Summary
Serotonin (5‐hydroxytryptamine or 5‐HT) is an important neurotransmitter regulating a wide range of physiological and pathological functions via activation of heterogeneously expressed 5‐HT receptors. Besides the important role of 5‐HT receptors in the pathogenesis of depressive disorders and in their clinical medications, underlying mechanisms are far from being completely understood. This review focuses on possible cross talk between two serotonin receptors, 5‐HT 1A and the 5‐HT 7. Although these receptors are highly co‐expressed in brain regions implicated in depression, and most agonists developed for the 5‐HT 1A or 5‐HT 7 receptors have cross‐reactivity, their functional interaction has not been yet established. It has been recently shown that 5‐HT 1A and 5‐HT 7 receptors form homo‐ and heterodimers both in vitro and in vivo. From the functional point of view, heterodimerization has been shown to play an important role in regulation of receptor‐mediated signaling and internalization, suggesting the implication of heterodimerization in the development and maintenance of depression. Interaction between these receptors is also of clinical interest, because both receptors represent an important pharmacological target for the treatment of depression and anxiety.
Keywords: 5‐HT1A/5‐HT7 receptor cross talk, Depression, G protein‐coupled receptor (GPCR), Receptor dimerization, Serotonin
Introduction
The brain serotonergic system is known to be involved in the control of a wide range of physiological functions as well as of different kinds of behavior. Such polyfunctionality of serotonin (5‐hydroxytryptamine or 5‐HT) is mediated by the existence of large number of serotonin receptors. Currently, 14 different 5‐HT receptor subtypes expressed in the mammals have been identified. To provide more detailed characterization of 5‐HT receptors, three main criteria are normally used: (1) structural features, including primary amino acid sequence, (2) signal transduction characteristics, including specific signal pathways, and (3) pharmacological profile 1. Based on these criteria, 5‐HT receptors were classified in seven subfamilies, which include both G protein‐coupled and ionotropic receptors 2, 3.
Despite the great clinical interest and large body of investigations dedicated to 5‐HT receptors, the mechanism regulating 5‐HT receptor functions are far from being completely understood. This review focuses on two serotonin receptors: the 5‐HT1A receptor representing a key player in the brain 5‐HT system 4, and the 5‐HT7 receptor. The 5‐HT1A receptor is known to be critically involved in major depression, anxiety, and suicide. More recently, functional studies have also implicated 5‐HT7 receptors in depression 5, 6, 7. As 5‐HT1A and 5‐HT7 receptors are co‐expressed in majority of the brain structures and modulate the same second messenger systems (even though in an opposite way), one intriguing question is the possible functional cross talk between these receptors. Since both receptors represent a pharmacological target for the treatment of depression, receptor–receptor interaction is also of great clinical significance.
5‐HT1A and 5‐HT7 Receptors: Genes, Signaling Pathways, and Distribution in the Brain
The 5‐HT1A receptor is one of the most extensively characterized members of the serotonin receptor family. Increased interest to the 5‐HT1A receptor is based on several reasons: (1) this receptor plays an important role in the regulation of neuronal development and plasticity 8, 9, (2) presynaptic 5‐HT1A autoreceptors are involved in the regulation of functions of 5‐HT neurons 2, (3) numerous data demonstrate implication of 5‐HT1A receptor in the control of various physiological functions 4, and (4) 5‐HT1A receptors are involved in the mechanisms of depression, anxiety, suicide, and schizophrenia 10, 11, 12, 13, 14.
The 5‐HT1A receptor gene was cloned in 1987 15. One year later, it has been demonstrated that the protein product of this genomic clone possesses all characteristics of the 5‐HT1A receptor 16. In following studies, the 5‐HT1A receptor gene was cloned from rat 17 and mouse 18. It was shown that 5‐HT1A receptor gene is localized at 5th chromosome in human 15 and at 13th chromosome in mice 19, and does not contain introns in its coding sequence 17. Mouse 5‐HT1A receptor gene demonstrates 94% homology with rat and 88% homology with human 5‐HT1A receptor gene (Ensemble Genome Browser, http://www.ensembl.org/index.html). The detailed structure of 5‐HT1A receptor gene promoter, including position of selective and nonselective enhancer as well as selective silencer, was also determined 20, 21. In addition, several rare single‐nucleotide polymorphisms (SNPs) were described for 5‐HT1A receptor gene 22. Between those, the SNP C(‐1019)G localized in the repressor region of promoter is of particular interest because of its association with depression, suicidal behavior, and schizophrenia 13 as well as sensitivity to the antidepressant drug treatment 23.
The 5‐HT1A receptor is known to activate a variety of effectors via Gi/o proteins, and receptor stimulation leads to inhibition of adenylyl cyclase (AC). The 5‐HT1A receptor is also involved in Gβγ‐mediated activation of a K+ current, inhibition of a Ca2+ current, and stimulation of the phospholipase C, as well as an activation of the mitogen‐activated protein kinase Erk2 2, 24. One important mechanism regulating 5‐HT1A receptor function is receptor desensitization and internalization 10. Interestingly, that 5‐HT1A receptor activation leads to effective internalization of 5‐HT1A autoreceptors in the nucleus raphe dorsalis, but not of heteroreceptors resided in hippocampal neurons 25, indicating the difference in the regulation of pre‐ and postsynaptic 5‐HT1A receptors activity.
5‐HT1A receptors are widely distributed within the brain. In rodents, high receptor density was detected in limbic areas; in hippocampus, septum, and cortical areas; and in the midbrain dorsal and median raphe nuclei 2, 26. Similar distribution was reported in human by postmortem autoradiography 27 as well as in the living human brain using positron emission tomography (PET) 28. Several experimental data suggested correlation between depressive disorders and changes in the distribution of 5‐HT1A receptor in the brain, although results of such studies are often contradictory. For example, an increase in 5‐HT1A autoreceptors was reported in postmortem brain from depressed suicide victims 29, 30. In contrast, PET analysis in depressed (submissive) monkeys revealed a reduction in 5‐HT1A receptor binding throughout the all brain areas 31. At the same time, PET analysis of 5‐HT1A receptor binding in the brain of depressed patients before and after electroconvulsive therapy demonstrated a strong reduction in 5‐HT1A receptor binding in regions consistently reported to be altered in major depression 32.
The 5‐HT7 receptor is one of the most recently described members of the 5‐HT receptor family 33, 34, 35. This receptor is coupled to Gs protein and activates AC. In addition, the 5‐HT7 receptor is coupled to the G12 protein to activate small GTPases of the Rho family (i.e., Cdc42 and RhoA), leading to enhanced neurite outgrowth, synaptogenesis, and neuronal excitability 36, 37, 38. It has been also demonstrated in cell lines that the 5‐HT7 receptor can stimulate intracellular calcium release 39.
The gene encoding the 5‐HT7 receptor is localized at 19th chromosome in mouse and at 7th chromosome in human. Comparison of amino acid sequence of the transmembrane regions of 5‐HT7 receptor from different species showed homology between 39% and 50%. Interestingly, the homology of transmembrane domains of 5‐HT7 receptor with those for 5‐HT1A receptor gene is relatively high (about 40%), which can partly explain the fact that most agonists developed for the 5‐HT1A and/or 5‐HT7 receptors have cross‐reactivity between these receptors 40 (see below). The human 5‐HT7 receptor gene contains three introns within the coding region 41, 42. Detailed analysis of human 5‐HT7 receptor gene revealed existence of eight SNPs. Noteworthy that two of these polymorphisms (rs3808932 and rs12412496) were shown to be associated with schizophrenia 43. A number of 5‐HT7 receptor splice variants including 5‐HT7(a), 5‐HT7(b) 5‐HT7(c) 5‐HT7(e) and 5‐HT7(a), 5‐HT7(b) and 5‐HT7(d), differing in the amino acid sequence of their C‐terminus, but possessing similar pharmacological profile were cloned from rat and human, respectively 41, 42, 44, 45. Noteworthy that the human 5‐HT7(d) receptor isoform possesses a differential pattern of receptor internalization, which can affect receptor‐mediated signaling 46. The 5‐HT7 receptors are abundantly expressed in brain limbic structures. Significant density of 5‐HT7 receptor was also observed in hippocampus and in raphe nuclei area. Moderate or relatively low receptor density was obtained in cortex, caudate putamen, and cerebellum 47.
The 5‐HT7 receptor is associated with a number of physiological and pathological responses, including serotonin‐induced phase shifting of the circadian rhythm 34 and age‐dependent changes of the circadian timing 48, 49. There are some data implicating 5‐HT7 receptor in the control of memory 50, emotionality, locomotor, and exploratory activity 51, 52. A large body of evidence indicates involvement of 5‐HT7 receptor in the anxiety and depression, and recent studies suggest that the 5‐HT7 receptor can be highly relevant for the treatment of major depressive disorders 53.
Pharmacological Properties of 5‐HT1A and 5‐HT7 Receptors
Over the years, several agonists and antagonists for 5‐HT1A and 5‐HT7 receptors have been developed and applied to investigate the pharmacology of these receptors. Most frequently used molecules along with their binding affinities at 5‐HT1A and 5‐HT7 receptors are summarized in Table 1. The 5‐HT1A receptor was first identified in 1983 pharmacologically as a distinct binding site for 8‐OH‐DPAT 54, which was considered as the selective 5‐HT1A receptor reference agonist 55. The affinity of 8‐OH‐DPAT for 5‐HT1A receptors in rat hippocampal membranes resides in the low nanomolar range (Ki = 1.78 nM) 56. 8‐OH‐DPAT demonstrated the properties of a full 5‐HT1A receptor agonist in the forskolin‐stimulated cAMP assay for both native and cloned 5‐HT1A receptors 56. However, when the 5‐HT7 receptor was cloned in 1993, it became apparent that 8‐OH‐DPAT possesses affinity also for this receptor (Ki = 52 nM) 57 and behaves as a partial agonist (Emax = 62% of 5‐CT response; EC50 = 2345 nM).
Table 1.
Binding affinity values of agonists and antagonists acting on 5‐HT 1A or 5‐HT 7 receptors
| Compound | 5‐HT1A receptor | 5‐HT7 receptor | ||||
|---|---|---|---|---|---|---|
| Binding affinity | System | Reference | Binding affinity | System | Reference | |
| 8‐OH‐DPAT | Ki = 1.78 nM | Rat hippocampal membranes | 52 | Ki = 52 nM | Rat cloned receptor | 53 |
| WAY‐100635 | IC50 = 1.35 nM | Rat hippocampal membranes | 54 | Ki > 10000 nM | Human cloned receptor | 59 |
| WAY‐100135 | IC50 = 34 nM | Rat hippocampal membranes | 55 | Not available | ||
| NAN‐190 | pKi = 9.2 | Rat hippocampal membranes | 126 | Ki > 1000 nM | Rat cloned receptor | 60 |
| NAD‐299 | Ki = 0.59 nM | Rat hippocampal membranes | 58 | Ki > 1000 nM | Rat cloned receptor | 58 |
| 5‐CT | Ki = 0.53 nM | Human cloned receptor | 127 | IC50 = 0.83 nM | Rat cloned receptor | 31 |
| AS‐19 | Ki = 89.7 nM | Human cloned receptor | 66 | Ki = 0.6 nM | Human cloned receptor | 66 |
| E‐55888 | Ki = 700 nM | Human cloned receptor | 66 | Ki = 2.5 nM | Human cloned receptor | 66 |
| LP‐211 | Ki = 15 nM | Human cloned receptor | 67 | Ki = 379 nM | Human cloned receptor | 67 |
| SB‐269970 | pKi < 5 | Human cloned receptor | 128 | pKi = 8.9 | Human cloned receptor | 128 |
| SB‐258719 | pKi < 5.1 | Human cloned receptor | 129 | pKi = 7.5 | Human cloned receptor | 129 |
| SB‐656104 | pKi = 6.25 | Human cloned receptor | 130 | pKi = 8.70 | Human cloned receptor | 130 |
Several antagonists of 5‐HT1A receptor became available in early 1990. One of them, WAY‐100635, is the prototypical silent 5‐HT1A receptor antagonist, which has been widely used as a pharmacological probe to investigate the distribution and function of 5‐HT1A receptors 58. WAY‐100635 displaces specific [3H]8‐OH‐DPAT binding to 5‐HT1A receptors in the rat hippocampus with IC50 = 1.35 nM, and it is more then 100‐fold selective for the 5‐HT1A site in comparison with a range of other 5‐HT receptors. However, WAY‐100635 also display affinities for 5‐HT2B (Ki = 24 nM), dopamine D2 (Ki = 16.4 nM), and adrenergic α 1A (Ki = 19.9 nM) receptors. In addition, WAY‐100135 was initially considered as a silent 5‐HT1A antagonist 59, but it showed partial agonist properties in a subsequent study 60. Noteworthy that WAY‐100635 together with other 5‐HT1A receptor antagonists NAN‐190 61 and NAD‐299 62 did not reveal any affinity for 5‐HT7 receptors 62, 63, 64.
In case of the 5‐HT7 receptors, 5‐CT has been identified as a high‐affinity receptor agonist (IC50 = 0.83 nM, EC50 = 13 nM, 100% maximal efficacy) that is widely used to study the receptor pharmacology and functions 34. However, due to its high affinity for 5‐HT1A, 5‐HT1B, and 5‐HT1D receptors 65, analysis of 5‐HT7 receptor functions by 5‐CT requires parallel application of WAY‐100635 (5‐HT1A receptor antagonist) and GR‐127935 (5‐HT1B/1D receptor antagonists) 66. Interestingly, 8‐OH‐DPAT has also been used to study the functional effects of 5‐HT7 receptor in combination with application of the 5‐HT1A antagonists WAY‐100635, NAN‐190, and NAD‐299 67, 68, 69.
During the last decade, various selective 5‐HT7 receptor agonists became available in addition to 5‐CT. AS‐19 (Ki of 0.6 nM for the human 5‐HT7 receptor) was reported to display high selectivity for 5‐HT7 receptor (more then 100‐fold) in comparison with the other 5‐HT receptor subtypes with exception of 5‐HT1D receptors (only 11‐fold higher selectivity). AS‐19 was found to behave as a potent (EC50 = 9 nM), but partial 5‐HT7 receptor agonist with a maximal effect reaching 77% of the cAMP response evoked by the 5‐HT 70. Another selective and potent 5‐HT7 receptor agonist E‐55888 70 shows high affinity for 5‐HT7 receptors (Ki = 2.5 nM) and very low affinity for 5‐HT1A (Ki = 700 nM) with no significant affinity for other 5‐HT receptors. E‐55888 behaves as a full agonist with efficacy and potency (Emax = 99%; EC50 = 16 nM) to induce receptor‐mediated cAMP formation in HEK‐293F cells in a range similar to that obtained for 5‐HT 70. Selective 5‐HT7 receptor agonist LP‐211 displays high affinity for rat and human 5‐HT7 receptors (Ki = 0.58 and 15.0 nM, respectively) and moderate to low affinity for other 5‐HT receptors, including the 5‐HT1A receptor 71. LP‐211 possesses agonistic properties in vivo inducing hypothermia in 5‐HT7 +/+, but not in 5‐HT7 −/− mice.
Recently, several specific 5‐HT7 receptor antagonists were developed. The prototype of 5‐HT7 receptor antagonist SB‐269970 demonstrates a high binding affinity for human (pKi = 8.9) and guinea pig (pKi = 8.3) native 5‐HT7 receptors. This compound possesses very high selectivity for 5‐HT7 receptors (more than 100‐fold) in comparison with other G protein‐coupled receptors (GPCRs), except the human 5‐HT5A receptor for which selectivity was 50‐fold. SB‐269970 acts as a competitive antagonist of the human 5‐HT7 receptor in AC assay (pA2 = 8.5) as well as in the guinea pig native tissue assay (pKB = 8.3). Interestingly, SB‐269970 produces an inhibition of basal AC activity in the absence of any agonist, suggesting an inverse agonism 72. Other selective 5‐HT7 receptor antagonists are SB‐258719 73, that is less potent than SB‐269970 (pKi = 7.5; pA2 = 7.2), and SB‐656104 74. Although the latter has similar pharmacological properties with SB‐269970 (pKi = 8.7; pA2 = 8.4), it demonstrates only 10‐fold selectivity over the 5‐HT1D receptor.
Functional Cross Talk Between 5‐HT1A and 5‐HT7 Receptors
There is a set of studies suggesting functional cross talk between 5‐HT7 and 5‐HT1A receptors at the level of thermoregulation. Involvement of 5‐HT1A receptor in the regulation of the body temperature has been demonstrated more than 25 years ago by the observation that 5‐HT1A receptor agonist 8‐OH‐DPAT produced considerable hypothermic response 75, 76. This effect has been used as a test for 5‐HT1A receptor functional activity 75, 76, 77, 78. We have also shown that changes in the expression of 5‐HT1A receptor gene are linked to natural hibernation and associated hypothermia 79.
Based on the absence of 5‐HT‐induced hypothermic response in 5‐HT7 −/− mice, Hedlund and co‐authors suggested that 5‐HT‐induced hypothermia is mainly mediated via 5‐HT7 receptor 80. However, in this study, 5‐HT was applied peripherally. Because of the poor ability of 5‐HT to cross the blood–brain barrier 81, 82, the importance of the central 5‐HT7 receptor in thermoregulation suggested in this study should be therefore considered more carefully.
It was shown that at lower doses (0.3–0.6 mg/kg, i.p.), 8‐OH‐DPAT decreased body temperature in 5‐HT7 +/+ mice but not in 5‐HT7 −/− mice. At a higher dose (1 mg/kg, i.p.), 8‐OH‐DPAT induced hypothermia in both 5‐HT7 −/− and 5‐HT7 +/+ mice. At the same time, it was found that pretreatment of mice with a 5‐HT1A receptor selective antagonist WAY‐100135 completely blocked the hypothermic response of 8‐OH‐DPAT in 5‐HT7 +/+ mice 83. Moreover, we have shown that intracerebroventricular, but not intraperitoneal administration of selective 5‐HT7 receptor agonist LP44 produced considerable hypothermic response in mice, indicating involvement of central rather then peripheral 5‐HT7 receptors in thermoregulation. The comparison of 5‐HT7 and 5‐HT1A receptor‐induced hypothermia in eight mouse strains did not reveal any correlation, suggesting the low functional interaction between 5‐HT7 and 5‐HT1A receptors in the control of the body temperature. Together with the observation that the 5‐HT7 receptor selective antagonist did not affect 8‐OH‐DPAT‐induced hypothermia, these data suggest that central 5‐HT7 and 5‐HT1A receptors can be independently involved in thermoregulation 84.
Antidepressants effect of selective serotonin reuptake inhibitors (SSRIs) partly depends on 5‐HT1A autoreceptor functions. Chronic treatment with SSRIs is associated with a progressive recovery of 5‐HT neurons firing rate, correlating with clinical amelioration of depression 10. It is noteworthy that 5‐HT7 receptors can also be involved in the therapeutic effects of antidepressants. For example, both tricyclic antidepressants as well as SSRIs induced c‐Fos expression in rats in a manner consistent with the 5‐HT7 receptor activation within the suprachiasmatic nucleus. Moreover, chronic antidepressants treatment led to a decreased 5‐HT7 receptor binding 85. These data suggest possible interplay between 5‐HT7 and 5‐HT1A receptors during antidepressant drug action. One possible mechanism of such cross talk can be selective and regulated heterodimerization (see below).
Oligomerization of 5‐HT1A and 5‐HT7 Receptors
Results of multiple biochemical, structural, and functional studies collected during the last decade clearly indicate that GPCRs can exist as oligomeric complexes 86, 87. Moreover, a growing body of evidence points to the functional importance of GPCR oligomers for receptor trafficking, receptor activation, and G protein coupling in native tissues 88. The clinical significance of GPCR oligomerization has also become more evident during recent years, leading to identification of oligomeric complexes as novel therapeutic targets 89, 90.
Oligomerization can occur between identical receptor protomers (homomerization) or between different receptors belonging to the same or different GPCR families (heteromerization). Heteromerization may result in changed receptor pharmacology either by affecting the ligand binding on individual monomers or by the formation of new binding sites 91, 92. More importantly, heteromerization may also influence signaling pathways regulated by a given receptor monomer 93, 94, 95, 96, 97, which can lead to a switch in G protein coupling 98. Thus, heteromerization provides an additional mechanism regulating cellular processes through the fine tuning of receptor‐mediated signaling.
Using a novel Förster resonance energy transfer (FRET) technique based on the spectral analysis, we have recently demonstrated that 5‐HT1A receptors can form homodimers at the plasma membrane 99, 100. We also showed that FRET efficiency measured for the 5‐HT1A receptor oligomers significantly decreased in response to agonist stimulation. Combined results of our studies suggest that this decrease was mediated by accumulation of FRET‐negative complexes rather than by dissociation of oligomers to monomers. Formation of 5‐HT1A homomers (including the higher‐order oligomers) was further confirmed by several recent publications 101, 102. By combination of computational protein–protein docking, site‐directed mutagenesis, and FRET‐based analysis, we have recently demonstrated that transmembrane domains TM4/TM5 can be involved in the formation of 5‐HT1A receptor dimers and indicated that specific amino acids interactions (e.g., W1754.64, Y1985.41, R1514.40, and R1524.41) maintain the interaction interface 103.
The 5‐HT7 receptor has been shown to form homooligomers as well. This was demonstrated in recombinant system by pharmacological studies using the antagonist risperidone, which binds in a pseudo‐irreversible manner to one protomer of a dimer as well as by the Bioluminiscence resonance energy transfer approach 104. Recently, homooligomerization of 5‐HT7 receptor was confirmed using two different FRET assays 101. Existence of 5‐HT7 receptor homodimers has been also shown under in vivo like condition in primary cultures of rat cortical astrocytes 105.
Heterooligomerization between 5‐HT1A and 5‐HT7 receptors has been demonstrated by combined application of biochemical (co‐immunoprecipitation) and biophysical (FRET) approaches 106. In the same study, it has been also demonstrated that hippocampal neurons express both 5‐HT1A and 5‐HT7 receptors and that these receptors are highly co‐localized at the plasma membrane. Moreover, co‐immunoprecipitation studies in mouse brain provided direct evidence that these receptors can form heteromers in neurons in vivo. Functional analysis of oligomerization between 5‐HT1A and 5‐HT7 receptors revealed that heterooligomers decreased the 5‐HT1A receptor‐mediated activation of Gi protein without affecting 5‐HT7 receptor‐mediated Gs protein activation. In addition to reduced Gi protein coupling of 5‐HT1A receptor, heterodimerization affected 5‐HT1A receptor‐mediated activation of G protein‐gated potassium (GIRK) channels, an effect mediated through the Gβγ‐subunits of Gi proteins 107, 108. Noteworthy that inhibitory effect of heterooligomerization on GIRK currents was preserved in hippocampal neurons, suggesting a physiological relevance of heteromerization in vivo 106.
We have also demonstrated that heterodimerization can initiate agonist‐mediated internalization of 5‐HT1A receptor, which is highly resistant to internalization when expressed alone. Once internalized, 5‐HT1A receptor can activate G protein‐independent signaling pathways such as a β‐arrestin‐mediated coupling to mitogen‐activated protein kinase (MAPK) 106. Thus, dependent on the relative amount of 5‐HT1A receptors participating in heterooligomers, the same ligand (serotonin) can activate distinct ERK‐mediated pathways (i.e., G protein dependent or β‐arrestin dependent). This also raises the possibility that conditions selectively promoting or inhibiting heterodimerization could have a significant physiological relevance.
Possible Role of Heterodimerization between 5‐HT1A and 5‐HT7 Receptors in Anxiety and Depression
Modulation of receptor signaling by heterodimerization, in particular enhancement of 5‐HT1A receptor internalization, is also likely to play an important role in pathophysiological processes in CNS. The 5‐HT1A receptor is expressed as postsynaptic receptor in multiple brain regions including hippocampus and cortex 109, 110. In addition, the 5‐HT1A receptor is highly expressed as a presynaptic autoreceptor in 5‐HT neurons of raphe nuclei, where it controls serotonin release through feedback inhibition 111, 112. The role of 5‐HT1A autoreceptor in the regulation of presynaptic serotonin release has led to the hypothesis that its selective and progressive desensitization is a key element responsible for the therapeutic action of the SSRIs. Although widely accepted, this hypothesis still cannot explain the fact that the chronic in vivo SSRI treatment results in preferential functional desensitization of only 5‐HT1A autoreceptors without affecting the postsynaptic 5‐HT1A receptors 113, 114. We propose that higher amount of heterodimers produced in presynaptic versus postsynaptic neurons might represent a mechanism responsible for the differential desensitization obtained for the 5‐HT1A auto‐ and heteroreceptors (Figure 1). Such asymmetric distribution of heterodimers will result in effective co‐internalization of 5‐HT1A receptor within 5‐HT1A‐5‐HT7 heterodimers upon 5‐HT release, which can take place either under physiological conditions or as a consequence of SSRI treatment. It has been demonstrated that the expression level of postsynaptic 5‐HT7 receptors in hippocampus and forebrain progressively decreased during the postnatal development, while the expression of 5‐HT1A receptor remained relatively constant 36, 106, 115. This suggests that under physiological conditions, 5‐HT1A receptor homodimers represent a dominant postsynaptic population in adulthood ([5‐HT1A‐5‐HT1A] ≫ [5‐HT1A‐5‐HT7]). As 5‐HT1A receptor homodimers are resistant to the agonist‐mediated internalization, the amount of the postsynaptic 5‐HT1A receptor will not be affected by 5‐HT. Different relative concentration of 5‐HT1A‐5‐HT7 heterodimers between serotonergic presynaptic autoreceptors and target (postsynaptic receptors) neurons can explain not only the differences in desensitization between pre‐ and postsynaptic 5‐HT1A receptors, but also suggests that the regulated and balanced ratio of homo‐ and heterodimerization on pre‐ and postsynaptic neurons may be critically involved in both the onset as well as the response to treatment of psychiatric diseases such as depression and anxiety (Figure 1). Moreover, differences in relative concentration of 5‐HT1A‐5‐HT7 heterodimers in raphe and hippocampus represent an intrigue possibility to explain regional differences in the coupling of 5‐HT1A receptor to G proteins. It has been shown that in raphe, Gα i3 is the predominat coupling partner of 5‐HT1A receptor, while in hippocampus, receptor couples to both Gα o and Gα i3 proteins 116. Therefore, future investigations will be needed to more precisely evaluate to which extent the 5‐HT1A and 5‐HT7 oligomerization can contribute to selective activation of different G proteins.
Figure 1.
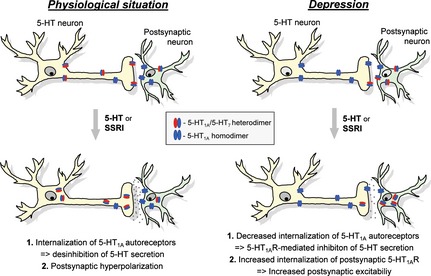
Hypothetical model explaining the functional role of 5‐HT 1A‐5‐HT 7 heterodimerization. Under physiological conditions (left), the amount of 5‐HT 1A‐5‐HT 7 heterodimers in presynaptic 5‐HT neurons is higher than in postsynaptic neurons, representing a mechanism responsible for the differential 5‐HT or SSRI‐mediated internalization obtained for the 5‐HT 1A auto‐ versus heteroreceptors. By depression (right), the relationship between 5‐HT 1A‐5‐HT 1A homodimers and 5‐HT 1A‐5‐HT 7 heterodimers in presynaptic 5‐HT neurons becomes shifted toward 5‐HT 1A‐5‐HT 1A homodimers. This will result in decreased 5‐HT or SSRI‐mediated internalization of 5‐HT 1A autoreceptors, which in turn will lead to 5‐HT 1A receptor‐mediated inhibition of 5‐HT release. On the postsynaptic neurons, higher amount of heterodimers ([5‐HT 1A‐5‐HT 7] > [5‐HT 1A‐5‐HT 1A]) is expected during depression. Consequently, internalization of postsynaptic 5‐HT 1A receptors will be increased, leading to the increased neuronal excitability.
Abnormalities in 5‐HT1A receptors have been previously noted in depressed patients and implicated in the pathophysiology of depression. Analysis of the postmortem brains of depressed subjects in comparison with control samples revealed a specific up‐regulation of 5‐HT1A autoreceptors in the raphe area, with no changes in postsynaptic 5‐HT1A receptor sites 30. In addition, it has been recently shown that transgenic mice with low level of 5‐HT1A autoreceptor possess increased resilience to chronic stress and faster response to SSRI treatment as compared to mice with the high level of presynaptic 5‐HT1A receptor 117. On the other hand, postmortem studies of major depression demonstrated a decrease in postsynaptic 5‐HT1A receptor expression in hippocampus and prefrontal cortex, consistent with decreased postsynaptic 5‐HT1A receptor‐mediated signaling observed in depressed suicide tissue 118, 119. Decreased 5‐HT1A receptor density in the prefrontal cortex was also found in multiple PET studies of human patients with major depression 120, 121, 122. All these results demonstrate that in depression, the number of presynaptic 5‐HT1A receptors increased and this is accompanied by the concomitant decrease in postsynaptic 5‐HT1A receptor level.
Although the detailed analysis of 5‐HT7 receptor distribution in patients with depression is not available yet, recent studies in rats have shown that expression of 5‐HT7 receptor is up‐regulated in the hippocampus after exposure to stress, and these changes were inhibited by treatment with fluoxetine 123. Similar results were obtained in mice, where chronic fluoxetine treatment led to a down‐regulation of 5‐HT7 receptor binding in hypothalamus 124. Moreover, analysis of 5‐HT7 knockout mice revealed that those mice exhibit a behavioral phenotype similar to that of antidepressant‐treated mice 6, 125. These data suggest that in depression, the relationship between 5‐HT1A homodimers and 5‐HT1A‐5‐HT7 heterodimers in presynaptic neurons may be shifted from [5‐HT1A‐5‐HT7] > [5‐HT1A‐5‐HT1A] to [5‐HT1A‐5‐HT1A] > [5‐HT1A‐5‐HT7], while in postsynaptic neurons, the opposite effect is expected (i.e., [5‐HT1A‐5‐HT7] > [5‐HT1A‐5‐HT1A]) (Figure 1). Functionally, an increased concentration of 5‐HT1A‐5‐HT7 heterodimers in hippocampus (and consequently inhibition of 5‐HT1A mediated signaling via its internalization) may cause depression or accentuate the effect of stress. In combination with decreased presynaptic activity due to a relative increase in concentration of 5‐HT1A homodimers in presynaptic neurons, serotonergic transmission particularly through 5‐HT1A receptor appears to be compromised in depression.
Conclusion
Serotonin receptors 5‐HT1A and 5‐HT7 are co‐expressed in multiple brain regions, where they are critically involved in regulation of various physiological and pathological brain functions. Recent studies demonstrated that these receptors can form heterodimers both in vitro as well as in vivo. From the functional point of view, heterodimerization decreases Gi protein coupling of 5‐HT1A receptor without substantial changes in the coupling of the 5‐HT7 receptor to the Gs protein. In addition, heterodimerization facilitates internalization of 5‐HT1A receptors as well as its ability to activate MAP kinase. Thus, differences in relative concentration of 5‐HT1A‐5‐HT7 heterodimers on presynaptic serotonergic neurons and target (post‐synaptic) neurons can explain the differences in desensitization patterns between pre‐ and postsynaptic 5‐HT1A receptors. More importantly, regulated and balanced ratio of homo‐ and heterodimerization on pre‐ and postsynaptic neurons may be critically involved in both the onset as well as the response to the treatment of psychiatric diseases such as depression and anxiety. As heterodimerization can exist between other 5‐HT receptor subtypes and between 5‐HT receptors and nonserotonergic GPCRs, it would be therefore interesting to analyze in future studies relevance of such receptor complexes for the pathophysiology and treatment of depression.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The work was supported by Deutsche Forschungsgemeinschaft through the Grant PO732 and Cluster of Excellence REBIRTH, program of the RAS “Molecular and Cell Biology” (grant No. 6.7), the Russian Foundation for Basic Research (grant No. 12‐04‐00082). The authors thank EU COST Action CM1103 “Structure‐based drug design for diagnosis and treatment of neurological diseases: dissecting and modulating complex function in the monoaminergic systems of the brain.”
References
- 1. Humphrey PP, Hartig P, Hoyer D. A proposed new nomenclature for 5‐HT receptors. Trends Pharmacol Sci 1993;14:233–236. [DOI] [PubMed] [Google Scholar]
- 2. Barnes NM, Sharp T. A review of central 5‐HT receptors and their function. Neuropharmacology 1999;38:1083–1152. [DOI] [PubMed] [Google Scholar]
- 3. Saudou F, Hen R. 5‐Hydroxytryptamine receptor subtypes: Molecular and functional diversity. Adv Pharmacol 1994;30:327–380. [DOI] [PubMed] [Google Scholar]
- 4. Popova NK, Naumenko VS. 5‐HT1A receptor as a key player in the brain 5‐HT system. Rev Neurosci 2013;24:191–204. [DOI] [PubMed] [Google Scholar]
- 5. Bonaventure P, Dugovic C, Kramer M, et al. Translational evaluation of JNJ‐18038683, a 5‐hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther 2012;342:429–440. [DOI] [PubMed] [Google Scholar]
- 6. Hedlund PB, Huitron‐Resendiz S, Henriksen SJ, Sutcliffe JG. 5‐HT7 receptor inhibition and inactivation induce antidepressant like behavior and sleep pattern. Biol Psychiatry 2005;58:831–837. [DOI] [PubMed] [Google Scholar]
- 7. Thomas DR, Hagan JJ. 5‐HT7 receptors. Curr Drug Targets CNS Neurol Disord 2004;3:81–90. [DOI] [PubMed] [Google Scholar]
- 8. Azmitia PM, Witaker‐Azmitia EC. Anatomy, cell biology and plasticity of the serotonergic system. Neuropsychopharmacological implications for the action of psychotropic drugs In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York: Raven Press, 1995;443–449. [Google Scholar]
- 9. Rasmuson S, Olsson T, Henriksson BG, et al. Environmental enrichment selectively increases 5‐HT1A receptor mRNA expression and binding in the rat hippocampus. Brain Res Mol Brain Res 1998;53:285–290. [DOI] [PubMed] [Google Scholar]
- 10. Albert PR, Lemonde S. 5‐HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist 2004;10:575–593. [DOI] [PubMed] [Google Scholar]
- 11. Blier P, Ward NM. Is there a role for 5‐HT1A agonists in the treatment of depression? Biol Psychiatry 2003;53:193–203. [DOI] [PubMed] [Google Scholar]
- 12. Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C‐1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J 2006;6:27–33. [DOI] [PubMed] [Google Scholar]
- 13. Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5‐hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 2003;23:8788–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meltzer HY, Sumiyoshi T. Does stimulation of 5‐HT(1A) receptors improve cognition in schizophrenia? Behav Brain Res 2008;195:98–102. [DOI] [PubMed] [Google Scholar]
- 15. Kobilka BK, Frielle T, Collins S, et al. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature 1987;329:75–79. [DOI] [PubMed] [Google Scholar]
- 16. Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G‐21 which resembles a beta‐adrenergic receptor sequence encodes the 5‐HT1A receptor. Nature 1988;335:358–360. [DOI] [PubMed] [Google Scholar]
- 17. Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5‐hydroxytryptamine1A receptor gene. J Biol Chem 1990;265:5825–5832. [PubMed] [Google Scholar]
- 18. Charest A, Wainer BH, Albert PR. Cloning and differentiation‐induced expression of a murine serotonin1A receptor in a septal cell line. J Neurosci 1993;13:5164–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oakey RJ, Caron MG, Lefkowitz RJ, Seldin MF. Genomic organization of adrenergic and serotonin receptors in the mouse: Linkage mapping of sequence‐related genes provides a method for examining mammalian chromosome evolution. Genomics 1991;10:338–344. [DOI] [PubMed] [Google Scholar]
- 20. Ou XM, Jafar‐Nejad H, Storring JM, Meng JH, Lemonde S, Albert PR. Novel dual repressor elements for neuronal cell‐specific transcription of the rat 5‐HT1A receptor gene. J Biol Chem 2000;275:8161–8168. [DOI] [PubMed] [Google Scholar]
- 21. Storring JM, Charest A, Cheng P, Albert PR. TATA‐driven transcriptional initiation and regulation of the rat serotonin 5‐HT1A receptor gene. J Neurochem 1999;72:2238–2247. [DOI] [PubMed] [Google Scholar]
- 22. Wu S, Comings DE. A common C‐1018G polymorphism in the human 5‐HT1A receptor gene. Psychiatr Genet 1999;9:105–106. [DOI] [PubMed] [Google Scholar]
- 23. Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(‐1019)G 5‐HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 2004;7:501–506. [DOI] [PubMed] [Google Scholar]
- 24. Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. The serotonin1A receptor: A representative member of the serotonin receptor family. Cell Mol Neurobiol 2005;25:553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist‐induced internalization of serotonin‐1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors). J Neurosci 2001;21:8378–8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knapp DJ, Overstreet DH, Crews FT. Brain 5‐HT1A receptor autoradiography and hypothermic responses in rats bred for differences in 8‐OH‐DPAT sensitivity. Brain Res 1998;782:1–10. [DOI] [PubMed] [Google Scholar]
- 27. Hall H, Lundkvist C, Halldin C, et al. Autoradiographic localization of 5‐HT1A receptors in the post‐mortem human brain using [3H]WAY‐100635 and [11C]way‐100635. Brain Res 1997;745:96–108. [DOI] [PubMed] [Google Scholar]
- 28. Takano H, Ito H, Takahashi H, et al. Serotonergic neurotransmission in the living human brain: A positron emission tomography study using [(1)(1)C]dasb and [(1)(1)C]WAY100635 in young healthy men. Synapse 2011;65:624–633. [DOI] [PubMed] [Google Scholar]
- 29. Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001;25:892–903. [DOI] [PubMed] [Google Scholar]
- 30. Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin‐1A autoreceptors in the midbrain of suicide victims with major depression‐postmortem evidence for decreased serotonin activity. J Neurosci 1998;18:7394–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shively CA, Friedman DP, Gage HD, et al. Behavioral depression and positron emission tomography‐determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry 2006;63:396–403. [DOI] [PubMed] [Google Scholar]
- 32. Lanzenberger R, Baldinger P, Hahn A, et al. Global decrease of serotonin‐1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry 2013;18:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5‐HT7) positively linked to adenylate cyclase. J Biol Chem 1993;268:23422–23426. [PubMed] [Google Scholar]
- 34. Lovenberg TW, Baron BM, de Lecea L, et al. A novel adenylyl cyclase‐activating serotonin receptor (5‐HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993;11:449–458. [DOI] [PubMed] [Google Scholar]
- 35. Plassat JL, Amlaiky N, Hen R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol Pharmacol 1993;44:229–236. [PubMed] [Google Scholar]
- 36. Kobe F, Guseva D, Jensen TP, et al. 5‐HT7R/G12 signaling regulates neuronal morphology and function in an age‐dependent manner. J Neurosci 2012;32:2915–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kvachnina E, Liu G, Dityatev A, et al. 5‐HT7 receptor is coupled to G alpha subunits of heterotrimeric G12‐protein to regulate gene transcription and neuronal morphology. J Neurosci 2005;25:7821–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ponimaskin EG, Profirovic J, Vaiskunaite R, Richter DW, Voyno‐Yasenetskaya TA. 5‐Hydroxytryptamine 4(a) receptor is coupled to the Galpha subunit of heterotrimeric G13 protein. J Biol Chem 2002;277:20812–20819. [DOI] [PubMed] [Google Scholar]
- 39. Baker LP, Nielsen MD, Impey S, et al. Stimulation of type 1 and type 8 Ca2+/calmodulin‐sensitive adenylyl cyclases by the Gs‐coupled 5‐hydroxytryptamine subtype 5‐HT7A receptor. J Biol Chem 1998;273:17469–17476. [DOI] [PubMed] [Google Scholar]
- 40. Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5‐HT7 receptor agents: Structure‐activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther 2011;129:120–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erdmann J, Nothen MM, Shimron‐Abarbanell D, et al. The human serotonin 7 (5‐HT7) receptor gene: Genomic organization and systematic mutation screening in schizophrenia and bipolar affective disorder. Mol Psychiatry 1996;1:392–397. [PubMed] [Google Scholar]
- 42. Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. Four 5‐hydroxytryptamine7 (5‐HT7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron‐exon organization. J Neurochem 1997;68:1372–1381. [DOI] [PubMed] [Google Scholar]
- 43. Ikeda M, Iwata N, Kitajima T, et al. Positive association of the serotonin 5‐HT7 receptor gene with schizophrenia in a Japanese population. Neuropsychopharmacology 2006;31:866–871. [DOI] [PubMed] [Google Scholar]
- 44. Heidmann DE, Szot P, Kohen R, Hamblin MW. Function and distribution of three rat 5‐hydroxytryptamine7 (5‐HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 1998;37:1621–1632. [DOI] [PubMed] [Google Scholar]
- 45. Liu H, Irving HR, Coupar IM. Expression patterns of 5‐HT7 receptor isoforms in the rat digestive tract. Life Sci 2001;69:2467–2475. [DOI] [PubMed] [Google Scholar]
- 46. Guthrie CR, Murray AT, Franklin AA, Hamblin MW. Differential agonist‐mediated internalization of the human 5‐hydroxytryptamine 7 receptor isoforms. J Pharmacol Exp Ther 2005;313:1003–1010. [DOI] [PubMed] [Google Scholar]
- 47. Horisawa T, Ishiyama T, Ono M, Ishibashi T, Taiji M. Binding of lurasidone, a novel antipsychotic, to rat 5‐HT7 receptor: Analysis by [3H]SB‐269970 autoradiography. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:132–137. [DOI] [PubMed] [Google Scholar]
- 48. Duncan MJ, Congleton MR. Neural mechanisms mediating circadian phase resetting by activation of 5‐HT(7) receptors in the dorsal raphe: Roles of GABAergic and glutamatergic neurotransmission. Brain Res 2010;1366:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duncan MJ, Short J, Wheeler DL. Comparison of the effects of aging on 5‐HT7 and 5‐HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res 1999;829:39–45. [DOI] [PubMed] [Google Scholar]
- 50. Cifariello A, Pompili A, Gasbarri A. 5‐HT(7) receptors in the modulation of cognitive processes. Behav Brain Res 2008;195:171–179. [DOI] [PubMed] [Google Scholar]
- 51. Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor‐like activity involving spinal 5‐HT7 and 5‐HT2A receptors. J Neurophysiol 2005;94:1392–1404. [DOI] [PubMed] [Google Scholar]
- 52. Takeda H, Tsuji M, Ikoshi H, et al. Effects of a 5‐HT7 receptor antagonist DR4004 on the exploratory behavior in a novel environment and on brain monoamine dynamics in mice. Eur J Pharmacol 2005;518:30–39. [DOI] [PubMed] [Google Scholar]
- 53. Gellynck E, Heyninck K, Andressen KW, et al. The serotonin 5‐HT7 receptors: Two decades of research. Exp Brain Res 2013;230:555–568. [DOI] [PubMed] [Google Scholar]
- 54. Middlemiss DN, Fozard JR. 8‐Hydroxy‐2‐(di‐n‐propylamino)‐tetralin discriminates between subtypes of the 5‐HT1 recognition site. Eur J Pharmacol 1983;90:151–153. [DOI] [PubMed] [Google Scholar]
- 55. Dourish CT, Ahlenius S, Hutson PH, editors. Brain 5‐HT1A receptors. Chichester, UK: Ellis Horwood Ltd, 1987;26–33. [Google Scholar]
- 56. Hall MD, el Mestikawy S, Emerit MB, Pichat L, Hamon M, Gozlan H. [3H]8‐hydroxy‐2‐(di‐n‐propylamino)tetralin binding to pre‐ and postsynaptic 5‐hydroxytryptamine sites in various regions of the rat brain. J Neurochem 1985;44:1685–1696. [DOI] [PubMed] [Google Scholar]
- 57. Ruat M, Traiffort E, Leurs R, et al. Molecular cloning, characterization, and localization of a high‐affinity serotonin receptor (5‐HT7) activating cAMP formation. Proc Natl Acad Sci USA 1993;90:8547–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fletcher A, Forster EA, Bill DJ, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY‐100635, a potent, selective and silent 5‐HT1A receptor antagonist. Behav Brain Res 1996;73:337–353. [DOI] [PubMed] [Google Scholar]
- 59. Fletcher A, Bill DJ, Bill SJ, et al. WAY100135: A novel, selective antagonist at presynaptic and postsynaptic 5‐HT1A receptors. Eur J Pharmacol 1993;237:283–291. [DOI] [PubMed] [Google Scholar]
- 60. Assie MB, Koek W. Effects of 5‐HT1A receptor antagonists on hippocampal 5‐hydroxytryptamine levels: (S)‐WAY100135, but not WAY100635, has partial agonist properties. Eur J Pharmacol 1996;304:15–21. [DOI] [PubMed] [Google Scholar]
- 61. Rydelek‐Fitzgerald L, Teitler M, Fletcher PW, Ismaiel AM, Glennon RA. NAN‐190: Agonist and antagonist interactions with brain 5‐HT1A receptors. Brain Res 1990;532:191–196. [DOI] [PubMed] [Google Scholar]
- 62. Johansson L, Sohn D, Thorberg SO, et al. The pharmacological characterization of a novel selective 5‐hydroxytryptamine1A receptor antagonist, NAD‐299. J Pharmacol Exp Ther 1997;283:216–225. [PubMed] [Google Scholar]
- 63. Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY‐100635 is a potent dopamine D4 receptor agonist. Psychopharmacology 2006;188:244–251. [DOI] [PubMed] [Google Scholar]
- 64. Shen Y, Monsma FJ Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5‐hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 1993;268:18200–18204. [PubMed] [Google Scholar]
- 65. Boess FG, Martin IL. Molecular biology of 5‐HT receptors. Neuropharmacology 1994;33:275–317. [DOI] [PubMed] [Google Scholar]
- 66. Freret T, Paizanis E, Beaudet G, et al. Modulation of 5‐HT receptor: Effect on object recognition performances in mice. Psychopharmacology 2013;231:393–400. [DOI] [PubMed] [Google Scholar]
- 67. Costa L, Spatuzza M, D'Antoni S, et al. Activation of 5‐HT7 serotonin receptors reverses metabotropic glutamate receptor‐mediated synaptic plasticity in wild‐type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry 2012;72:924–933. [DOI] [PubMed] [Google Scholar]
- 68. Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5‐HT(1A) and 5‐HT(7) receptors differently modulate AMPA receptor‐mediated hippocampal synaptic transmission. Hippocampus 2012;22:790–801. [DOI] [PubMed] [Google Scholar]
- 69. Eriksson TM, Holst S, Stan TL, et al. 5‐HT1A and 5‐HT7 receptor crosstalk in the regulation of emotional memory: Implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology 2012;63:1150–1160. [DOI] [PubMed] [Google Scholar]
- 70. Brenchat A, Romero L, Garcia M, et al. 5‐HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 2009;141:239–247. [DOI] [PubMed] [Google Scholar]
- 71. Hedlund PB, Leopoldo M, Caccia S, et al. LP‐211 is a brain penetrant selective agonist for the serotonin 5‐HT(7) receptor. Neurosci Lett 2010;481:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hagan JJ, Price GW, Jeffrey P, et al. Characterization of SB‐269970‐A, a selective 5‐HT(7) receptor antagonist. Br J Pharmacol 2000;130:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thomas DR, Gittins SA, Collin LL, et al. Functional characterisation of the human cloned 5‐HT7 receptor (long form); antagonist profile of SB‐258719. Br J Pharmacol 1998;124:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas DR, Melotto S, Massagrande M, et al. SB‐656104‐A, a novel selective 5‐HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol 2003;139:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goodwin GM, De Souza RJ, Green AR, Heal DJ. The pharmacology of the behavioural and hypothermic responses of rats to 8‐hydroxy‐2‐(di‐n‐propylamino)tetralin (8‐OH‐DPAT). Psychopharmacology 1987;91:506–511. [DOI] [PubMed] [Google Scholar]
- 76. Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8‐OH‐DPAT. J Neural Transm 1985;61:131–135. [DOI] [PubMed] [Google Scholar]
- 77. Overstreet DH, Rezvani AH, Knapp DJ, Crews FT, Janowsky DS. Further selection of rat lines differing in 5‐HT‐1A receptor sensitivity: Behavioral and functional correlates. Psychiatr Genet 1996;6:107–117. [DOI] [PubMed] [Google Scholar]
- 78. Popova NK, Naumenko VS, Plyusnina IZ, Kulikov AV. Reduction in 5‐HT1A receptor density, 5‐HT1A mRNA expression, and functional correlates for 5‐HT1A receptors in genetically defined aggressive rats. J Neurosci Res 2005;80:286–292. [DOI] [PubMed] [Google Scholar]
- 79. Naumenko VS, Tkachev SE, Kulikov AV, et al. The brain 5‐HT1A receptor gene expression in hibernation. Genes Brain Behav 2008;7:300–305. [DOI] [PubMed] [Google Scholar]
- 80. Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5‐HT7 receptor knockout mice. Proc Natl Acad Sci USA 2003;100:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carley DW, Radulovacki M. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest 1999;115:1397–1401. [DOI] [PubMed] [Google Scholar]
- 82. Yoshioka M, Goda Y, Togashi H, Matsumoto M, Saito H. Pharmacological characterization of 5‐hydroxytryptamine‐induced apnea in the rat. J Pharmacol Exp Ther 1992;260:917–924. [PubMed] [Google Scholar]
- 83. Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8‐OH‐DPAT acts on both 5‐HT1A and 5‐HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol 2004;487:125–132. [DOI] [PubMed] [Google Scholar]
- 84. Naumenko VS, Kondaurova EM, Popova NK. On the role of brain 5‐HT7 receptor in the mechanism of hypothermia: Comparison with hypothermia mediated via 5‐HT1A and 5‐HT3 receptor. Neuropharmacology 2011;61:1360–1365. [DOI] [PubMed] [Google Scholar]
- 85. Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5‐HT7 receptor research. Trends Pharmacol Sci 2004;25:481–486. [DOI] [PubMed] [Google Scholar]
- 86. Bulenger S, Marullo S, Bouvier M. Emerging role of homo‐ and heterodimerization in G‐protein‐coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 2005;26:131–137. [DOI] [PubMed] [Google Scholar]
- 87. Devi LA. Heterodimerization of G‐protein‐coupled receptors: Pharmacology, signaling and trafficking. Trends Pharmacol Sci 2001;22:532–537. [DOI] [PubMed] [Google Scholar]
- 88. Rivero‐Muller A, Chou YY, Ji I, et al. Rescue of defective G protein‐coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci USA 2010;107:2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gonzalez‐Maeso J, Ang RL, Yuen T, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2008;452:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Waldhoer M, Fong J, Jones RM, et al. A heterodimer‐selective agonist shows in vivo relevance of G protein‐coupled receptor dimers. Proc Natl Acad Sci USA 2005;102:9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Franco R, Casado V, Cortes A, et al. G‐protein‐coupled receptor heteromers: Function and ligand pharmacology. Br J Pharmacol 2008;153(Suppl 1):S90–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci 2010;31:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fuxe K, Ferre S, Canals M, et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci 2005;26:209–220. [DOI] [PubMed] [Google Scholar]
- 94. Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci 2000;20:RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hornigold DC, Mistry R, Raymond PD, Blank JL, Challiss RA. Evidence for cross‐talk between M2 and M3 muscarinic acetylcholine receptors in the regulation of second messenger and extracellular signal‐regulated kinase signalling pathways in Chinese hamster ovary cells. Br J Pharmacol 2003;138:1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Israilova M, Tanaka T, Suzuki F, Morishima S, Muramatsu I. Pharmacological characterization and cross talk of alpha1a‐ and alpha1b‐adrenoceptors coexpressed in human embryonic kidney 293 cells. J Pharmacol Exp Ther 2004;309:259–266. [DOI] [PubMed] [Google Scholar]
- 97. Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A‐adrenergic receptors. Mol Pharmacol 2003;64:1317–1324. [DOI] [PubMed] [Google Scholar]
- 98. Lee SP, So CH, Rashid AJ, et al. Dopamine D1 and D2 receptor Co‐activation generates a novel phospholipase C‐mediated calcium signal. J Biol Chem 2004;279:35671–35678. [DOI] [PubMed] [Google Scholar]
- 99. Kobe F, Renner U, Woehler A, et al. Stimulation‐ and palmitoylation‐dependent changes in oligomeric conformation of serotonin 5‐HT1A receptors. Biochim Biophys Acta 2008;1783:1503–1516. [DOI] [PubMed] [Google Scholar]
- 100. Woehler A, Wlodarczyk J, Ponimaskin EG. Specific oligomerization of the 5‐HT1A receptor in the plasma membrane. Glycoconj J 2009;26:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ganguly S, Clayton AH, Chattopadhyay A. Organization of higher‐order oligomers of the serotonin(1)(A) receptor explored utilizing homo‐FRET in live cells. Biophys J 2011;100:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Paila YD, Kombrabail M, Krishnamoorthy G, Chattopadhyay A. Oligomerization of the serotonin(1A) receptor in live cells: A time‐resolved fluorescence anisotropy approach. J Phys Chem B 2011;115:11439–11447. [DOI] [PubMed] [Google Scholar]
- 103. Gorinski N, Kowalsman N, Renner U, et al. Computational and experimental analysis of the transmembrane domain 4/5 dimerization interface of the serotonin 5‐HT(1A) receptor. Mol Pharmacol 2012;82:448–463. [DOI] [PubMed] [Google Scholar]
- 104. Teitler M, Toohey N, Knight JA, Klein MT, Smith C. Clozapine and other competitive antagonists reactivate risperidone‐inactivated h5‐HT7 receptors: Radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology 2010;212:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Smith C, Toohey N, Knight JA, Klein MT, Teitler M. Risperidone‐induced inactivation and clozapine‐induced reactivation of rat cortical astrocyte 5‐hydroxytryptamine(7) receptors: Evidence for in situ G protein‐coupled receptor homodimer protomer cross‐talk. Mol Pharmacol 2011;79:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Renner U, Zeug A, Woehler A, et al. Heterodimerization of serotonin receptors 5‐HT1A and 5‐HT7 differentially regulates receptor signalling and trafficking. J Cell Sci 2012;125:2486–2499. [DOI] [PubMed] [Google Scholar]
- 107. Kofuji P, Davidson N, Lester HA. Evidence that neuronal G‐protein‐gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci USA 1995;92:6542–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Reuveny E, Slesinger PA, Inglese J, et al. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature 1994;370:143–146. [DOI] [PubMed] [Google Scholar]
- 109. Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5‐HT1A serotonin receptor is located on calbindin‐ and parvalbumin‐containing neurons in the rat brain. Brain Res 2003;959:58–67. [DOI] [PubMed] [Google Scholar]
- 110. Beck SG, Choi KC, List TJ. Comparison of 5‐hydroxytryptamine1A‐mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther 1992;263:350–359. [PubMed] [Google Scholar]
- 111. Hamon M, Gozlan H, el Mestikawy S, Emerit MB, Bolanos F, Schechter L. The central 5‐HT1A receptors: Pharmacological, biochemical, functional, and regulatory properties. Ann N Y Acad Sci 1990;600:114–129; discussion 29–31. [DOI] [PubMed] [Google Scholar]
- 112. Riad M, Garcia S, Watkins KC, et al. Somatodendritic localization of 5‐HT1A and preterminal axonal localization of 5‐HT1B serotonin receptors in adult rat brain. J Comp Neurol 2000;417:181–194. [PubMed] [Google Scholar]
- 113. Jolas T, Haj‐Dahmane S, Kidd EJ, et al. Central pre‐ and postsynaptic 5‐HT1A receptors in rats treated chronically with a novel antidepressant, cericlamine. J Pharmacol Exp Ther 1994;268:1432–1443. [PubMed] [Google Scholar]
- 114. Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato‐dendritic 5‐HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 1995;352:141–148. [DOI] [PubMed] [Google Scholar]
- 115. Beique JC, Campbell B, Perring P, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: Coordinated expression of 5‐hydroxytryptamine (5‐HT)1A, 5‐HT2A, and 5‐HT7 receptors. J Neurosci 2004;24:4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5‐hydroxytryptamine‐1A receptors to G proteins in the rat brain. Mol Pharmacol 2006;70:1013–1021. [DOI] [PubMed] [Google Scholar]
- 117. Richardson‐Jones JW, Craige CP, Guiard BP, et al. 5‐HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010;65:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. Attenuated 5‐HT1A receptor signaling in brains of suicide victims: Involvement of adenylyl cyclase, phosphatidylinositol 3‐kinase, Akt and mitogen‐activated protein kinase. J Neurochem 2003;87:182–194. [DOI] [PubMed] [Google Scholar]
- 119. Lopez‐Figueroa AL, Norton CS, Lopez‐Figueroa MO, et al. Serotonin 5‐HT1A, 5‐HT1B, and 5‐HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry 2004;55:225–233. [DOI] [PubMed] [Google Scholar]
- 120. Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type‐1A receptor imaging in depression. Nucl Med Biol 2000;27:499–507. [DOI] [PubMed] [Google Scholar]
- 121. Neumeister A, Young T, Stastny J. Implications of genetic research on the role of the serotonin in depression: Emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology 2004;174:512–524. [DOI] [PubMed] [Google Scholar]
- 122. Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY‐100635: Effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000;57:174–180. [DOI] [PubMed] [Google Scholar]
- 123. Li YC, Wang FM, Pan Y, et al. Antidepressant‐like effects of curcumin on serotonergic receptor‐coupled AC‐cAMP pathway in chronic unpredictable mild stress of rats. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:435–449. [DOI] [PubMed] [Google Scholar]
- 124. Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5‐HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology 1999;21:352–367. [DOI] [PubMed] [Google Scholar]
- 125. Guscott M, Bristow LJ, Hadingham K, et al. Genetic knockout and pharmacological blockade studies of the 5‐HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 2005;48:492–502. [DOI] [PubMed] [Google Scholar]
- 126. Millan MJ, Canton H, Gobert A, et al. Novel benzodioxopiperazines acting as antagonists at postsynaptic 5‐HT1A receptors and as agonists at 5‐HT1A autoreceptors: A comparative pharmacological characterization with proposed 5‐HT1A antagonists. J Pharmacol Exp Ther 1994;268:337–352. [PubMed] [Google Scholar]
- 127. Sundaram H, Newman‐Tancredi A, Strange PG. Characterization of recombinant human serotonin 5HT1A receptors expressed in Chinese hamster ovary cells. [3H]spiperone discriminates between the G‐protein‐coupled and ‐uncoupled forms. Biochem Pharmacol 1993;45:1003–1009. [DOI] [PubMed] [Google Scholar]
- 128. Lovell PJ, Bromidge SM, Dabbs S, et al. A novel, potent, and selective 5‐HT(7) antagonist: (R)‐3‐(2‐(2‐(4‐methylpiperidin‐1‐yl)ethyl)pyrrolidine‐1‐sulfonyl) phen ol (SB‐269970). J Med Chem 2000;43:342–345. [DOI] [PubMed] [Google Scholar]
- 129. Forbes IT, Dabbs S, Duckworth DM, et al. (R)‐3, N‐dimethyl‐N‐[1‐methyl‐3‐(4‐methyl‐piperidin‐1‐yl) propyl]benzenesulfonamide: The first selective 5‐HT7 receptor antagonist. J Med Chem 1998;41:655–657. [DOI] [PubMed] [Google Scholar]
- 130. Forbes IT, Douglas S, Gribble AD, et al. SB‐656104‐A: A novel 5‐HT(7) receptor antagonist with improved in vivo properties. Bioorg Med Chem Lett 2002;12:3341–3344. [DOI] [PubMed] [Google Scholar]


