Summary
Semantic dementia (SD) is a neurodegenerative disorder characterized by the progressive loss of semantic memory and conceptual knowledge, coupled with asymmetric local brain atrophy concentrated in the anterior temporal lobe. Recent developments in neuroimaging techniques, especially the emergence of the “human connectomics,” have made possible the study of the brain's functional and structural connections and the topological properties of the brain networks. Recent studies applying these techniques have shown that SD manifests extensive structural and functional connectivity alterations, providing important insights into the pathogenesis of SD and the neural basis of semantic memory in general. In this review, we present and discuss the existing findings about the brain connectivity changes in SD and how they might be related to the various behavioral deficits associated with this disorder and propose important unanswered questions that warrant further investigation.
Keywords: Connectomics, Functional connectivity, Semantic dementia, Semantic memory, Structural connectivity
Introduction
Semantic dementia (SD), also referred to as the semantic variant of primary progressive aphasia, is a clinical syndrome prominently characterized by the insidious and progressive loss of semantic memory or conceptual knowledge 1, 2, 3. The core cognitive deficits in patients with SD typically manifest with reduced expressive and receptive vocabulary, anomia, impaired confrontation naming and single‐word comprehension, object recognition/use (especially for low‐frequency and/or low‐familiarity items), as well as surface dyslexia and dysgraphia. While some researchers argued that SD is a characteristic “amodal” semantic deficit, in the sense that the semantic impairments in SD were not found to be restricted to certain conceptual domains or modalities of knowledge 4, some SD cases have exhibited disproportional impairments in specific semantic categories, such as that of living things 5. In contrast, SD patients’ episodic memory, repetition, calculation, and reasoning are relatively preserved, at least in the early stage of the disease 1, 2, 3, 6, 7, 8, 9, 10, 11, 12. The gross anatomical signature of SD is locally degenerative in nature, primarily involving the temporal and inferior frontal lobes, and SD is considered to be a subtype of frontotemporal lobar degeneration from a nosological perspective 8.
The human brain is structurally and functionally organized into complex networks that enable the effective segregation and integration of information processing. Recent developments in neuroimaging techniques have made measuring the integrity of specific functional and structural connectivities and networks possible 13, 14. Increasing evidence has indicated that neurological and psychiatric disorders, such as Alzheimer's disease (AD) and depression, are specific “disconnection syndromes” (e.g. 15, 16, 17). In particular, two questions have been extensively explored: (1) How do diseased brains differ from healthy ones in terms of their structural and functional wiring patterns? and (2) How do these brain changes underlie the behavioral deficits associated with these diseases? The latter question is critical for understanding the brain–cognition relationship, which is a fundamental question in cognitive neuroscience. The former is also important, not only in setting the ground for studying the brain mechanisms of behavioral deficits but also for clinical purposes such as biomarker and therapeutic strategy development.
In the present review, we focus on studies related to the structural and functional connectivity patterns associated with SD, organizing the findings into the two questions raised above. We first review the findings of the brain connectivity changes associated with SD, and then, those assessing how these brain changes might be related to the various behavioral deficits in SD, followed by the proposals of some important unanswered questions that warrant further study. The regional changes in SD are briefly presented first for a better understanding of the connectivity results.
Regional Structural and Functional Abnormalities in SD
Extensive brain imaging studies have investigated the local structural and functional brain abnormalities in SD. Structural MRI studies using voxel‐based morphological methods have consistently reported that SD is associated with asymmetric (more commonly left‐predominant) gray matter loss concentrated in the temporal lobe and also involves other brain regions in the frontal and limbic regions. Specifically, the most consistently reported gray matter atrophic regions included the bilateral temporal pole (TP), the fusiform gyrus, the middle and inferior temporal gyrus (ITG), the ventromedial frontal cortex, the amygdala, the hippocampus, and the left insula 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32. The bilateral superior temporal gyrus, the orbital frontal regions, the parahippocampus, the caudate nucleus (mainly the left side), and the right insula have also often been reported to be atrophic 18, 19, 20, 21, 22, 23, 25, 26, 28, 29, 30, 31, 32. Other regions that have been less frequently associated with gray matter loss in SD include the thalamus 21, 22, the superior temporal sulcus 19, the frontal pole and prefrontal cortex (PFC), the postcentral gyrus 32, the putamen and the cingulate 22, 30. ROI‐based structural MRI studies have confirmed these atrophy patterns 20, 33, 34, 35. Regarding the potential behavioral significance of these atrophic regions, correlations have been reported between the gray matter loss (volume or density) in the left temporal lobe and a series of semantic tasks, including word/picture matching 18, picture naming 19, 36, and composite verbal and nonverbal semantic evaluations 11. However, the precise location of the temporal regions showing such correlations varied across studies, with the left anterior temporal lobe (ATL), ITG, lateral temporal, and ventral temporal regions being implicated in different studies. Such differences may partly be due to the different gray matter metrics and semantic tasks being employed. In addition to the temporal lobe, the greater gray matter volume loss in the insula, frontal, and parietal cortices has also been found to be associated with sentence comprehension impairment, especially that of grammatically complex sentences, in SD 37, 38.
Functional MRI studies using semantic processing tasks, such as single‐word reading 23, 39, auditory sentence to picture matching 37, and object sound listening 40, have revealed that in comparison with controls, SD patients showed decreased activation in the mid‐fusiform and superior temporal gyrus 23, 37, 39 and increased activation in the intraparietal sulcus 23, 39, the inferior frontal gyrus 37, 39, and the left superior temporal gyrus/sulcus 40,and a lack of deactivation in the ventral ATL 37. Based on resting‐state fMRI, Guo et al. 29 reported an SD‐associated reduction in the fractional amplitude of low‐frequency fluctuation (fALFF) in the atrophied brain areas mentioned above and additionally in many regions in the frontal, parietal and occipital lobes, such as the postcentral gyri, the superior, middle and inferior occipital gyri, and the precuneus. Among these areas, the fALFF of the left fusiform, middle temporal, and calcarine cortices was correlated with verbal semantic performance, while that of the right frontoinsula, anterior cingulate cortex, and orbitofrontal cortex was correlated with awareness of social inference performance 29. Farb et al. (2013)58 further reported that SD was associated with a decreased low‐frequency power in the right anterior insula and bilateral dorsolateral PFC and with a decreased regional homogeneity in the bilateral dorsolateral PFC and the cerebellum.
Although these results have provided important insights into the specific affected brain regions, they do not address how communication patterns across brain regions are altered in SD. Below, we first briefly present how the functional and structural connectivity can be measured with brain imaging approaches, followed by detailed reviews of the SD‐associated connectivity changes and then, more specifically, how such connectivity alterations might underlie the behavioral impairments observed in SD.
Measuring Brain Connectivity with Neuroimaging
A major advance in the brain sciences in the recent decade is the development of ways to measure structural and functional connectivity patterns in vivo and to depict the topological properties of such patterns. Most of these methods have been recently applied in SD research.
Brain functional connectivity (FC) has been defined as “statistical dependencies among remote neurophysiological events” 41 and is frequently measured by the correlation of time series of the blood oxygen level dependent (BOLD) signals across brain regions on various levels, for example, brain regions or voxels during a particular cognitive task or at rest 42, 43, 44. Structural connectivity (SC) describes the existence of anatomical connections. White matter, containing the neural fiber bundles, is the basic SC element of the brain and can be mapped by diffusion tensor imaging (DTI) and reconstructed by tractography. Based on DTI data, the integrity of white matter tracts can be inferred from several frequently used parameters, including the mean diffusivity (MD), fractional anisotropy (FA), axial diffusion (DA), and the radial diffusion (DR). MD reports the average barriers to free diffusion at each voxel, and its values are observed to be higher in damaged or atrophied white matter; FA reports the extent to which diffusion is directionally constrained, and its values decrease when coherence in the preferred direction of movement is lost; DA and DR represent the diffusion rate in the directions parallel and perpendicular to the direction of fastest diffusion, respectively, and are associated with structures in the corresponding directions 45, 46, 47. These parameters are not fully independent but may show varying sensitivity to different types of white matter alterations. As summarized in Thomason and Thompson's review (2011), the alterations in these variables have been observed in normal development and aging, as well as in various neuropsychiatric disorders 45. The 3D orientation of white matter tracts can be reconstructed from the DTI information through tractography (see reviews 45, 46, 47). SC can also be derived from cross‐region correlations in cortical thickness or volume across subjects 13, 48, 49.
When more than two regions are involved and connected in some manner, they form a “brain network”, with the description and quantification of the information communication properties in the network becoming more challenging. Graph theory approaches have been applied to quantify various types of topological properties of the network, including the segregation, integration, and efficiency of information processing in the network, and have been referred to as “human connectomics”13, 14, 50, 51. Connectomic studies have revealed many nontrivial characteristics of the human structural and functional brain networks, such as small‐worldness, the existence of hub regions and modularity 50, 51, 52, 53. Critically, in the current context, the connectomic approach has provided novel insights into the pathological mechanisms of many neurological and psychiatric disorders including AD, depression, and schizophrenia 15, 17, 54, 55 and is beginning to be applied to SD.
Functional Connectivity Alterations in SD
Comparing SD‐associated Regional Changes with Healthy Functional Connectivity Patterns
Although a first line of studies did not directly assess the FC patterns in SD patients, their results have implications as to whether and how brain functional integration is disrupted in SD. These studies examined the relationship between the SD‐associated atrophy/hypometabolism patterns and healthy subjects’ resting‐state functional connectivity patterns (Table 1). A potential convergence between these two types of patterns would suggest which functional connections are most likely to be compromised in SD, and more generally, that nodes that constitute a functional network in health tend to be simultaneously affected by disease.
Table 1.
Neuroimaging studies of brain functional and structural connectivity in SD
| Study | Imaging modality | SD Patients | Controls | Connectivity description | |||
|---|---|---|---|---|---|---|---|
| N | Age | Severity | N | Age | |||
| Seeley et al. (2009) 56 | rs‐fMRI; sMRI | *24 | *63.4 (7.8) |
*MMSE = 24.3 (5.6) *CDR = 0.6 (0.4) *CDR‐SB = 3.1 (2.4) |
*65 19 |
*65.3 (8.3) 64.7 (8.5) |
Seed (l‐TP)‐based ICA generated FC; Seed (l‐TP)‐based SCN |
| Zhou et al. (2012) 57 | rs‐fMRI | *24 | *63.4 (7.8) | *ditto | 16 | 65.4 (3.2) | Seed (all atrophied areas)‐based FC; FC Network: nodes: spheres in all atrophied areas, edges: regression analysis |
| Guo et al. (2013) 29 | rs‐fMRI | 17 | 63.4 (6.1) |
MMSE = 26.4 (2.3) CDR = 0.7 (0.4) CDR‐SB = 4.1 (2.8) |
†17 | †63.6 (5.8) | Seed (b‐ATL)‐based FC |
| Farb et al. (2013) 58 | rs‐fMRI | 8 | 64.5 (3.3) | CDR = 1.2 (0.2) | 16 | 67.2 (1.2) | ICA: executive network, DMN, SLN, and emotion‐related networks; Seed (r‐DLPFC)‐based FC |
| La Joie et al. (2014) 60 | rs‐fMRI | *13 | *66.2 (5.5) | *Mattis total score: 118.7 (8.6) | 58 | 64.8 (8.7) | Seed (SD/AD “specific” ROIs)‐based FC; |
| Agosta et al. (2014) 31 | rs‐fMRI | 13 | 65 (7.0) |
MMSE: 22.2 (7.2) CDR‐SB: 2.8 (2.7) |
55 | 61 (9) | FC network: nodes: AAL (90), edges: Pearson correlation |
| Acosta‐Cabronero et al. (2011) 62 | DTI | 10 | 62.5 (6.5) |
MMSE: 24.2 (18–27) ACE‐R: 60.1 (40–78) |
21 | 69.3 (6.1) | WM TBSS; tractography |
| Schwindt et al. (2013) 64 | DTI | 9 | 65.2 (10.8) |
MMSE: 19.9 (8.4) MoCA: 15.4 (7.4) DRS: 99.2 (27.2) |
16 | 70.1 (8.7) | WM TBSS |
| Mahoney et al. (2013) 68 | DTI | 10 | 63.4 (6.7) | MMSE: 20.6 (8.5) | 20 | 64.7 (5.5) | WM TBSS |
| Sajjadi et al. (2013) 63 | DTI | 9 | 65 (51–73) |
MMSE: 22 (15–27) ACE‐R: 53 (22–84) |
26 | 69 (57–79) | WM TBSS |
| Lam et al. (2014) 70 | DTI | 11 | 62 (6) | ACE ‐R: 63 (12) (BE) | 15 | 67 (6) | WM TBSS longitudinal study |
| Zhang et al. (2013) 69 | DTI | 6 | 64.8 (5.8) |
MMSE: 26.2 (4.8) CDR: 0.7 (0.4) |
19 | 63.1 (7.6) | WM voxel‐wise and ROI‐based analysis |
| Agosta et al. (2010) 39 | DTI | 5 | 62.6 (4.6) |
MMSE: 24.2 (4.8) CDR: 0.8 (0.3) |
8 | 66.2 (12.5) | WM tractography |
| Galantucci et al. (2011) 66 | DTI | 9 | 62.5 (7.6) |
MMSE: 19.1 (7.4) CDR‐SB: 5.5 (3.2) |
21 | 65.3 (3.6) | WM tractography |
| Agosta et al. (2013) 67 | DTI | 13 | 66 (8) |
MMSE: 21.3 (7.1) CDR‐SB: 3.2 (2.6) |
35 | 64 (9) | WM tractography; Tract‐restricted TBSS |
Data shown as mean (SD or range). SD, semantic dementia; AD, Alzheimer's disease; MMSE, Mini Mental State Examination; CDR, clinical dementia rating; CDR‐SB, CDR sum of boxes; ACE‐R, Addenbrooke's cognitive examination‐revised score out of 100‐point total; MoCA, Montreal Cognitive Assessment; Mattis, Mattis total score; DRS, Dementia Rating Scale; GM, gray matter; WM, white matter; rs, resting state; sMRI, structural MRI; FC, functional connectivity; SCN, structural covariance network; ICA, independent component analysis; TBSS, Tract‐based spatial statistics; ROI, region of interest; DMN, default mode network; SLN, salience network; l, left; r, right; b, bilateral; TP, temporal pole; ATL, anterior temporal lobe; DLPFC, dorsolateral prefrontal cortex; BE, baseline evaluation. *Indicates that the subjects were involved only in the atrophy or hypometabolism analysis, and not the connectivity analysis. †Indicates more than one healthy control group were included in the study, and we only present the one for comparison with SD patients.
Seeley et al. 56 studied the relationship between structural atrophy patterns and functional networks in the healthy population across several neurodegenerative diseases, and we focus on their findings related to SD here. They first compared the gray matter measures between 24 SD patients and 65 controls, obtaining an SD‐associated atrophy map, with the left fusiform/ITG exhibiting the greatest reduction, followed by the TP. They argued that fusiform/ITG was susceptible to scanning artifacts and selected the left TP as the primary seed region to construct an intrinsic connectivity network based on resting‐state fMRI data and a structural covariance network based on gray matter intensity measures in two separate groups of healthy controls. Importantly, the distribution pattern of brain atrophy in SD was reported to be largely consistent with the intrinsic functional network and the structural network of left TP in the healthy populations 56. Although the details of the left TP‐based functional and structural networks were not provided, they can be inferred from results of a later study 29, in which averaging the intrinsic FCs of bilateral TPs in the healthy control group resulted in the identification of connections between TPs and a wide range of brain areas more extensive than those typically atrophied in SD (see below, Guo et al. 29).
Beyond TP, Zhou et al. 57 treated all atrophied brain areas as seeds and obtained, in the healthy group, the FC patterns of each seed. They observed that the seeds, of which the FC patterns in the healthy brains showed the greatest overlap with the gray matter atrophy patterns in patients, corresponded to the set of most atrophic regions in SD, including the left parahippocampal gyrus, superior TP, ITG, and the bilateral inferior TP. Furthermore, using graph‐based analyses, the authors found that in SD, as well as four other neurodegenerative disorders (AD, behavioral variant frontotemporal dementia (bvFTD), progressive nonfluent aphasia, and corticobasal syndrome), a shorter functional path from a given region to these sets of seeds in healthy controls was associated with greater atrophy severity of the region in the patients and that the overall functional connectivity strength of a region in the healthy subjects was also correlated with its atrophy severity in patients 57.
While these lines of studies elegantly demonstrated the general principle that brain regional changes correspond with the intrinsic functional networks in neurodegenerative disease, including SD, it can also be indirectly inferred that the connectivity pattern changes associated with SD are likely to be concentrated in the regions showing the strongest atrophy, including TP, and are likely to follow the FC patterns of these regions.
Functional Connectivity Alterations in SD
Only very recently have the FC pattern changes in SD patients been directly examined using resting‐state fMRI (Table 1). Guo et al. 29 compared the FC pattern seeding from the bilateral ATL between SD patients and controls and observed that SD was associated with extensive FC disruptions between the ATL and a broad range of brain regions across the temporal, frontal, parietal, and occipital lobes, including the primary cortices, the visual and auditory association cortices, and the corticoid, the allocortical and the peri‐allocortical regions (Figure 1). Notably, this pattern of FC disruption was convergent with the distribution of the ATLs’ FC in healthy brains, as well as the lowered fALFF map in the same study, indicating that the connectivity and regional alterations in SD, at least to some extent, respect the intrinsic networks in the healthy system 29. Farb et al. 58 focused on the executive, default mode, salience, and five emotion subnetworks in bvFTD and SD patients. These networks were of interest because they or their constituent regions have been previously reported to be relevant to bvFTD and SD. ICA analysis indicated that both groups showed reduced FC in the limbic part of the executive network and elevated FC in the medial PFC. Uniquely in SD, reduced FC strengths were observed in the bilateral lateral PFC and the anterior cingulate. FC reduction and elevation were also observed in different components within the default mode, salience, and emotion networks 58.
Figure 1.
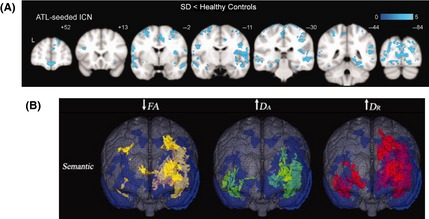
Disrupted brain functional and structural connectivity patterns in semantic dementia patients. (A) ATL‐seeded resting‐state functional connectivity findings of SD patients relative to controls. SD patients showed distributed reductions in functional connectivity with bilateral ATL. (B) Diffusion findings of SD patients relative to controls using an 8‐channel head coil with an array spatial sensitivity encoding a technique parallel imaging factor of 2 along 23 noncollinear directions with a b value of 1000 in reference to the gray matter atrophy distributions (blue). Relative to controls, the SD patients showed the most widespread FA (yellow) reductions and DR (green) increases but limited DA (red) increases. ATL, anterior temporal pole; ICN, intrinsic connectivity network; FA, fractional anisotropy; DA, axial diffusion; DR, radial diffusion. Adapted from 29 (Figure 2A) and 64 (Figure 3) with slight modifications. Information about the studies, including the imaging modality, the subject properties and the analyses methods, is shown in Table 1.
Beyond specific functional connection/networks, graph theoretical analyses have also recently been applied to elucidate the overall topological abnormalities in SD. Agosta et al. 31 constructed a whole‐brain functional network based on the AAL90 atlas 59 in 13 SD patients and 55 healthy controls, reporting that both groups exhibited small‐world properties. Importantly, relative to controls, the SD patients exhibited a lower mean network degree, clustering coefficient and global efficiency, and a higher characteristic path length and assortativity, indicating reduced network integration. Furthermore, compared with healthy controls, SD was associated with the absence of hubs (defined as regions with the highest nodal degree or betweenness centrality), including, but not restricted to, those areas reported to be atrophied in this disease. Notably, the distribution pattern of the lost hubs was partly overlapped with that of the ATL FC disturbances reported by Guo et al. 29. The emergence of hubs was also seen in SD relative to controls, mainly involving the bilateral superior temporal gyrus, the middle frontal gyrus, the thalamus, and the motor cortices. These SD‐specific hub regions may be related to some type of compensatory or release changes, and more studies are clearly needed to understand the underlying mechanisms.
These studies converged in showing the extensive changes of FC in SD, including those associated with the core atrophic region (i.e., ATL) and those distributed in multiple functional systems, which affected the overall network communication patterns.
Relationship Between the FC Alterations and Behavioral Deficits in SD
While the above studies have revealed widely distributed, extensive changes in terms of FCs associated with SD, most did not examine how these changes were related to the major symptoms in these patients, particularly the signature semantic impairment. To our knowledge, only two studies investigated the correlations between SD‐associated FC patterns and the behavioral deficits.
In the study by Farb et al. 58, after examining the FC alterations in patients with bvFTD and SD, they further showed that in both groups, the reduction in the anterior thalamus and the elevation in PFC connectivity were associated with greater apathy, and the left insula FC reduction in the salience network were correlated with disinhibition measurements. Only in SD patients, the lateral PFC FC was also correlated with disinhibition measurements. However, the relevance of these FC changes to the semantic deficits in SD was not examined.
Another recent study 60 focused on the hippocampus. The hippocampus is a commonly observed atrophied area in both SD and AD patients and has long been considered to underlie the episodic memory deficits in AD patients. To understand why the episodic memory of SD patients is relatively preserved, the authors compared the metabolic differences between SD and AD patients using 18Fluorodeoxyglucose positron emission tomography (PET) and identified the areas specifically exhibiting metabolic reduction in these two disorders: SD patients exhibited a significant metabolic reduction relative to AD patients in the bilateral anterior temporal areas, the subgenual and the right anterior cingulate cortex; the reverse pattern was observed in the bilateral precuneus/posterior cingulate cortex and the right angular gyrus. The peaks of the six disease‐selective regions showing between‐group metabolism differences were defined as seeds to derive resting‐state FC maps in the healthy group. All six maps included the right anterior hippocampus. Notably, however, while the connectivity between the crossroad hippocampus cluster and the two AD‐relevant ROIs (the right precuneus and angular gyrus) was significantly correlated with episodic memory performance in the healthy group, the strength of the connectivity between the hippocampus and the four SD‐relevant ROIs (the left perirhinal cortex, right TP, left subgenual and right anterior cingulate) did not significantly correlate with semantic performances in the same group, raising questions about the behavioral relevance of these altered FCs in SD 60.
To summarize, while the FC changes relatively specific to SD and how they may underlie the apathy and executive control changes in SD have been illustrated, the exact FC mechanisms underlying the core semantic deficits are yet to be established. Note that relative to the SD‐control comparison, comparisons between SD and other neurodegenerative disease highlight the aspects that are relatively more specific to SD (e.g., more salient semantic deficits). However, a direct comparison across these specific studies is difficult, as they measured rather different aspects of the brain and behavior.
Structural Connectivity Alterations in SD
Whole‐brain Level White Matter Alterations in SD
Where in the white matter do SD patients differ from the controls? First, SD has been found to be associated with a reduction of white matter volume in the left temporal lobe, the periventricular white matter and the corpus callosum 20 and in white matter tract areas of the fornix, the inferior longitudinal fasciculus (ILF), and the uncinate fasciculus (UF) 61. Studies examining SD‐associated SC changes on the whole‐brain level using DTI data have consistently reported that SD patients exhibited reduced FA and increased diffusivity (measured by MD, DA, and DR) 39, 62, 63, 64, 65, 66, 67, 68, 69 in the anterior ventral temporal lobe, extending dorsally and posteriorly in the temporal lobe and further into the ventral frontal areas, typically affecting the UF, the arcuate fasciculus (AF), the superior longitudinal fasciculus (SLF), and the ILF fiber bundles 62, 63, 64, 68, 69, 70 (Table 1, Figure 1). In addition, Schwindt et al. 64 observed an SD‐associated FA reduction and diffusivity increase in the inferior fronto‐occipital fasciculi (IFOF), the corpus callosum, the corona radiata, the internal capsule, and the cingulum (Figure 1). Note that although different DTI metrics were found to be more sensitive across these different studies, the abnormal areas revealed by DTI metrics largely overlapped and are mostly adjacent to gray matter regions showing atrophy 62, 64, 68.
To understand the SC changes in the degeneration process of SD, Lam et al. 70 conducted a longitudinal study and observed that at the baseline analyses, reduced FA and increased diffusivity were only identified in the left hemisphere, involving the left cingulum, SLF, ILF, and UF. Within one year, the white matter abnormalities extended to the IFOF and the splenium of the corpus callosum, while less progression was observed in the left temporal regions and no progression was seen in the cingulum, probably due to floor effects in these regions 70. Given that IFOF has been implicated in semantic processing from various paradigms 71, 72, this SC progression pattern is consistent with the semantic decline pattern in SD.
Tract‐based White Matter SC Alterations in SD
A number of recent studies have employed tractography, focusing on some specific tracts of interest because of (1) their relevance in semantic processing, (2) their possible relationship with the most atrophied gray matter areas in SD, or (3) the results of prior related studies (Table 1). Agosta et al. 39 tracked the left ILF, AF, fronto‐parietal SLF, UF and the genu and splenium of the corpus callosum in both SD patients and healthy controls and reported increased MD, DR, DA and decreased FA in all of these tracts in SD patients compared with healthy controls, except in the splenium of the corpus callosum. Convergent results were reported by Galantucci et al. 66, who tracked the ILF, UF, and SLF and observed decreased FA and increased diffusivity in the bilateral UF and anterior ILF, and the middle ILF, the arcuate and the temporoparietal SLF in the left hemisphere. No significant differences were seen in the more dorsal components of the SLF, which do not involve the temporal lobes 66. Agosta et al. 67 tracked the bilateral SLF, ILF, UF, the corpus callosum, and the corticospinal tract and applied TBSS‐based voxel‐wise analyses to these tract masks. They found that while nearly the entire UF was affected in SD, the ILF, the corpus callosum, and the SLF/AF were more severely affected in the left hemisphere and in the anterior portion, while little difference was observed between SD and healthy controls in the corticospinal tracts 67. In summary, these studies showed that SD is associated with disruptions of SC related to the gray matter atrophy regions, most severely affecting the white matter tracts connecting the temporal regions with the frontal, parietal, and occipital regions, that is, the UF, AF/SLF, and ILF.
Morphometry Covariance SC Alterations in SD
SC can also be established based on cross‐regional gray matter morphometry covariance across subjects. Seeley et al. 56 compared the gray matter atrophy pattern of SD patients (and other neurodegenerative diseases) with various types of network patterns in the healthy controls and reported that the atrophy pattern in patients was in accordance with the structural covariance network associated with the left TP constructed in healthy subjects. Although the morphometry covariance network has not yet been directly examined in SD patients, it could be inferred that those involving the gray matter regions with atrophy are likely to be altered. The potential changing patterns of SCs between these atrophy regions and other regions are more difficult to predict without direct assessments.
Relationship Between SC Alterations and Behavioral Deficits in SD
To our knowledge, only one study has directly examined how the white matter SC changes in SD patients are related to their behavioral deficits. Agosta et al. 39 reported that the mean MD of the left ILF and AF was significantly and negatively correlated with scores on verbal semantic tests, while the MD of the left SLF connecting the frontal and parietal regions was significantly and negatively correlated with grapheme–phoneme‐conversion ability. These results indicated that the changes of the left ILF and AF, and not the SLF, are likely to account for the semantic deficits in SD patients.
Relationship Between the Functional and Structural Connectivity Changes in SD
No study has yet directly compared the functional and structural connectivity changes in SD. Nonetheless, looking at the results of the two types of connectivity studies reviewed above, some accordant patterns can be observed: Both white matter damages 62, 64, 68 and FC disruptions 56, 57 correspond to the gray matter atrophy patterns in SD, which has similarity with the FC patterns of TP in healthy populations. This pattern is consistent with the hypothesis that SD starts from its key region (TP) and progresses along its anatomical and functional networks. However, inconsistencies should also be noted. For example, Guo et al. 29 reported much more extensive FC disturbances than the SC changes in SD 62, 63, 64, 68, 70. Such differences might reflect earlier changes in the functional network compared with the structural network, and/or be due to the different sensitivities of the two imaging modalities or the severity of disease in the patient populations. Furthermore, although graph analyses have been applied to the functional networks to unravel the SD‐associated topological changes, little is known about the topological properties of the structural network in SD.
Future Prospective of Connectivity Studies in SD
Patient Sampling and Neurobehavioral Evaluation
SD is degenerative by nature. Thus, the cognitive, behavioral and brain profiles of patients are dynamically changing. Additionally, important heterogeneities exist in this population. In terms of neuroanatomical changes, there are patients who show a different lateralization of brain atrophy. In terms of behaviors, we noticed that not only did the mean MMSE scores differ greatly across studies, but the deviations were also large within the same studies (Table 1). As shown in Table 1, we specifically presented the MMSE scores (or other routine general cognitive assessments) of the patient groups in the studies being reviewed whenever these scores were provided. For white matter connectivity, there seems to be a trend that patients with less severe overall cognitive impairments exhibited fewer extensive white matter changes; for FC, given the variability in analyses methods, it is difficult to determine any clear trends of the relationship between disease severity and FC changes. Importantly, the scores of MMSE (and other dementia‐screening batteries) are at best approximations of the disease severity for SD, as they are often developed to be tailored to Alzheimer's disease. Most items depend on language ability, which is particularly challenging for SD patients, given their semantic deficits. Thus, it would be critical, in future studies, to more carefully consider the patient population properties, using more specific cognitive neuropsychological assessments methods.
Understanding the Relationship Between Brain Connectivity Alterations and Semantic Behaviors
Although the recent imaging studies have revealed extensive changes in the structural and functional connections/networks, whether and how such brain changes are related to the behavioral symptoms is largely unclear. As reviewed above, only one FC study 60 and one SC study 39 examined the relationship of the connectivity changes with the signature semantic deficits, and the FC study found no positive results. However, studies involving healthy participants and patients with other types of brain damage have indeed illustrated the semantic effects of some white matter tracts reported to be affected by SD 71, 73, 74. What is the behavioral relevance of the observed brain connectivity changes in SD? What are the actual brain origins of the behavioral impairments, especially the selective, predominant loss of semantic memory? What are merely the byproducts of brain pathology that are irrelevant to semantic behavior? These questions are central to understanding the mechanisms underlying the behavioral profile of SD and semantic processing in general and require the systematic examination of the brain measures together with comprehensive semantic behavioral assessments.
Further Methodological Considerations of the Connectivity Analyses of the SD Brain
Although a few studies have analyzed the SD brain from the network perspective, as reviewed above (e.g. 31), there are still many open issues about the network mechanisms of SD. For example, most of the seed‐based connectivity studies have selected the TP or ATL as the seed, the rationale of which is rooted largely in the gray matter atrophy results. Such an approach results in a lack of detailed investigation of the connectivity roles of other relevant regions (e.g., the fusiform gyrus). In addition, it is unclear how SD affects the brain's topological properties such as the modularity structure of the brain networks and how such properties change as the disease progresses. Finally, the functional and structural network results have been shown to be affected by many parameters of the network construction and analyses, including the node definition, edge definition, and preprocessing procedures 29, 56, which varied in the current network studies of SD (Table 1). The influences of these parameters are general issues in connectomic research and may be particularly important in patient studies due to the greater variations in patients’ brain functional and structural properties and potentially greater head motion. The reproducibility of the network change patterns in SD across different methodological parameters remains to be established.
Development of Diagnostic Biomarkers from a Connectomic Perspective
The diagnosis of SD can be challenging, especially at early stages. The combination of machine learning and neuroimaging techniques has recently generated a decent classification accuracy for diagnosing many disorders, including AD 75 and depression 76. The commonly used approach is multivariate pattern analysis based on support vector machine (SVM) methods. Structural and functional connectivity and graph network metrics are potentially useful features for the SVM classification analysis of SD.
Development of Potential Therapeutic Targets from a Connectomic Perspective
The specific connections or regions that are compromised in SD, especially those underlying the core semantic deficits, constitute primary targets for various potential treatment programs, including pharmacological and brain stimulation therapies (e.g., repetitive transcranial magnetic stimulation, rTMS). Transcranial magnetic stimulation allows for the modulation of the excitability of both the brain areas directly targeted by stimulation and those connected to the targets. rTMS could potentially induce long‐lasting changes of cortical excitability and has been explored in the therapy of neuropsychiatric disorders such as AD and depression 77, 78, 79. rTMS to the anterior temporal lobes has been shown to affect semantic processing in healthy individuals (e.g., 80, 81, 82). It is desirable to examine whether stimulating similar sites and other relevant sites in SD patients can elevate the semantic performance or delay the degeneration process. Combining connectomic studies and brain stimulation techniques to develop such treatment programs and test their effectiveness will also increase our understanding of the biological mechanisms underlying this disorder.
Pathological, Genetic, and Patho‐physiological Basis of Network Changes in SD
Knowledge of the pathological and genetic basis of SD is scarce. As revealed by histopathologic studies, SD is most commonly associated with the FTLD‐TDP pathological subtype, which is characterized by TAR DNA‐binding protein 43 (TDP protein) deposition in the brain; in addition, the FTLD‐tau subtype (characterized by the microtubule‐associated protein tau deposition) has also been reported 2, 83, 84, 85. Genetic studies, mainly involving familial cases, have revealed several important genes acting in FTD, including MAPT (encoding microtubule‐associated protein tau), PGRN (encoding the protein progranulin), and others, such as C9ORF72. However, as SD cases are rarely found to be familial, these FTD‐related genes may not necessarily be relevant to SD 85, 86, and more studies about the genetic basis of SD are clearly needed. Regarding patho‐physiological mechanisms, studies employing fluorodeoxyglucose‐PET have revealed hypometabolism in the rostral and inferior/anterior temporal lobes, which is closely coupled with a regional atrophy pattern in SD 8, 62, 87. In addition, autoimmune mechanisms have also been suggested to play a role in the pathogenesis of SD 88. Relating these findings to the brain network changes discussed here poses further intriguing questions about the general mechanisms of the disorder and the origins of the heterogeneity within the disorder. For example, what are the underlying mechanisms of the different lateralization of atrophy patterns in different SD patients? What are the mechanisms for the variations in SD patients’ symptoms, such as the affected domain of knowledge? Only by the combination of multiple approaches can these questions be addressed, and this will lead to a comprehensive understanding of this disorder.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgment
We thank Jun‐Hua Ding and Yan Chen for their helpful comments. The study was supported by the National Basic Research Program of China (2013CB837300; 2014CB846103), the National Natural Science Foundation of China (81171019, 31171073, 31222024), NCET (NCET‐12‐0055) and Fok Ying Tong Education Foundation (141020).
References
- 1. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 2. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain 2012;135(Pt 5):1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007;8:976–987. [DOI] [PubMed] [Google Scholar]
- 5. Lin N, Guo Q, Han Z, Bi Y. Motor knowledge is one dimension for concept organization: further evidence from a Chinese semantic dementia case. Brain Lang 2011;119:110–118. [DOI] [PubMed] [Google Scholar]
- 6. Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain 1992;115:1783–1806. [DOI] [PubMed] [Google Scholar]
- 7. Hodges JR, Garrard P, Perry R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early alzheimer's disease: a comparative neuropsychological study. Neuropsychology 1999;13:31–40. [DOI] [PubMed] [Google Scholar]
- 8. Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007;6:1004–1014. [DOI] [PubMed] [Google Scholar]
- 9. Mesulam M. Primary progressive aphasia. Ann Neurol 2001;49:425–432. [PubMed] [Google Scholar]
- 10. Mesulam M. Primary Progressive Aphasia — a language‐based dementia. N Engl J Med 2003;349:1535–1542. [DOI] [PubMed] [Google Scholar]
- 11. Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 2006;129(Pt 11):3066–3080. [DOI] [PubMed] [Google Scholar]
- 12. Mesulam M. Primary progressive aphasia: a dementia of the language network. Dement Neuropsychol 2013;7:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–198. [DOI] [PubMed] [Google Scholar]
- 14. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 2010;52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 15. Crossley NA, Mechelli A, Scott J, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014;137(Pt 8):2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai Z, Yan C, Li K, et al. Identifying and mapping connectivity patterns of brain network hubs in alzheimer's disease. Cereb Cortex. doi: 10.1093/cercor/bhu246 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry 2015;77:223–235. [DOI] [PubMed] [Google Scholar]
- 18. Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel‐based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000;47:36–45. [PubMed] [Google Scholar]
- 19. Rosen HJ, Gorno‐Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002;58:198–208. [DOI] [PubMed] [Google Scholar]
- 20. Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. NeuroImage 2002;17:29–46. [DOI] [PubMed] [Google Scholar]
- 21. Gorno‐Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desgranges B, Matuszewski V, Piolino P, et al. Anatomical and functional alterations in semantic dementia: a voxel‐based MRI and PET study. Neurobiol Aging 2007;28:1904–1913. [DOI] [PubMed] [Google Scholar]
- 23. Wilson SM, Brambati SM, Henry RG, et al. The neural basis of surface dyslexia in semantic dementia. Brain 2009;132(Pt 1):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ash S, Moore P, Vesely L, et al. Non‐fluent speech in frontotemporal lobar degeneration. J Neurolinguistics 2009;22:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogar JM, Baldo JV, Wilson SM, et al. Semantic dementia and persisting Wernicke's aphasia: linguistic and anatomical profiles. Brain Lang 2011;117:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsieh S, Hornberger M, Piguet O, Hodges JR. Neural basis of music knowledge: evidence from the dementias. Brain 2011;134(Pt 9):2523–2534. [DOI] [PubMed] [Google Scholar]
- 27. Hsieh S, Hornberger M, Piguet O, Hodges JR. Brain correlates of musical and facial emotion recognition: evidence from the dementias. Neuropsychologia 2012;50:1814–1822. [DOI] [PubMed] [Google Scholar]
- 28. Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 2012;135(Pt 7):2178–2191. [DOI] [PubMed] [Google Scholar]
- 29. Guo CC, Gorno‐Tempini ML, Gesierich B, et al. Anterior temporal lobe degeneration produces widespread network‐driven dysfunction. Brain 2013;136:2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumfor F, Irish M, Hodges JR, Piguet O. Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS ONE 2013;8:e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agosta F, Galantucci S, Valsasina P, et al. Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol Aging 2014;35:2646–2655. [DOI] [PubMed] [Google Scholar]
- 32. Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014;137(Pt 4):1241–1253. [DOI] [PubMed] [Google Scholar]
- 33. Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 2001;57:216–225. [DOI] [PubMed] [Google Scholar]
- 34. Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and alzheimer's disease. Ann Neurol 2001;49:433–442. [PubMed] [Google Scholar]
- 35. Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. The human perirhinal cortex and semantic memory. Eur J Neurosci 2004;20:2441–2446. [DOI] [PubMed] [Google Scholar]
- 36. McMillan C, Gee J, Moore P, Dennis K, DeVita C, Grossman M. Confrontation naming and morphometric analyses of structural MRI in frontotemporal dementia. Dement Geriatr Cogn Disord 2004;17:320–323. [DOI] [PubMed] [Google Scholar]
- 37. Wilson SM, DeMarco AT, Henry ML, et al. What role does the anterior temporal lobe play in sentence‐level processing? Neural correlates of syntactic processing in semantic variant primary progressive aphasia. J Cogn Neurosci 2014;26:970–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peelle JE, Troiani V, Gee J, et al. Sentence comprehension and voxel‐based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008;21:418–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agosta F, Henry RG, Migliaccio R, et al. Language networks in semantic dementia. Brain 2010;133(Pt 1):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goll JC, Ridgway GR, Crutch SJ, Theunissen FE, Warren JD. Nonverbal sound processing in semantic dementia: a functional MRI study. NeuroImage 2012;61:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Friston KJ. Functional and effective connectivity: a review. Brain Connect 2011;1:13–36. [DOI] [PubMed] [Google Scholar]
- 42. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 43. Raichle ME. Two views of brain function. Trends Cogn Sci 2010;14:180–190. [DOI] [PubMed] [Google Scholar]
- 44. Smith SM, Vidaurre D, Beckmann CF, et al. Functional connectomics from resting‐state fMRI. Trends Cogn Sci 2013;17:666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 2011;7:63–85. [DOI] [PubMed] [Google Scholar]
- 46. Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003;4:469–480. [DOI] [PubMed] [Google Scholar]
- 47. Le Bihan D, Johansen‐Berg H. Diffusion MRI at 25: exploring brain tissue structure and function. NeuroImage 2012;61:324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol 2005;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alexander‐Bloch A, Giedd JN, Bullmore E. Imaging structural co‐variance between human brain regions. Nat Rev Neurosci 2013;14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012;13:336–349. [DOI] [PubMed] [Google Scholar]
- 51. Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol 2013;23:162–171. [DOI] [PubMed] [Google Scholar]
- 52. Meunier D, Lambiotte R, Bullmore ET. Modular and hierarchically modular organization of brain networks. Front Neurosci 2010;4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci 2013;17:683–696. [DOI] [PubMed] [Google Scholar]
- 54. Filippi M, van den Heuvel MP, Fornito A, et al. Assessment of system dysfunction in the brain through MRI‐based connectomics. Lancet Neurol 2013;12:1189–1199. [DOI] [PubMed] [Google Scholar]
- 55. Rubinov M, Bullmore E. Fledgling pathoconnectomics of psychiatric disorders. Trends Cogn Sci 2013;17:641–647. [DOI] [PubMed] [Google Scholar]
- 56. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large‐scale human brain networks. Neuron 2009;62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012;73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Farb NA, Grady CL, Strother S, et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 2013;49:1856–1873. [DOI] [PubMed] [Google Scholar]
- 59. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 60. La Joie R, Landeau B, Perrotin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia‐targeted networks. Neuron 2014;81:1417–1428. [DOI] [PubMed] [Google Scholar]
- 61. Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. NeuroImage 2010;49:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Acosta‐Cabronero J, Patterson K, Fryer TD, et al. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain 2011;134(Pt 7):2025–2035. [DOI] [PubMed] [Google Scholar]
- 63. Sajjadi SA, Acosta‐Cabronero J, Patterson K, Diaz‐de‐Grenu LZ, Williams GB, Nestor PJ. Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 2013;136(Pt 7):2253–2261. [DOI] [PubMed] [Google Scholar]
- 64. Schwindt GC, Graham NL, Rochon E, et al. Whole‐brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum Brain Mapp 2013;34:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitwell JL, Avula R, Senjem ML, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 2010;74:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Galantucci S, Tartaglia MC, Wilson SM, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 2011;134(Pt 10):3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Agosta F, Galantucci S, Canu E, et al. Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang 2013;127:157–166. [DOI] [PubMed] [Google Scholar]
- 68. Mahoney CJ, Malone IB, Ridgway GR, et al. White matter tract signatures of the progressive aphasias. Neurobiol Aging 2013;34:1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Tartaglia MC, Schuff N, et al. MRI signatures of brain macrostructural atrophy and microstructural degradation in frontotemporal lobar degeneration subtypes. J Alzheimers Dis 2013;33:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lam BY, Halliday GM, Irish M, Hodges JR, Piguet O. Longitudinal white matter changes in frontotemporal dementia subtypes. Hum Brain Mapp 2014;35:3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain 2013;136(Pt 10):2952–2965. [DOI] [PubMed] [Google Scholar]
- 72. Duffau H, Gatignol P, Moritz‐Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language? A cerebral stimulation study J Neurol 2009;256:382–389. [DOI] [PubMed] [Google Scholar]
- 73. Harvey DY, Wei T, Ellmore TM, Hamilton AC, Schnur TT. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia 2013;51:789–801. [DOI] [PubMed] [Google Scholar]
- 74. Harvey DY, Schnur TT. Distinct loci of lexical and semantic access deficits in aphasia: Evidence from voxel‐based lesions‐symptom mapping and diffusion tensor imaging. Cortex 2015;67:37–58. [DOI] [PubMed] [Google Scholar]
- 75. Dai Z, Yan C, Wang Z, et al. Discriminative analysis of early Alzheimer's disease using multi‐modal imaging and multi‐level characterization with multi‐classifier (M3). NeuroImage 2012;59:2187–2195. [DOI] [PubMed] [Google Scholar]
- 76. Zeng LL, Shen H, Liu L, et al. Identifying major depression using whole‐brain functional connectivity: a multivariate pattern analysis. Brain 2012;135(Pt 5):1498–1507. [DOI] [PubMed] [Google Scholar]
- 77. Ljubisavljevic M, Ismail F, Filipovic S. Transcranial magnetic stimulation of degenerating brain: a compar of normal aging, alzheimer's, parkinson's and huntington's disease. Curr Alzheimer Res 2013;10:578–596. [DOI] [PubMed] [Google Scholar]
- 78. Devi G, Voss HU, Levine D, et al. Open‐label, short‐term, repetitive transcranial magnetic stimulation in patients with Alzheimer's disease with functional imaging correlates and literature review. Am J Alzheimers Dis Other Demen 2014;29:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Micallef‐Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta‐analysis. Depress Res Treat 2014;2014:135049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pobric G, Lambon Ralph MA, Jefferies E. The role of the anterior temporal lobes in the comprehension of concrete and abstract words: rTMS evidence. Cortex 2009;45:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pobric G, Jefferies E, Lambon Ralph MA. Category‐specific versus category‐general semantic impairment induced by transcranial magnetic stimulation. Curr Biol 2010;20:964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Binney RJ, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion‐corrected fMRI, rTMS, and semantic dementia. Cereb Cortex 2010;20:2728–2738. [DOI] [PubMed] [Google Scholar]
- 83. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol 2010;6:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 2011;122:137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seltman RE, Matthews BR. Frontotemporal lobar degeneration epidemiology, pathology, diagnosis and Management. CNS Drugs 2012;26:841–870. [DOI] [PubMed] [Google Scholar]
- 86. Kirshner HS. Frontotemporal dementia and primary progressive aphasia, a review. Neuropsychiatr Dis Treat 2014;10:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008;64:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miller ZA, Rankin KP, Graff‐Radford NR, et al. TDP‐43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry 2013;84:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]


