The inflammatory immune response plays an important role in the pathophysiological process of cerebral ischemic injury, especially during the enlargement of damage and the secondary damage after cerebral ischemia. There are high cytokine expression and proinflammatory infiltration in damage area after ischemic cerebral injury, which aggravates brain injury especially in reperfusion period 1, 2. Interleukin‐1β (IL‐1β) is an inflammatory factor regulating the complex pathologic changes in acute ischemic brain injury. Increase in IL‐1β mRNA and protein level was observed after cerebral ischemia–reperfusion. IL‐1β plays an important role in inflammation of focal cerebral ischemia. IL‐1β contributes to brain tissue injury following cerebral ischemia–reperfusion 3, 4.
Anakinra, a recombinant, nonglycosylated form of human interleukin‐1 receptor antagonist (rhIL‐1ra), is identical to a native IL‐1 receptor antagonist except for a methionine residue that has been added to the beginning of the amino acid sequence 5. Intracerebroventricular injection of IL‐1ra (10 mg) significantly reduced the lesion volume after middle cerebral artery occlusion (MCAO) in rats 6. There are no articles about animal studies of anakinra being administered i.v. and therapeutic time window in cerebral ischemia. This study was designed to document the therapeutic effect and therapeutic time window of anakinra after transient focal MCA occlusion and its mechanism at molecular level.
Male Wistar rats (250–350 g) were divided into 18 groups, normal groups, sham groups, control groups, and anakinra groups (5, 10, or 20 mg/kg, i.v.) at 3, 6, or 12 h after ischemia, respectively. Twenty‐four hours after cerebral ischemia, rats were euthanized and decapitated. The brains were removed, and then, RNA was extracted. The mRNA primer of intercellular adhesion molecule 1 (ICAM‐1), inducible nitric oxide synthase (iNOS), IL‐1β, and tumor necrosis factor‐α (TNF‐α) were synthesized.
Anakinra decreased infarct percentage and improved neurological deficits induced by MCAO in male Wistar rats dose dependently and time dependently in Figure 1A–C. Significant difference was found between anakinra 5 mg/kg i.v. at 3 or 6 h after ischemia and the control group. Similar findings were observed in anakinra 10 or 20 mg/kg i.v. at 3, 6, or 12 h.
Figure 1.
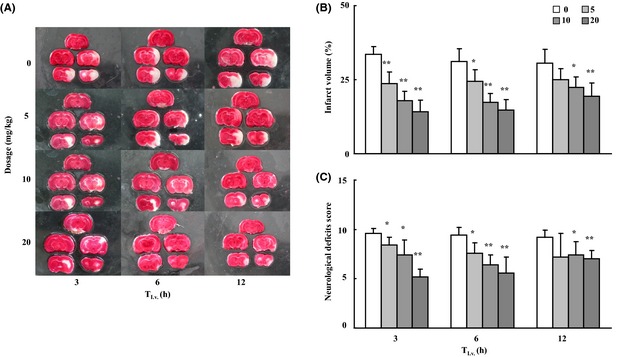
Effects of anakinra on neurological deficits in transient focal MCA occlusion rats. A nylon suture was advanced from the lumen of the ECA into the ICA to block the origin of the right MCA. After 2 h ischemia, the nylon suture was withdrawn to establish reperfusion. Animals were treated with shamoperation, or given saline control or 5/10/20 mg/kg. Anakinra was administrated i.v. via the tail vein at 3, 6 or 12 h after occlusion. Twenty‐four hours later, animals were killed and the brains were removed and stained by TTC, which stains viable tissue red but does not stain infarcted tissue. (A) Histological evidence for anakinra reduction infarct size. (B) The effects of anakinra on cerebral infarct volume. The values for the infarct volume are relative to whole brain of the rat. (C) Effects of anakinra on neurological deficits score. The values of percentage and score are expressed as mean ± SD. Dunnett t‐test, *P < 0.05, **P < 0.01 for treatment groups vs. control group. n = 5 in each group.
The effect of anakinra on ICAM‐1 mRNA, iNOS mRNA, IL‐1β mRNA, and TNF‐α mRNA was assessed by semiquantitative reverse transcription PCR 7. The integral gray value of normal group was set as 1, and the values of other groups were expressed as a ratio versus normal group. As shown in Figure 2A–D, ischemia–reperfusion upregulated ICAM‐1, iNOS, IL‐1β, and TNF‐α mRNA level. Anakinra 5~20 mg/kg i.v. at 3, 6, or 12 h after ischemia could significantly downregulate their mRNA levels (P < 0.01 or P < 0.05, vs. control group). Results have demonstrated that anakinra has an inhibitory effect on these overexpression cytokine.
Figure 2.
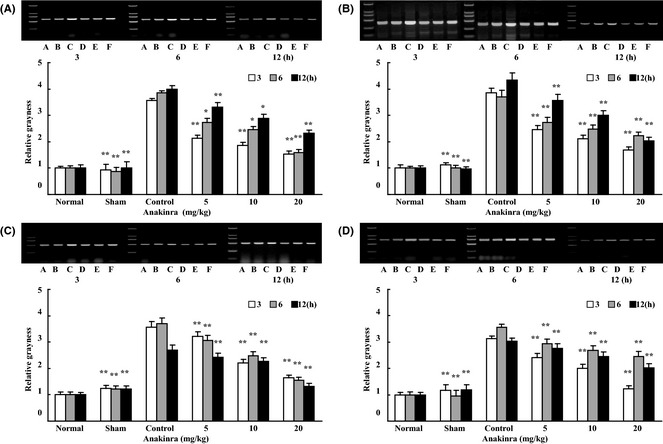
Effects of anakinra on mRNA levels in cerebral tissues after transient focal MCA occlusion in rats. Ischemia‐reperfusion (IR) markedly increased mRNA level in cerebral tissues after IR. Compared with control group, when administrated 3, 6, or 12 h after ischemia, anakinra of three doses (5, 10 or 20 mg/kg, i.v. via the tail vein) significantly decreased (A) ICAM‐1 mRNA, (B) iNOS mRNA, (C) IL‐1β mRNA, and (D) TNF‐α mRNA levels. A: normal; B: sham; C: control; D: anakinra 20 mg/kg; E: anakinra 10 mg/kg; F: anakinra 5 mg/kg. The values of relative grayness are expressed as mean ± SD. Dunnett t‐test, *P < 0.05, **P < 0.01 for sham/treatment groups vs control group. n = 5 in each group.
Anakinra is a kind of biomacromolecule, which is composed of 153 amino acids, and it cannot cross blood–brain barrier by i.v. administration in normal animals. It has been reported that natural IL‐1ra could improve brain injury resulting from focal cerebral ischemia through cerebral ventricle injection 8. In this study, anakinra can significantly decrease the damage of neural function and brain tissue by i.v. administration. This result has indicated that anakinra can cross blood–brain barrier and then generate therapeutic effects in transient MCAO rats. The center necrotic region came into being quickly when cerebral ischemia occurred, but the ischemic penumbra came slowly and lasted for a longer time. In this study, we have found that anakinra remained having therapeutic effects at 12 h after ischemia by i.v. administration. And the therapeutic time window was much longer than that of known drugs, which had been clinically applied to cure ischemic stroke. This might be related to the antiinflammatory response mediated by interleukin of anakinra. From these results, we can conclude that the drugs with the character of antiinflammatory response will have longer therapeutic time window against cerebral ischemic injury.
In this study, we have found that the mRNA expression of ICAM‐1, iNOS, IL‐1,β and TNF‐α was significantly upregulated in cerebral tissue after ischemia. These results indicated that these inflammatory factors were involved in the neuronal injury induced by cerebral ischemia and ischemia–reperfusion. The mRNA expression of ICAM‐1, iNOS, IL‐1,β and TNF‐α was significantly decreased after intravenous injection of anakinra, which demonstrated that anakinra could decrease the infiltration of leukocyte and could improve focal perfusion. Anakinra can inhibit the expression of ICAM‐1 and iNOS and decrease the generation of oxygen free radical and leukocytic infiltration in cerebral ischemia. And then, it would lessen the cerebral injury and the expression of IL‐1β and TNF‐α, which were induced by ischemic injury. Therefore, these results indicate that anakinra's therapeutic effects might result from the inhibitive effects of IL‐1R and the generation of inflammatory cytokine and/or the expression of iNOS and then inhibit the extension of inflammatory reactions or inflammatory cytotoxicity and toxicity which was induced by oxygen free radical.
Conflict of Interest
The author declare no conflict of interest.
The first two authors contributed equally to this work.
References
- 1. Ma XJ, Cheng JW, Zhang J, et al. E‐selectin deficiency attenuates brain ischemia in mice. CNS Neurosci Ther 2012;18:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothwell NL, Hopkis SJ. Cytokies and nervous system II: Actions and mechanisms of action. Trends Neurosci 2001;223:130–136. [DOI] [PubMed] [Google Scholar]
- 3. Cui YQ, Jia YJ, Zhang T, Zhang QB, Wang XM. Fucoidan protects against lipopolysaccharide‐induced rat neuronal damage and inhibits the production of proinflammatory mediators in primary microglia. CNS Neurosci Ther 2012;18:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Touzani O, Boutin H, LeFeuvre R, et al. Interleukin‐1 influences ischemic brain damage in the mouse independently of the interleukin‐1 type I receptor. J Neurosci 2002;22:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fleischmann RM, Schechtman J, Bennett R, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist, in patients with rheumatoid arthritis: A large, international, multicenter, placebo‐controlled trial. Arthritis Rheum 2003;48:927–934. [DOI] [PubMed] [Google Scholar]
- 6. Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin‐1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol 2003;140:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu MX, Tan BC, Zhou W, et al. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation‐related signaling pathways in microglial cells, prevents lipopolysaccharide‐induced inflammatory responses. CNS Neurosci Ther 2013;19:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin‐1 in acute brain injury. Trends Pharmacol Sci 2011;32:617–622. [DOI] [PubMed] [Google Scholar]


