Numerous advances have been made in the understanding of the mechanism of action of the antiparkinsonian medicine L‐DOPA. This has allowed for the development of interesting predictive models regarding the positive and side effects of this medication. Recently, a mathematical model has been developed to investigate the impact of L‐DOPA on dopamine (DA) release from serotonergic (5‐HT) terminals in the striatum 1. The parameters used to elaborate this model were partially based on experimental data and may not be sufficient to illustrate the biochemical effects of L‐DOPA inside 5‐HT terminals.
Parkinson's disease, the second most devastating neurodegenerative disease in terms of prevalence, has benefited from efficient treatments for 50 years. The disease is characterized by the progressive loss of mesencephalic DA neurons from the substantia nigra pars compacta innervating the striatum 2. L‐DOPA, the precursor of DA, was introduced in the mid‐60s to limit the decrease in DA associated with the degeneration of DA neurons. Upon chronic use of L‐DOPA, numerous side effects emerged such as L‐DOPA‐induced dyskinesia (LID). Among the various theories on LID 3, 4, the presynaptic hypothesis postulates that the increase in DA release induced by L‐DOPA is directly implicated in LID 5. After several years of skepticism, it is now accepted that 5‐HT neurons are mainly responsible for the release of DA induced by L‐DOPA 6. 5‐HT neurons are also responsible for the behavioral effects of L‐DOPA including locomotor activity, LID, and cognitive effects 7. Thus, the activity of 5‐HT neurons has become the most relevant parameter in predicting the DA output of L‐DOPA.
The model by Reed et al. (2012) focuses on the consequences of the import of L‐DOPA into 5‐HT neurons on DA and 5‐HT releases in the striatum. The main biochemical effects of L‐DOPA inside 5‐HT terminals allowed the authors to predict the shortening of the L‐DOPA therapeutic window and the synergistic benefit of 5‐HT1A and 5‐HT1B agonists against LIDs. So far, this mathematical model closely approximates the mechanism of action of L‐DOPA and raises interesting hypothesis that can be tested experimentally. Nevertheless, significant experimental findings dampen the full validity of the model. They concern the function of the striatal 5‐HT terminal itself in the presence of L‐DOPA and the ectopic influence of L‐DOPA‐derived DA release in the Parkinsonian brain.
First, the mechanisms triggered by L‐DOPA to enhance DA extracellular levels from striatal 5‐HT terminals may not be directly related to the firing rate of 5‐HT neurons. The main portion of L‐DOPA‐induced DA release comes from 5‐HT neurons 6, but the mechanism is not entirely impulse‐dependent 8, 9 (Figure 1). Indeed, L‐DOPA may trigger a nonexocytotic release of DA via the reversal of 5‐HT uptake sites 6. This may explain why the dampening effect of 5‐HT1A agonist alone or in combination with a 5‐HT1B agonist on L‐DOPA‐induced striatal DA release is only partial 8, 10. Moreover, the dampening effect of a 5‐HT1B agonist alone on L‐DOPA‐induced striatal DA release has not yet been reported 10. Consequently, the control of the electrical activity of 5‐HT neurons does not exclude the output of DA from 5‐HT terminals. It is also noteworthy that LID is not related to a higher striatal DA release compared with non‐dyskinetic animals 11 and that both the antiparkinsonian and dyskinetic actions of L‐DOPA can occur independently of changes in striatal DA release 12.
Figure 1.
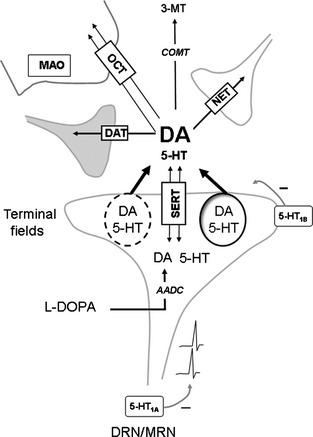
Regulation of DA extracellular levels in DA neuron‐depleted conditions after L‐DOPA administration. L‐DOPA enters 5‐HT neurons and is converted by AADC into DA, which competes with 5‐HT on numerous proteins (not represented here). 5‐HT terminals are responsible for the release of the newly synthesized DA, in part through an impulse‐dependent mechanism (depicted by action potentials at the level of DRN/MRN). This release of DA from 5‐HT terminals can be reduced by 5‐HT1A/1B receptor stimulation, although the role of the 5‐HT1B receptor in the striatum remains unclear. These mechanisms described above constitute the main parameters for the mathematical model of L‐DOPA effects developed by Reed et al. 1. However, L‐DOPA‐induced DA release involves other mechanisms. First, an impulse‐independent output of DA may occur through the reversal of the SERT. Second, the relative proportion of impulse‐dependent and ‐independent release of DA may differ with regard to the heterogeneity of 5‐HT terminals (symbolized here by the dashed or plain line of the vesicle of exocytosis). Third, an important source of regulation of DA extracellular levels comes from mechanisms of clearance including OCT, DAT (if any), NET, COMT, MAO and SERT itself. The relative contribution of these mechanisms depends on the brain region targeted by 5‐HT terminals and 5‐HT may also compete on these mechanisms. AADC, amino acid decarboxylase; COMT, catechol‐O‐methyl transferase; DAT, DA transporters; DRN/MRN, dorsal and median raphe nuclei; MAO, monoamine oxidase; 3‐MT, 3‐methoxytyramin; NET, noradrenalin transporter; OCT, organic cation transporter; SERT, 5‐HT transporter.
Secondly, LID can be generated from brain regions other than the striatum 13. Therefore, it is not clear whether the mechanisms of DA release induced by L‐DOPA from any other 5‐HT terminal in the brain correspond to what has been modeled in the striatum. Unfortunately, the mechanisms involved in the regulation of extracellular levels of DA and 5‐HT are different in each brain region. This may account for the heterogeneity of 5‐HT terminals that results from the ontogeny of 5‐HT neurons, their anatomical origin within the dorsal or median raphe nuclei associated with different autoregulatory mechanisms and/or various heterologous regulatory proteins at 5‐HT terminals 14. In addition to these differences, one has to include the numerous mechanisms involved in the clearance of these neurotransmitters away from the synaptic cleft. They include organic carbocation transporters, norepinephrine transporters, catechol‐O‐methyl transferase, monoamine oxidase, and DA transporters 15 (Figure 1). The combination of this 5‐HT terminal heterogeneity, together with heterologous clearance mechanisms, leads to region‐dependent effects of L‐DOPA on 5‐HT and DA releases that are exacerbated after chronic treatment 14. Besides, the ectopic release of DA leads to an ectopic and aberrant synthesis of DA receptors in regions outside the striatum, which may also play a fundamental role in the side effects induced by L‐DOPA 13.
In conclusion, additional experimental data are needed to determine to what extent 5‐HT electrical activity contributes to the effect of L‐DOPA on both DA and 5‐HT releases. Modeling the action of L‐DOPA inside 5‐HT terminals must be extended to brain regions other than the striatum to predict the consequences of brain DA and 5‐HT transmission imbalances created by L‐DOPA on its efficacy and side effects.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Reed MC, Nijhout HF, Best JA. Mathematical insights into the effects of levodopa. Front Integr Neurosci 2012;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esposito E, Di Matteo V, Di Giovanni G. Death in the substantia nigra: a motor tragedy. Expert Rev Neurother 2007;7:677–697. [DOI] [PubMed] [Google Scholar]
- 3. Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa‐induced dyskinesias in patients with Parkinson's disease: filling the bench‐to‐bedside gap. Lancet Neurol 2010;9:1106–1117. [DOI] [PubMed] [Google Scholar]
- 4. Cenci MA, Lundblad M. Post‐ versus presynaptic plasticity in L‐DOPA‐induced dyskinesia. J Neurochem 2006;99:381–392. [DOI] [PubMed] [Google Scholar]
- 5. de la Fuente‐Fernandez R, Sossi V, Huang Z, et al. Levodopa‐induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain 2004;127:2747–2754. [DOI] [PubMed] [Google Scholar]
- 6. Navailles S, Bioulac B, Gross C, De Deurwaerdere P. Serotonergic neurons mediate ectopic release of dopamine induced by L‐DOPA in a rat model of Parkinson's disease. Neurobiol Dis 2010;38:136–143. [DOI] [PubMed] [Google Scholar]
- 7. Navailles S, De Deurwaerdere P. Contribution of serotonergic transmission to the motor and cognitive effects of high‐frequency stimulation of the subthalamic nucleus or levodopa in Parkinson's disease. Mol Neurobiol 2012;45:173–185. [DOI] [PubMed] [Google Scholar]
- 8. Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L‐DOPA‐induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 2010;112:1465–1476. [DOI] [PubMed] [Google Scholar]
- 9. Navailles S, Carta M, Guthrie M, De Deurwaerdere P. L‐DOPA and serotonergic neurons: functional implication and therapeutic perspectives in Parkinson's disease. Cent Nerv Syst Agents Med Chem 2011;11:305–320. [DOI] [PubMed] [Google Scholar]
- 10. Kannari K, Yamato H, Shen H, Tomiyama M, Suda T, Matsunaga M. Activation of 5‐HT(1A) but not 5‐HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L‐DOPA in the striatum with nigrostriatal denervation. J Neurochem 2001;76:1346–1353. [DOI] [PubMed] [Google Scholar]
- 11. Nevalainen N, Af Bjerken S, Lundblad M, Gerhardt GA, Stromberg I. Dopamine release from serotonergic nerve fibers is reduced in L‐DOPA‐induced dyskinesia. J Neurochem 2011; 118:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Porras G, De Deurwaerdere P, Li Q, et al. L‐dopa‐induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep 2014;4:3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bastide MF, Dovero S, Charron G, et al. Immediate‐early gene expression in structures outside the basal ganglia is associated to l‐DOPA‐induced dyskinesia. Neurobiol Dis 2014;62:179–192. [DOI] [PubMed] [Google Scholar]
- 14. Navailles S, De Deurwaerdere P. Imbalanced dopaminergic transmission mediated by serotonergic neurons in L‐DOPA‐induced dyskinesia. Parkinsons Dis 2012;2012:323686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hensler JG, Artigas F, Bortolozzi A, et al. Catecholamine/Serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol 2013;68:167–197. [DOI] [PMC free article] [PubMed] [Google Scholar]


