the anterior new bone formation with less than 2 mm of distance between the bony structures OR a full connection of the bone in a maximum of two sagittal or coronal CT sections (visualized on 1-mm-thick sections); and a score of 3, complete bridging between the vertebral bodies above and below the disk with abundant new bone formation in more than two sagittal or coronal CT sections (visualized on 1-mm-thick sections).
Figure 2:
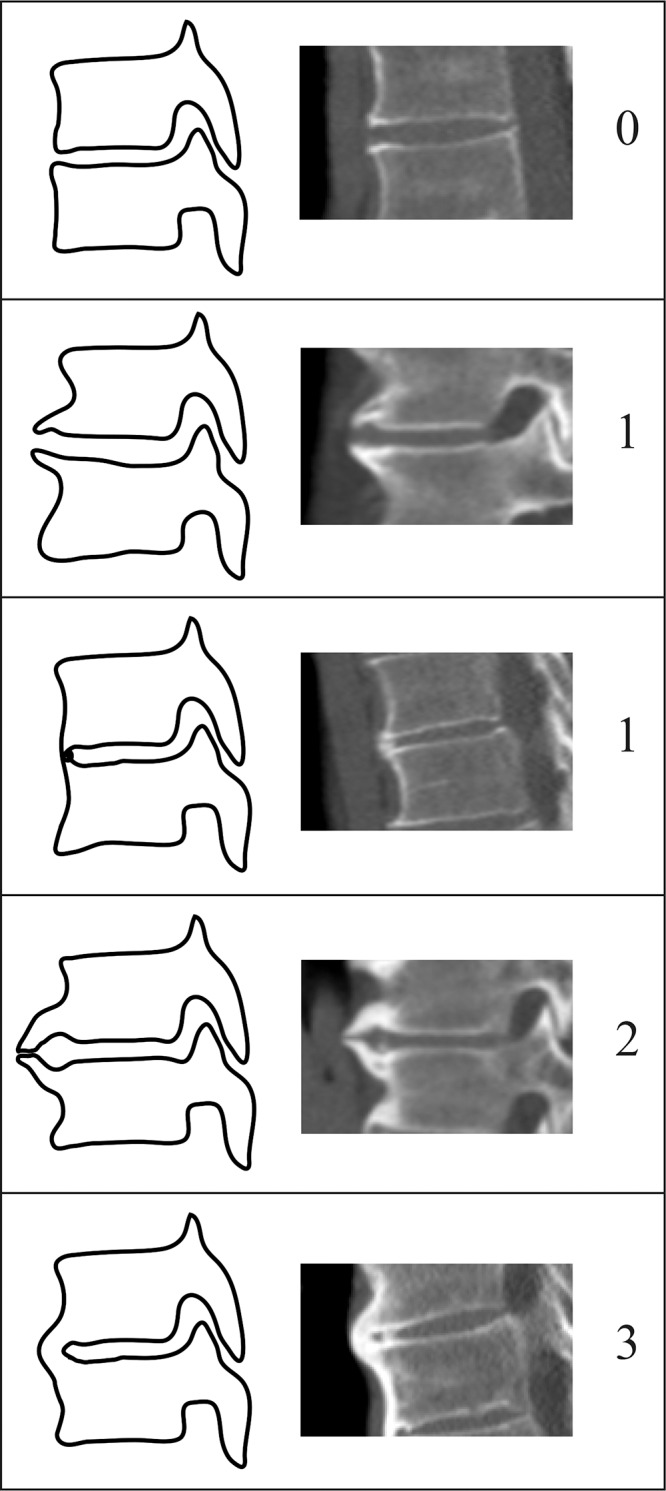
Scoring system used for definition of early diffuse idiopathic skeletal hyperostosis. A score from 0 to 3 was assigned for each vertebral segment. A score of 0 indicated normal vertebral bodies without formation of new bone; a score of 1, anterior new bone formation—connected or unconnected to the vertebral body above or below (eg, nodular soft-tissue calcifications)—without a solid bony bridge (osteophytes with ≥ 2 mm distance between two bony structures) OR a connection between two adjacent vertebral bodies without abundantly formed bone; a score of 2, near-complete bridging by the anterior new bone formation with less than 2 mm of distance between the bony structures OR a full connection of the bone in a maximum of two sagittal or coronal CT sections (with a section thickness of 1 mm); and a score of 3, complete bridging between the vertebral bodies above and below the disk with abundant new bone formation in more than two sagittal or coronal CT sections (with a section thickness of 1 mm).
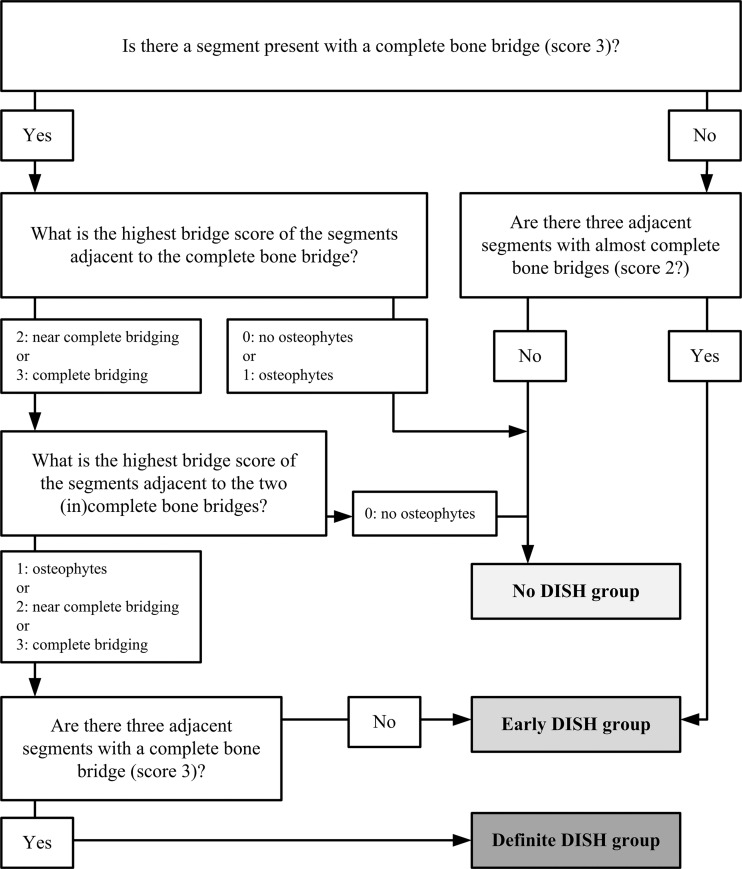
The aim was to discriminate between no DISH, early DISH, and definite DISH at baseline CT. The definite DISH stage was defined according to the first Resnick and Niwayama criterion: the presence of bridging over four continuous vertebral bodies (three consecutive complete bony bridges—representing score 3 bone formation) (7). The optimal set of criteria had to be able to discriminate between no DISH and early DISH and was selected by comparing receiver operating characteristic curves for continuous outcome measures, sensitivity and specificity for dichotomous outcome measures, and the clinical usability of different potential criteria combinations (consisting of the presence of new bone and/or the shape of the new bone [degree of flow]).
Cohort II
Chest CT studies in the participants in cohort II were used to validate the criteria for early DISH and to calculate intra- and interobserver reliability. For cohort II, participants involved in a study of chronic obstructive pulmonary disease (COPD)—the Genetic Epidemiology of COPD (COPDGene) study—were selected (12). Cohort II constituted of 2267 randomly selected participants from the COPDGene cohort with two CT studies available that were performed between 2008 and 2016 (mean interval, 5.4 years). Inclusion criteria for the COPDGene cohort were age between 45 and 80 years, racial/ethnic category of either non-Hispanic white or African-American, and a minimum of 10 pack-years of smoking (Fig E1 [online]) (12). The relevant exclusion criteria were a history of lung disease other than COPD or asthma, active cancer currently being treated, and myocardial infarction. All inclusion and exclusion criteria have previously been published (12). The CT studies were performed with 16-detector or higher CT scanners and were stored as axial reconstructions with a section thickness of 0.5 mm.
Validation of Criteria
On the last CT study in all participants in cohort II, the presence of early DISH was recorded by using the proposed criteria. A random sample was drawn (equal to the number of participants with definite DISH) from the participants with no DISH and from the participants with early DISH (1:4 case-control ratio) by following efficient nested case-control design (13). Subsequently, a single observer (J.S.K.) applied the criteria to the first CT study of the random sample plus the definite DISH cases while being blinded to the presence of (early) DISH on the last CT study.
The CT studies were also evaluated for the presence of substantial disk height loss and/or facet joint ankylosis, and the results of the criteria were presented with exclusion of potential other causes of ankylosis (classification criteria) and without exclusion of such cases (diagnostic criteria). From a clinical perspective, the co-occurrence of DISH and other diseases such as ankylosing spondylitis or spondylosis is conceivable (14). However, in the research setting, the comparison between participants with and those without early DISH should not be confounded by other conditions that could cause spinal ankylosis and the formation of new bone (15).
A selection of the CT studies was used to calculate interrater reliability. Seven observers with different levels of expertise (observer 1 [J.W., a nonradiologist research student with 0 years of experience], observer 2 [W.P.G., a nonradiologist PhD candidate with 1 year of experience], observer 3 [J.S.K., a nonradiologist PhD candidate with 3 years of experience], observer 4 [W.F., a radiologist-in-training with 2 years of experience], observer 5 [S.F.O., a radiologist with 6 years of experience], observer 6 [F.A.M.H., a radiologist with 6 years of experience], and observer 7 [J.J.V., a nonradiologist orthopedic surgeon with 12 years of experience]) were trained in the use of the criteria for early DISH with 10 CT studies. Each observer successively scored a total of 90 CT studies with no, early, and definite DISH. For intrarater reliability, a single observer (J.S.K.) repeated the assessment of the 90 CT studies after 3 months.
Statistical Analysis
All data analyses were performed by using R statistical software (R, version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria). For each potential set of criteria derived from the results of cohort I, sensitivity, specificity, and the receiver operating characteristic curve were calculated for comparison of the no-DISH and early-DISH groups. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and risk ratio were all calculated from the results in cohort II, with the reference standard of definite DISH at the last CT study after multiplying the nondefinite DISH samples (1/sample fraction) to correct for the nested case-control design using by the epiR package 0.9–93 (https://cran.r-project.org/web/packages/epiR/epiR.pdf [13,16]). The intra- and interrater reliability of the selected diagnostic and classification criteria were calculated by using a linear κ value between each of the seven observers, a Fleiss κ value between all observers, and a linear κ value between the two assessments of a single observer (3-month interval) by using the irr package 0.84 (https://cran.r-project.org/web/packages/irr/irr.pdf [17]).
Results
Demographics of Cohort I
Cohort I consisted of 1367 men with a mean age of 60.0 years ± 5.6 (standard deviation) at the first CT study and an interval of 5.0 years ± 1.1 between the first and last CT studies (Table 1). The mean age was 61.6 years ± 5.8 for the 55 patients in the early DISH group and 59.6 years ± 4.5 for the 55 randomly selected patients without DISH.
Table 1:
Baseline Characteristics of Cohorts I and II
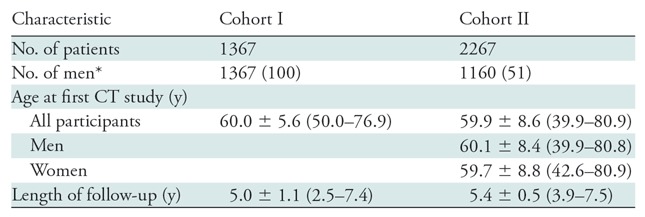
Note.—Unless otherwise specified, data are means ± standard deviations, with ranges in parentheses.
*Data in parentheses are percentages.
Development of Early DISH Criteria
We assessed various combinations of scores from cohort I and selected the criteria with the best sensitivity and specificity between the no-DISH and early-DISH groups (Fig E2 [online]). In the optimal criteria, early DISH was identified as follows: a segment with a complete bone bridge (score 3) with an adjacent segment of at least a near-complete bone bridge (score 2 or 3) AND another adjacent segment with at least the presence of newly formed bone (score 1 or 2; if the first adjacent segment was score 2, the second adjacent segment may be score 3); OR when at least three adjacent segments were recorded as showing a near-complete bone bridge (score 2).
Definite DISH was defined (as per the original Resnick and Niwayama criteria) by the presence of at least three adjacent segments (four vertebral bodies) with a complete bone bridge (score 3). All other combinations of results were considered to indicate no DISH. The flowchart in Figure 3 summarizes these results and facilitates quick assessment of (early) DISH. Examples of the use of the flowchart are shown in Figure 4 and Movie 1 (online).
Figure 3:
Flowchart of the criteria for (early) diffuse idiopathic skeletal hyperostosis (DISH). The flowchart was used to differentiate between no DISH, early DISH, and definite DISH by using the criteria. The corresponding scores for the presence and completeness of a bone bridge are presented in Figure 2. For the diagnostic criteria, simply the flowchart should be used to diagnose DISH. For the classification criteria, the overall height of the intervertebral disks and the presence of ankylosis of the facet joints should be evaluated to exclude potential cases of other diseases that cause formation of new bone in the spine.
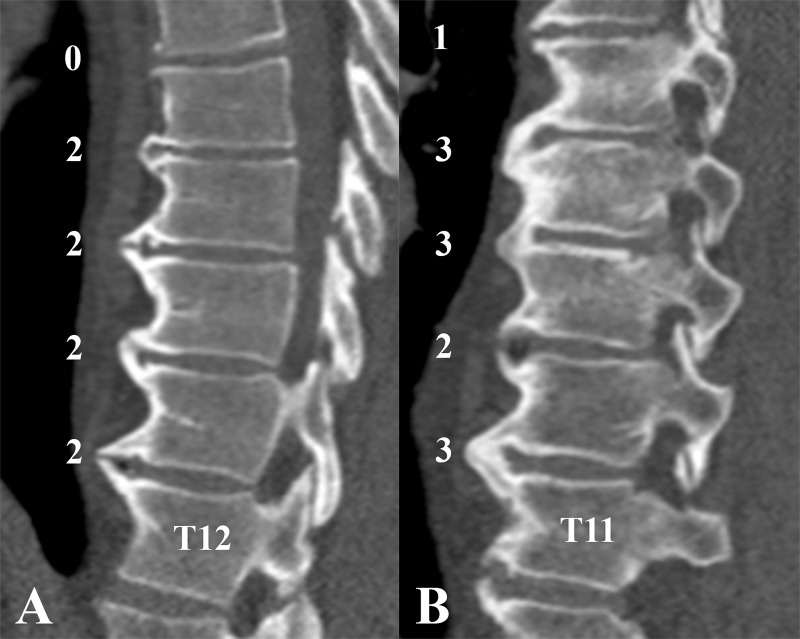
Figure 4:
Example images in patients with early diffuse idiopathic skeletal hyperostosis (DISH). A, Sagittal CT image in a 54-year-old man with early DISH with grades based on the flowchart shown in Figure 3. No complete bridge was present at any spinal segment; however, four segments were scored as showing a near-complete bone bridge (three or more segments with a score of 2). B, Sagittal CT image in a 67-year-old man with early DISH is shown. Three complete bone bridges (score of 3) were present, but not in an adjacent fashion, as is required to fulfill the criteria for DISH of Resnick and Niwayama (7). According to the flowchart in Figure 3, a complete bone bridge was present, and the highest bridge score of an adjacent segment was also a complete bone bridge (score of 3). Adjacent to these two complete bridge scores, the highest bridge score was a near-complete bone bridge (score of 2), and thus no adjacent three segments with complete bridges were present, excluding definite DISH and resulting in the diagnosis of early DISH. See also Movie 1 (online).
Movie 1.
A short summary is given regarding the use of the criteria for diffuse idiopathic skeletal hyperostosis.
For early DISH, the above criteria in cohort I had a sensitivity of 96% (52 of 54; 95% confidence interval [CI]: 87%, 100%) and a specificity of 94% (51 of 54; 95% CI: 85%, 99%).
Demographics of Cohort II
Cohort II consisted of 2267 participants (1160 men [51%]) with a mean age of 59.9 years ± 8.6 and a mean interval of 5.4 years ± 0.5 between the first and last CT studies (Table 1).
Validation of Criteria
According to the diagnostic criteria, the prevalence of definite DISH in cohort II in the last CT study was 10.2% (231 of 2267) and the prevalence of early DISH was 19.7% (447 of 2267) (Table E1 [online]). A random sample of 924 participants (four times 231 participants with definite DISH) was drawn from the group without definite DISH at the last CT study. The sampling fraction for the 1:4 ratio of the nested case-control design was 0.454 [924/(2267 − 231)]. The prevalence of definite DISH at the first CT study was 5.7% (130 of 2267) after correction for the full study population with the sampling fraction (Table E1 [online]). In cohort II, the diagnostic criteria had a sensitivity of 96% (99 of 103; 95% CI: 90%, 99%), a specificity of 83% (1695 of 2034; 95% CI: 82%, 85%), a PPV of 23% (99 of 438; 95% CI: 19%, 27%), an NPV of 100% (1695 of 1699; 95% CI: 99%, 100%), and a risk ratio of 96.0 (95% CI: 35.5, 259.3) (Table 2, Table E1 [online]).
Table 2:
Results of Diagnostic Criteria for DISH in Cohort II
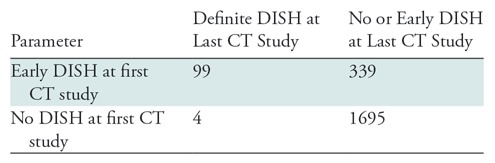
Note.—Data are numbers of participants. For cohort II, (early) DISH was diagnosed by using the diagnostic criteria on both the first and last CT studies (Figs 3, 4). The data from cohort II were corrected for the nested case-control design by using the sampling fraction. A more-detailed table of the primary outcomes is available as a supplement (Table E1 [online]). DISH = diffuse idiopathic skeletal hyperostosis.
The intrarater reliability was 0.89 (95% CI: 0.82, 0.96) for the diagnostic criteria. The interrater reliability of the criteria for DISH was “substantial” to “almost perfect” for each combination of observers (κ values ranged from 0.75 [95% CI: 0.65, 0.86] to 0.89 [95% CI: 0.81, 0.96]) (Table E2 [online]). The Fleiss κ was 0.78 (95% CI: 0.77, 0.78) between all observers. The results of the validation and the intra- and interrater reliability of the classification criteria can be found in Appendix E1 (online).
Discussion
In this study, we developed and validated criteria for early diffuse idiopathic skeletal hyperostosis (DISH) by using longitudinal CT imaging in two large-scale patient cohorts for both the clinical and research settings. The proposed criteria are based on longitudinal data and were selected by comparing different sets of criteria. Validation in a second longitudinal cohort showed acceptable-to-good results regarding sensitivity and specificity. The PPV for the classification of early DISH was 23%; this indicates that 23% of the participants with early DISH developed definite DISH within 5 years. In contrast, the NPV was 100%, and only four of the 1699 patients without (early) DISH would develop definite DISH after 5 years. The relative risk of 96.0 describes the ratio of the probability of developing definite DISH after 5 years in the group with early DISH to the probability of developing definite DISH after 5 years in the no-DISH group. The criteria for (early) DISH were highly reproducible between seven observers with different levels of expertise.
The most frequently used Resnick and Niwayama criteria are dichotomous and probably reflect end-stage disease (3,7,11). Research in the early phase of the condition is hampered by these strict criteria, and for this reason, the development of criteria with consecutive stages is considered essential (11). The main goal of our study was to create criteria that could discriminate between three groups: no DISH, early DISH, and definite DISH. These three groups, however, are still a (categorical) simplification of the natural progression of DISH, which is known for its slow and ongoing character (8–10).
The proposed criteria for DISH were based on the original criteria described by Resnick and Niwayama in 1976 (7). In addition to the fact that these are the most commonly used criteria in the literature, the presence of newly formed bone in the spine as an important item in the diagnosis of DISH was further supported by a Delphi exercise among eight expert participants in 2013 (3,18).
In our study, a distinction was made between diagnostic and classification criteria. The criteria coined by Resnick and Niwayama and those coined by multiple other authors excluded cases with loss of intervertebral disk height, ankylosis of the apophyseal (facet) joints, and erosion, sclerosis, or bony ankylosis of the sacroiliac joints (3,7). Nevertheless, multiple case reports and small case series have demonstrated the clinical co-occurrence of DISH and other conditions, such as ankylosing spondylitis, resulting in ankylosis of facet and sacroiliac joints (14). The simultaneous occurrence of multiple spinal ankylosing conditions could thus occasionally be expected in a clinical setting, but it would lead to confusion in a research environment. For this reason, the diagnostic criteria for the clinical setting do not exclude cases with potential other conditions, while, in contrast, the research criteria do exclude these cases (15).
Our study had some limitations. The proposed criteria for early DISH were developed based on longitudinal data and were validated within a second longitudinal cohort. The mean follow-up of both cohorts was 5 years, which might have not been long enough for the new incomplete bridges to develop fully into complete bridges (8–10). However, we were able to define criteria that clearly reflect the stage prior to definite DISH. Studies with longer follow-up may be able to explore even earlier phases of DISH. In our study, we used CT studies of the thoracic spine only. In current clinical practice, a radiograph is usually obtained in patients with DISH, and the criteria are not yet validated for use on radiographs. Furthermore, the presence of new bone in the cervical and lumbar spine was not included in our study. Considering the high frequency of DISH reported in the lower thoracic spine, our study was regarded as representative of most cases of DISH (11). Our criteria for early DISH were based only on radiographic imaging (ie, CT) of the spine. Finally, the criteria for early DISH were developed in male patients only; however, they were validated in a cohort with male and female participants by the same group of investigators. Sex bias is expected to be low, because the morphology of DISH is regarded to be the same in male and female patients. Stratified analysis of the validation cohort by sex showed similar diagnostic performance for men and women. Preferably, an independent research group should replicate our study, possibly including parameters such as sex, body mass index, and ethnic background.
In conclusion, our proposed criteria for early diffuse idiopathic skeletal hyperostosis (DISH) were developed for both the clinical and research settings and were validated with good results. These criteria could be used to investigate the early phase of DISH to gain a better understanding of its pathogenesis. Future diagnostic research should focus on peripheral manifestations and biochemical and genetic characteristics related to bone turnover to create and expand the diagnostic model for DISH.
APPENDIX
SUPPLEMENTAL FIGURES
Supported by the COPD Foundation (NCT00608764) and the National Heart, Lung, and Blood Institute (U01 HL089856, U01 HL089897). The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board consisting of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations:
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- COPDGene
- Genetic Epidemiology of COPD
- DISH
- diffuse idiopathic skeletal hyperostosis
- NPV
- negative predictive value
- PPV
- positive predictive value
Disclosures of Conflicts of Interest: J.S.K. disclosed no relevant relationships. S.F.O. disclosed no relevant relationships. W.F. disclosed no relevant relationships. F.A.M.H. disclosed no relevant relationships. W.P.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: APPROACH has received support from the Innovative Medicines Initiative Joint Undertaking under Grant Agreement no. 115770, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind contributions. See www.imi.europa.eu. Other relationships: disclosed no relevant relationships. J.W. disclosed no relevant relationships. E.A.R. disclosed no relevant relationships. D.A.L. disclosed no relevant relationships. F.C.O. disclosed no relevant relationships. P.A.d.J. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: the Department of Radiology of the University Medical Center Utrecht receives research support from Philips Healthcare. Other relationships: disclosed no relevant relationships. J.J.V. disclosed no relevant relationships.
References
- 1.Mader R, Verlaan JJ, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol 2013;9(12):741–750. [DOI] [PubMed] [Google Scholar]
- 2.Westerveld LA, van Bemmel JC, Dhert WJA, Oner FC, Verlaan JJ. Clinical outcome after traumatic spinal fractures in patients with ankylosing spinal disorders compared with control patients. Spine J 2014;14(5):729–740. [DOI] [PubMed] [Google Scholar]
- 3.Kuperus JS, de Gendt EEA, Oner FC, et al. Classification criteria for diffuse idiopathic skeletal hyperostosis: a lack of consensus. Rheumatology (Oxford) 2017;56(7):1123–1134. [DOI] [PubMed] [Google Scholar]
- 4.Westerveld LA, van Ufford HM, Verlaan JJ, Oner FC. The prevalence of diffuse idiopathic skeletal hyperostosis in an outpatient population in the Netherlands. J Rheumatol 2008;35(8):1635–1638. [PubMed] [Google Scholar]
- 5.Kim SK, Choi BR, Kim CG, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in Korea. J Rheumatol 2004;31(10):2032–2035. [PubMed] [Google Scholar]
- 6.Holton KF, Denard PJ, Yoo JU, et al. Diffuse idiopathic skeletal hyperostosis and its relation to back pain among older men: the MrOS Study. Semin Arthritis Rheum 2011;41(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976;119(3):559–568. [DOI] [PubMed] [Google Scholar]
- 8.Yaniv G, Bader S, Lidar M, et al. The natural course of bridging osteophyte formation in diffuse idiopathic skeletal hyperostosis: retrospective analysis of consecutive CT examinations over 10 years. Rheumatology (Oxford) 2014;53(11):1951–1957. [DOI] [PubMed] [Google Scholar]
- 9.Baraliakos X, Listing J, Buschmann J, von der Recke A, Braun J. A comparison of new bone formation in patients with ankylosing spondylitis and patients with diffuse idiopathic skeletal hyperostosis: a retrospective cohort study over six years. Arthritis Rheum 2012;64(4):1127–1133. [DOI] [PubMed] [Google Scholar]
- 10.Kuperus JS, Buckens CF, Šprem J, Oner FC, de Jong PA, Verlaan JJ. The natural course of diffuse idiopathic skeletal hyperostosis in the thoracic spine of adult males. J Rheumatol 2018;45(8):1116–1123. [DOI] [PubMed] [Google Scholar]
- 11.Mader R, Verlaan JJ, Eshed I, et al. Diffuse idiopathic skeletal hyperostosis (DISH): where we are now and where to go next. RMD Open 2017;3(1):e000472 [Published correction appears in RMD Open 2017;3(2):e000472corr1.] 10.1136/rmdopen-2017-000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biesheuvel CJ, Vergouwe Y, Oudega R, Hoes AW, Grobbee DE, Moons KG. Advantages of the nested case-control design in diagnostic research. BMC Med Res Methodol 2008;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuperus JS, Waalwijk JF, Regan EA, et al. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: a systematic review. Rheumatology (Oxford) 2018;57(12):2120–2128. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken) 2015;67(7):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson M, Nunes T, Heuer C, et al. epiR: Tools for the Analysis of Epidemiological Data. R package. R package version 0.9-93. 2017-12-11 10:58:39 UTC. http://fvas.unimelb.edu.au/veam. Published 2017. Accessed October 17, 2018.
- 17.Gamer M, Lemon J, Fellows I, Singh P. irr: Various Coefficients of Interrater Reliability and Agreement. R package version 0.84. https://cran.r-project.org/package=irr. Published 2012. Accessed October 17, 2018.
- 18.Mader R, Buskila D, Verlaan JJ, et al. Developing new classification criteria for diffuse idiopathic skeletal hyperostosis: back to square one. Rheumatology (Oxford) 2013;52(2):326–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.