Intravenous (IV) thrombolysis is currently the most established treatment for acute ischemic stroke 1. Traditionally, patients with unruptured intracranial aneurysms have been deemed ineligible due to a theoretical increased risk of intracranial hemorrhage (ICH) from post‐thrombolytic rupture of aneurysm. Recently, four retrospective studies indicated no significant difference in symptomatic ICH after intravenous thrombolysis between stroke patients with or without intracranial aneurysms 2, 3, 4, 5. However, in most of these patients, the ischemic stroke mechanism was unrelated to the unruptured intracranial aneurysm. The safety of IV thrombolysis in acute ischemic stroke in the same territory of unruptured intracranial aneurysms remains unknown. We therefore present a case of pontine infarction due to a large unruptured intracranial aneurysm (17 mm in diameter) of the basilar artery where the patient received IV recombinant tissue plasminogen activator (rtPA) thrombolysis based on multimodal MR imaging.
A 59‐year‐old man was admitted to the emergency room with a 4‐h history of abrupt‐onset right hemiparesis and dysarthria. He reported that the symptom severity increased during transport to the hospital. The patient had a past medical history of treated hypertension for 20 years and known basilar artery aneurysm for 2 years that had been managed conservatively. His blood pressure was 160/102 mmHg on admission. Neurological examination revealed normal conscious state, dysarthria, right hemiparesis, and sensory loss. His score on the National Institutes of Health Stroke Scale (NIHSS) was 6.
A brain magnetic resonance imaging (MRI) scan (3.0T) was performed immediately. Diffusion‐weighted imaging (DWI) revealed increased signal intensity in the left ventral pons (Figure 1A), indicating the presence of hyperacute infarction. Magnetic resonance angiography (MRA) showed a large unruptured intracranial aneurysm of the basilar artery (17 mm in maximal diameter) without basilar artery occlusion (Figure 1B). IV rtPA was administered immediately at a dose of 0.9 mg/kg body weight. His focal neurological deficit dramatically improved by the next morning. Twenty‐four hours later, he was also administered an antiplatelet agent (aspirin 200 mg). He made a complete recovery and was discharged 6 days later (NIHSS 0).
Figure 1.
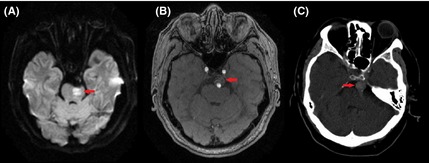
Diffusion MRI on admission revealed increased signal intensity in the left lateral medulla (arrow) (A). Magnetic resonance angiography (MRA) on admission showed a large intracranial aneurysm of the basilar artery (arrow) without basilar artery occlusion (B). Noncontrast CT (2 h after thrombolysis) indicated unruptured intracranial aneurysm of the basilar artery (arrow) and no evidence of ICH or subarachnoid hemorrhage (C).
Our patient did not have any evidence of aneurysm rupture or ICH on his initial susceptibility‐weighted imaging (SWI) scan, or on computed tomographic (CT) scan 2 h after thrombolysis (Figure 1C), and SWI at 24 h. There was no evidence of atherosclerotic disease on cervical CT angiography or carotid duplex ultrasonography.
Noncontrast and contrast‐enhanced T1 fast spin echo (FSE) sequences demonstrated abnormal blood flow with partial thrombosis of the aneurysm (Figure 2A and B). We hypothesize that the abnormal flow within the aneurysm (Figure 2C) leads to gradual thrombosis.
Figure 2.
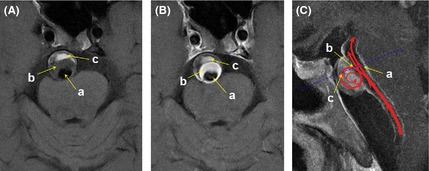
Noncontrast T1 fast spin echo (FSE) image was divided into three regions: Region “a”, characterized as hypointensity both on noncontrast and contrast T1‐FSE due to flow void phenomenon, reflected normal blood flow velocity and direction in basilar artery without occlusion; Region “b”, characterized as relatively low signal intensity on noncontrast T1‐FSE and hyperintensity on contrast T1‐FSE, indicated relatively slow blood flow velocity; Region “c”, characterized as hyperintensity both on noncontrast and contrast T1‐FSE, is obviously the thrombus in the aneurysm (A). The same regions on contrast T1‐FSE image (B). Illustration of hemodynamic change on a sagittal projection T1‐FSE image (C).
Approximately 9.3% of stroke patients receiving intravenous thrombolysis have incidental aneurysms 2. Sometimes the intracranial aneurysms which presence is already known either being managed conservatively or already treated with clipping or coil embolization. On other occasions, the aneurysm is diagnosed during multimodal imaging performed to investigate the stroke.
In acute stroke patients with pre‐existing intracranial aneurysms, recent studies have suggested that intravenous thrombolysis may be safe given the similar rates of hemorrhagic transformation 2, 3, 4, 5 and favorable 3‐month clinical outcomes 5. There has been one report of anterior‐communicating artery aneurysm rupture after IV rtPA for acute ischemic stroke 6. However, this case may have had a sentinel bleed from an intracranial aneurysm before the onset of ischemic stroke symptoms. Thus, it does highlight the necessity of carefully excluding previous aneurysm rupture before the administration of rtPA.
Symptomatic acute ischemia in the territory of an unruptured intracranial aneurysm occurs rarely (approximately 1.3% of ischemic stroke patients) 7. Only two patients who have received thrombolysis in this context have been previously reported. One patient had a left middle cerebral artery aneurysm and received intravenous rtPA for their stroke 8. The other had a basilar tip aneurysm, and the stroke was treated with delayed intra‐arterial rtPA 9. The largest aneurysms reported in patients who received rtPA have been approximately 8 mm in diameter 2, 3. Our case is the first report of acute ischemic stroke associated with an unruptured large intracranial aneurysm (17 mm diameter) of the basilar artery with successful treatment using IV rtPA. According to multimodal imaging analysis, thrombus was clearly observed in the aneurysm of the basilar artery. We hypothesize that the pontine infarct may have a potential association with the thrombus in the aneurysm. The fresh extension of intraluminal thrombus may have had lower platelet content, making it more susceptible to pharmacological thrombolysis.
In conclusion, whilst it is difficult to estimate the risk of ICH after thrombolysis in acute ischemic stroke patients associated with intracranial aneurysms, our case indicates that at least some patients may benefit from IV rtPA. This case also suggests that multimodal imaging may assist physicians to identify the stroke mechanism and predict response to thrombolytic therapy.
Conflict of Interest
The authors have no actual or potential conflicts of interest to disclose.
Acknowledgments
Dr. Lou receives research support from Zhejiang Provincial Natural Science Foundation of China (LR12H09001), the National Natural Science Foundation of China (81171095), and the Health Bureau of Zhejiang Province (WKJ2010‐2‐010). We thank Prof. Bruce Campbell (Royal Melbourne Hospital, University of Melbourne, Parkville, Vic., Australia) for English language correction.
References
- 1. The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2. Edwards NJ, Kamel H, Josephson SA. The safety of intravenous thrombolysis for ischemic stroke in patients with pre‐existing cerebral aneurysms: a case series and review of the literature. Stroke 2012;43:412–416. [DOI] [PubMed] [Google Scholar]
- 3. Kim JT, Park MS, Yoon W, Cho KH. Detection and significance of incidental unruptured cerebral aneurysms in patients undergoing intravenous thrombolysis for acute ischemic stroke. J Neuroimaging 2012;22:197–200. [DOI] [PubMed] [Google Scholar]
- 4. Sheth KN, Shah N, Morovati T, Hermann LD, Cronin CA. Intravenous rt‐PA is not associated with increased risk of hemorrhage in patients with intracranial aneurysms. Neurocrit Care 2012;17:199–203. [DOI] [PubMed] [Google Scholar]
- 5. Mittal MK, Seet RC, Zhang Y, Brown RD Jr, Rabinstein AA. Safety of intravenous thrombolysis in acute ischemic stroke patients with saccular intracranial aneurysms. J Stroke Cerebrovasc Dis 2013;22:639–643. [DOI] [PubMed] [Google Scholar]
- 6. Rammos SK, Neils DM, Fraser K, Klopfenstein JD. Anterior communicating artery aneurysm rupture after intravenous thrombolysis for acute middle cerebral artery thromboembolism: case report. Neurosurgery 2012;70:E1603–E1607; discussion E1607. [DOI] [PubMed] [Google Scholar]
- 7. McLaughlin N, Bojanowski MW. Unruptured cerebral aneurysms presenting with ischemic events. Can J Neurol Sci 2008;35:588–592. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzaki T, Yoshino A, Sakatani K, Katayama Y. Recanalization of middle cerebral artery and intracranial aneurysm in the same ischemic territory with intravenous administration of recombinant tissue plasminogen activator: case report. J Stroke Cerebrovasc Dis 2011;20:269–272. [DOI] [PubMed] [Google Scholar]
- 9. Fakhouri T, McCullough LD. Delayed treatment of basilar thrombosis in a patient with a basilar aneurysm: a case report. J Med Case Rep 2008;2:353. [DOI] [PMC free article] [PubMed] [Google Scholar]


