Summary
Aims
Dopamine and glutamate receptors are densely expressed in the nucleus accumbens (NAc). Active interactions between these receptors contribute to the development of neuropsychiatric diseases, such as drug addiction and relapse. However, the molecular mechanisms underlying these interactions remain unclear.
Methods
This study established a mouse model of intermittent morphine‐induced mouse behavioral sensitization model. Western blot and electrophysiological recording methods were performed to directly identify the affective components of morphine behavioral sensitization.
Results
Interval morphine administration could cause significant locomotor sensitization. Hyperlocomotion and behavioral locomotor sensitization were significantly suppressed when ifenprodil (5 mg/kg), a selective NR2B subunit‐containing N‐methyl‐d‐aspartate (NMDA) receptor antagonist, or nafadotride (25 μg/kg), a dopamine D3 receptor (D3R)‐preferring antagonist, was coadministered with morphine. Western blot analysis showed that morphine behavioral sensitization induced a region‐specific increase in phosphorylation of NR2B (pNR2B) and total levels of NR2B (NR2B) expression in the NAc. Systemically administered nafadotride attenuated behavioral locomotor sensitization induced by morphine and significantly reversed the overexpression of pNR2B and NR2B subunit‐containing NMDA receptor in the NAc. NMDA receptor‐mediated excitatory postsynaptic currents in the NAc were also significantly reduced by nafadotride.
Conclusions
These findings suggest that D3Rs are involved in morphine‐induced behavioral locomotor sensitization in mice by regulating the NR2B subunits of NMDA receptors in the NAc.
Keywords: Morphine, Behavioral locomotor sensitization, NR2B, Dopamine receptor 3, Nucleus accumbens
Introduction
Opioids are analgesics highly effective for treating postoperative and cancer pain. However, the rewarding effects and behavioral sensitization caused by the chronic use of morphine may lead to morphine addiction and relapse.
Originating from the ventral tegmental area (VTA) and projecting to the NAc, the mesolimbic dopaminergic system mediates the rewarding properties of abused drugs. As one of the most significant neurotransmitters in the central nervous system, dopamine regulates many physiological functions, such as movement, decision making, learning, and reward 1. The pathophysiology of many diseases, such as Parkinson's disease, Schizophrenia, Alzheimer's disease, and drug addiction, and relapse after abstinence have been linked to dysfunctional dopamine signaling 2. The D1 class (D1 and D5 subtypes) and the D2 class (D2, D3, and D4 subtypes) are the two types of dopamine receptors 3. Dopamine D3 receptors (D3Rs), which are preferentially expressed in mesolimbic dopaminergic projection areas, including the NAc, are involved in the effects of abused drugs 4, 5. Our previous study found that D3Rs regulate basal nociception and are involved in the development of morphine‐induced tolerance and withdrawal 6. We also found that loss of the D3R gene may inhibit acute morphine‐induced hyperlocomotor activity and chronic morphine‐induced behavioral sensitization 7. However, the mechanisms involved in these phenomena are not clearly understood.
Aside from the dopamine system, the glutamate system is another key element in drug addiction. The principal members of ionotropic glutamate receptors include N‐methyl‐d‐aspartate (NMDA) receptor and non‐NMDA receptors (AMPA and kainate) subclasses. NMDA receptors are composed of an obligatory NR1 subunit and one or more modulatory NR2A‐D subunits. Previous studies have shown that the NR2B (GRIN2B) receptors contribute to the rewarding effects 8, 9 and behavioral sensitization 10, 11 of morphine.
Other researchers have extensively studied the functional interaction between dopamine and NMDA receptors. It is known that the two transmitter systems intimately interact with each other to mediate drug actions 12, 13. However, studies focusing on the effects of D3Rs on morphine‐induced locomotor activity as well as on the regulation of NR2B receptors in the NAc are lacking.
This study investigated behavioral locomotor sensitization in mice to identify drug relapse‐related behavior. Results showed that the dopamine D3R antagonist nafadotride decreased both pNR2B and NR2B receptors expression in the NAc and inhibited morphine‐induced behavioral locomotor sensitization. These results indicate that the D3R‐regulated NR2B receptors in the NAc are involved in morphine‐induced locomotor activity.
Materials and methods
Animals
Eight‐week‐old male C57BL/6 mice (25–30 g) were housed in groups of four per cage at constant temperature (20–22°C) and humidity (50–55%) under a 12‐h light/dark cycle with food and water provided ad libitum. Before experimental manipulations were undergone, the mice were allowed to habituate in the colony room for 1 week. The experiments were performed during the light phase of the cycle. The Animal Care and Use Committee of the Xi'an Jiaotong University approved all animal protocols.
Drug Treatments and Behavioral Testing
Morphine HCl (the First Pharmaceutical Factory of Shenyang, China), ifenprodil, the selective NR2B receptor antagonist (5 mg/kg, Sigma), and the D3R‐preferring antagonist nafadotride (25 μg/kg, Sigma) were dissolved in 0.9% sodium chloride solution.
As previously described, the sensitization protocol was based on intermittent intraperitoneal (i.p.) administration of 10 mg/kg/10 mL morphine every 72 h 7. Behavioral experiments were performed between 08:00 and 13:00. We evaluated the basal activities of the mice by measuring their horizontal locomotion on day 0. Within the following 13 d, morphine was injected five times at an interval of 72 h. Similarly, the controls were treated with 0.9% saline (10 mL/kg). Nafadotride (25 μg/kg) was injected into the nafadotride–morphine mice, and ifenprodil (5 mg/kg) was injected into the ifenprodil–morphine mice 30 min before each morphine injection.
An animal activity measurement system (Jiliang Software Technology Co., Ltd. JLBehv‐LAM‐1, Shanghai, China) consisting of four testing boxes (42 cm × 42 cm × 42 cm) with a TV camera was used to test individual mice. The interior bottoms of the testing boxes were painted white, whereas the interior sides were painted black. The boxes were set in an isolated dark room, and two standard laboratory lamps were used for illumination. After the experimental animals were placed in the boxes, their locomotor activities were recorded by the TV camera for 90 min and analyzed off line using a PC. Horizontal trajectories of the mice were video recorded and analyzed to determine their travelled distances in 90 min or per 10‐min period. Recording commenced within 2 min to 5 min after each injection. The mice were killed after the last test.
Tissue Preparation for Western Blot
Tissue samples were prepared as our previously described 14. The frontal association cortex (FrA), NAc, caudate putamen (CPu), and hippocampus (Hip) of the killed mice were dissected on ice, frozen immediately in liquid nitrogen, and then stored at −80°C until use. The samples were homogenized in 50 mM RIPA lysis buffer (20 μL/mg) with protease inhibitor cocktail. Homogenates were incubated on ice for 30 min and then centrifuged at 12,000 × g for 15 min at 4°C. The samples containing 20 mg of total protein were denatured at 95°C for 5 min, separated by 12% SDS–PAGE, and then transferred onto 0.45‐μm nitrocellulose membranes. The membranes were blocked in 5% nonfat dry milk with TTBS for 1 h at room temperature and then incubated with primary antibody pNR2B (pNR2B‐Ser1303, dilution ratio, 1:1000; Epitomics), NR2B (dilution ratio, 1:2000; ProteinTech Group) and with β‐actin (dilution ratio, 1:10,000; Sigma) as a loading control. The membranes were agitated overnight at 4°C, incubated with antirabbit secondary antibody conjugated to horseradish peroxidase, and then developed using enhanced chemiluminescence. All western blot analyses were performed at least thrice, and parallel results were obtained.
Slice Preparation and Whole‐Cell Patch Clamp Recordings
NAc slices from fresh brain tissue of male C57BL/6 mice were prepared as previously described 10. The mice were rendered unconscious by 4% isoflurane in air and then killed by cervical dislocation. The brains were rapidly removed. A tissue block was glued to a vibration slicer (1000plus, USA) containing NAc, cut into slices 300 μm thick, and then immediately transferred to oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) containing 2.5 mM KCl, 124 mM NaCl, 2 mM MgSO4, 2 mM CaCl2, 1 mM NaH2PO4, 25 mM NaHCO3, and 10 mM glucose at room temperature for at least 1 h. Experiments were performed in a recording chamber on the stage of an Olympus microscope with infrared DIC optics for visualization of whole‐cell patch clamp recording. Excitatory postsynaptic currents (EPSCs) were recorded from the shell of NAc neurons with an Axon 200B amplifier (Axon Instruments, Union City, CA, USA), and the stimulations were delivered by a bipolar tungsten‐stimulating electrode placed in the shell of NAc ~100 μm apart from the recording electrode. The NMDA component of EPSCs was observed in voltage‐clamp mode. The input–output relationships measuring EPSC amplitudes were evoked at different membrane potentials. The slices were superfused with antagonists specific for non‐NMDA (kainate and AMPA) glutamate receptors (20 μM CNQX) and GABAA receptors (100 μM picrotoxin) for at least 0.5 h before analysis to pharmacologically isolate the NMDA‐EPSC component. Patch electrodes containing 3.7 mM NaCl, 102 mM cesium gluconate, 11 mM BAPTA, 5 mM TEA chloride, 0.2 mM EGTA, 2 mM MgATP, 20 mM HEPES, 0.3 mM NaGTP, and 5 mM QX‐314 chloride (adjusted to pH 7.2 with CsOH) were used to record NMDA receptor‐mediated EPSCs. Neurons were voltage clamped at −30 mV, and NMDA receptor‐mediated EPSCs were evoked at 0.05 Hz. To test for synaptic excitation effects, AMPA receptor‐mediated miniature excitatory synaptic currents (mEPSCs) were recorded in the neurons clamped at −70 mV with 0.5 μM TTX added in the ACSF. Access resistance ranged from 15 MΩ to 30 MΩ and was monitored throughout the experiment. Data were discarded if access resistance changed by more than 15% during an experiment.
Data Analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical significance was assessed via one‐way or two‐way analysis of variance (ANOVA), followed by a post hoc multiple comparison. Individual comparisons were performed using unpaired Student' s t test. The significance level was set at P < 0.05.
Results
Immunodetection of NR2B Receptor Subunits
To detect the NR2B antibody's specificity and suitability for western blotting, we used western blotting to immunodetection of NR2B receptor subunits. Antibodies recognizing the carboxyl‐terminal regions of NR2B receptor subunits were used to immunoblot membranes from the naive mouse whole brain. Immunodetection by carboxyl‐terminal antibody was eliminated by preabsorption of the antibody with synthetic peptide with sequence CVAGASKARPDFRALVTNK (1411–1428 aa of human GRIN2B; Figure 1). These data demonstrate that the antibody of NR2B have the characteristics of specificity and selectivity.
Figure 1.

Characterization of antibody to NR2B subunit‐containing N‐methyl‐d‐aspartate (NMDA) receptor in western blotting. Antibody recognizing the carboxyl‐terminal regions of NR2B receptor subunits was used to immunoblot membranes from the naive mouse whole brain. Immunodetection by the carboxyl‐terminal antibody was eliminated by preabsorption of the antibody with the synthetic peptide immunogen.
Effects of D3R and NR2B Subunit‐Containing NMDA Receptor on Behavioral Responsiveness to Morphine
The effects of D3R and NR2B subunit‐containing NMDA receptor on behavioral responsiveness to morphine were tested. Intermittent administration of the same dose of morphine (i.p. 10 mg/kg) gradually increased locomotor activity during the 90‐min video‐tracking session following each administration (Figure 2B), effect of treatment: F(1, 14) = 6765.316, P < 0.001; effect of day: F(4, 35) = 13.954, P < 0.001; effect of day, versus day 1: day 4 (P < 0.001), day 7 (P < 0.001), day 10 (P < 0.001), and day 13 (P < 0.001). The results indicated that interval morphine administration caused significant locomotor sensitization. Hyperlocomotion (P < 0.01, compared with the morphine group) and behavioral locomotor sensitization (P > 0.05, compared with day 1 in the same group) were significantly suppressed when ifenprodil (5 mg/kg) or nafadotride (25 μg/kg) or both ifenprodil and nafadotride were coadministered with morphine (Figure 2B). The mice treated alone with saline, ifenprodil, or nafadotride showed constant locomotor activity after each injection (Figure 2A). Although ifenprodil and nafadotride did not show behavioral locomotor sensitization effects, they affected morphine‐induced behavioral locomotor sensitization.
Figure 2.
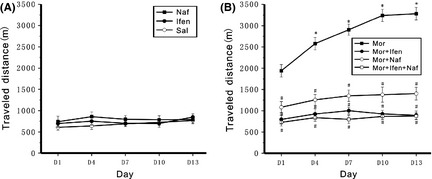
Development of sensitization to morphine‐induced hyperlocomotion and the effect of nafadotride or ifenprodil on locomotor activity in chronic morphine‐treated mice. Mice were i.p. injected at an interval of 72 h with morphine (Mor), saline (Sal), ifenprodil (Ifen), or nafadotride (Naf) alone or ifenprodil (Ifen) and nafadotride (Naf) administered 30 min before each morphine injection, respectively (as Mor + Ifen and Mor + Naf), or both ifenprodil and nafadotride are coadministered with morphine (as Mor + Ifen + Naf). n = 8 mice in each group. Locomotor activity was monitored for 90 min following each injection using a video camera. Mean ± SEM was used to evaluate the distance travelled during each 90 min recording session. (A) Saline, ifenprodil, or nafadotride alone did not induce locomotor sensitization. (B) Ifenprodil or nafadotride was coadministered with morphine or both ifenprodil and nafadotride are coadministered with morphine can significantly suppress morphine caused locomotor sensitization. Data were subjected to repeated‐measures ANOVA followed by Tukey's post hoc test (*P < 0.01 compared with day 1 in the same group; # P < 0.01 compared with the morphine group in the same days).
Enhanced NR2B Expression in NAc After Morphine‐Induced Behavioral Locomotor Sensitization
After intermittent administration of the same dose of morphine (i.p. 10 mg/kg) for 13 days in vivo, changes in NR2B expression in the forebrain region were observed. Chronic morphine injection upregulated both pNR2B and NR2B expression (P < 0.05) in the NAc compared with saline control. In addition, other forebrain areas were tested for changes in NR2B expression. Chronic morphine did not alter pNR2B and NR2B levels in FrA, Hip, and CPu (Figure 3). Thus, morphine behavioral sensitization induced a region‐specific increase in NR2B expression in the NAc.
Figure 3.
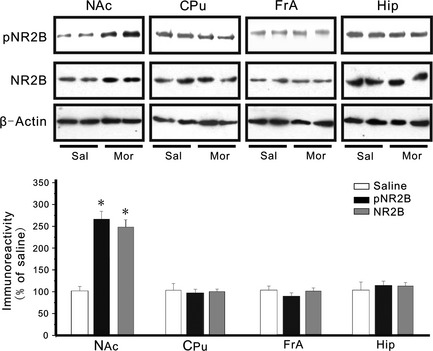
Effects of chronic morphine on NR2B expression in the mouse forebrain. Western blot analysis of NR2B immunoreactivities in the NAc, Cpu, FrA, and Hip of the chronic morphine‐treated mice. The upper part of the figure shows the NR2B bands in four mouse forebrains. The lower parts of the figure depict the immunoreactivities of the pNR2B and the NR2B expressed as their proportions to those of the corresponding Sal (mean ± SEM). n = 8 mice in each group. Unpaired samples t tests showed significant differences in pNR2B and NR2B immunoreactivity in the NAc but not in the FrA, Hip, or Cpu between the morphine‐treated groups. *P < 0.05, compared with the saline control.
Regulatory Effect of D3Rs on NR2B Expression
A previous study revealed that D3Rs are involved in morphine‐induced behavioral sensitization 7. However, the mechanisms underlying the sensitization process remain unclear. The effects of nafadotride on the morphine‐induced increase in NR2B in the NAc were measured to evaluate the capacity of D3Rs to mediate morphine effects. Nafadotride alone did not alter basal levels of both pNR2B and NR2B expression in the NAc (P > 0.05) compared with saline control (Figure 4).Under normal conditions, endogenous dopaminergic tone via D3R slightly influenced NR2B expression. By contrast, nafadotride blocked the morphine‐induced increase in both pNR2B and NR2B expression in the NAc (P < 0.05) compared with the morphine group (Figure 4). Thus, nafadotride reversed the overexpression of NR2B receptors in the NAc of morphine‐sensitized mice.
Figure 4.
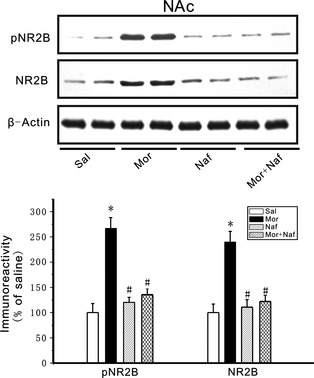
Role of dopamine D3 receptors in regulating NR2B expression. The upper part of the figure shows the pNR2B and NR2B bands in four groups in the NAc. The lower parts of the figure depict the immunoreactivities of the pNR2B and the NR2B expressed as their proportions to those of the corresponding Sal. Nafadotride alone did not affect basal levels of pNR2B and NR2B but nafadotride can block the morphine‐induced increases in pNR2B and NR2B expression in the NAc. Mice were given morphine (10 mg/kg) at an interval of 72 h for five times. The expression of pNR2B and NR2B levels increased compared with the saline control mice. Both pNR2B and NR2B expression was significantly reduced in the NAc when nafadotride (25 μg/kg) was coadministered with morphine (10 mg/kg). n = 8 mice in each group. Data were subjected to repeated‐measures ANOVA followed by Tukey's post hoc test *P < 0.05, compared with the saline control; # P < 0.05 compared with the morphine group, respectively .
D3R Regulation of NMDA Receptor‐Mediated Currents
Whole‐cell NMDA receptor‐mediated currents were recorded in acutely dissociated, medium‐sized striatal neurons to determine whether or not D3Rs can modulate the functions of NMDA receptors. The slope of the NMDA receptor‐mediated EPSC curves was dramatically greater in the neurons from morphine‐dependent mice compared with saline controls (Figure 5B,C). These data suggested that the synaptic efficacy increased in the NAc neurons from the morphine‐sensitized mice. Coadministration of nafadotride (25 μg/kg) with morphine significantly reduced NMDA receptor currents enhanced by chronic morphine injection (Figure 5B,C). Thus, nafadotride can partially downregulate the enhancement of excitatory synaptic transmission in the NAc by chronic morphine administration. Subsequently, mEPSCs in the shell of NAc were recorded to determine further the presynaptic or the postsynaptic components associated with increased synaptic transmission (Figure 6A). mEPSC frequency and amplitude significantly increased in the morphine‐ sensitized mice compared with saline controls (Figure 6B,C). Nafadotride (25 μg/kg) can significantly reverse the enhancement of mEPSC amplitude and frequency induced by chronic morphine injection. These results indicate that the nafadotride‐induced downregulation of excitatory synaptic transmission is probably due to presynaptic and postsynaptic modifications in the NAc synapses of morphine‐dependent mice.
Figure 5.
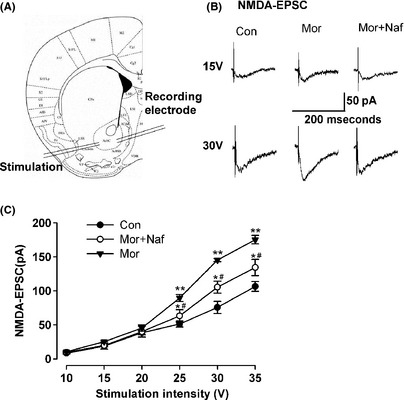
D3R regulation of NMDAR‐mediated currents. (A) Diagram of a slice showing the placement of whole‐cell patch recording and a stimulation electrode in the shell of NAc. (B) Representative traces show NMDA‐EPSC in the saline control, morphine‐sensitized, and nafadotride–morphine‐administered mice on D13. (C) Plot of input–output curves shows the enhancement of NMDA‐EPSC in the shell of NAc of morphine‐sensitized mice. Nafadotride partially inhibited morphine‐induced enhancement of NMDA‐EPSC. n = 8 neurons/4 mice in each group, *P < 0.05, **P < 0.01 compared with the saline control; # P < 0.05 compared with the morphine‐injected mice.
Figure 6.
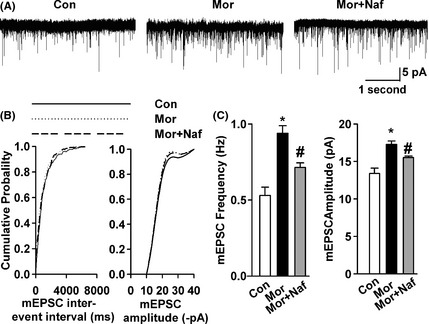
Effects of nafadotride on basal glutamatergic synaptic transmission. (A) mEPSCs recorded a holding potential of −70 mV in pyramidal neurons from saline control, morphine‐sensitized, and nafadotride–morphine‐administered mice on D13. (B) Cumulative frequency (left) and amplitude (right) histograms of the mEPSCs in neurons from saline control, morphine‐injected, and nafadotride–morphine‐administered mice. (C) Summary of mEPSC frequency (left) and amplitude (right) in neurons from saline control (n = 10 neurons/4 mice), morphine‐treated (n = 11 neurons/4 mice), and nafadotride–morphine‐treated (n = 12 neurons/4 mice) mice. Nafadotride reversed the enhancement of AMPA receptor‐mediated mEPSC amplitude and frequency induced by chronic morphine injection. *P < 0.05, compared with the saline control; # P < 0.05 compared with the morphine‐sensitized mice.
Discussion
To reveal the neural mechanisms underlying drug craving and relapse, locomotion sensitization is commonly studied in animal models. Previous reports 7, 15 confirmed that repeated, intermittent administration of morphine increases locomotion activity in mice, suggesting the development of locomotion sensitization.
Substantial evidence has proven that the mesolimbic DA system is essential in the mediation of locomotion sensitization to morphine 7, 16. The present results are similar to our previous study that D3R knockout mice did not display an enhanced behavioral response to acute morphine administration or did not develop an increased rate of locomotor sensitization to intermittent morphine administration. Coadministration of morphine with nafadotride can effectively suppress morphine‐induced behavioral sensitization 7. Our findings indicated that dopamine D3Rs were involved in morphine behavioral sensitization. These results are consistent with those of previous pharmacological studies 16, 17, 18, 19, in which the absence or inhibition of D3R can prevent the development of amphetamine or morphine‐induced behavioral sensitization.
Aside from the dopamine system, the glutamate system is another key element in drug addiction 20, 21, 22. Glutamate receptors are involved in many drug‐induced activities, such as conditioned place preference 23, self‐administration 24, amphetamine sensitization 25, 26, 27, and opioid dependence 28. The present study found that ifenprodil can significantly inhibit the formation of morphine‐induced behavioral sensitization. This result is consistent with that of Wu et al. 29. By contrast, the present results were inconsistent with previous reports 10, 30. This difference can be attributed to the different experimental protocols used. Previous reports focused on the roles of NMDA receptor antagonists on the expression of morphine sensitization. Meanwhile, the present study found that NR2B receptor antagonists are important in the development of morphine‐induced behavioral sensitization.
Substantial evidence has proven that NMDA receptors in NAc neurons influence chronic opiate treatment effects. However, the cellular and molecular mechanisms underlying this phenomenon remain unclear. The present study is the first to prove that the upregulation of NR2B phosphorylation and total levels of NR2B expression in the NAc contributes to morphine‐induced behavioral locomotor sensitization. The results are consistent with those of previous studies 23, 31. These findings indicate that NR2B receptors in the NAc but not in the FrA, CPu, and Hip of the limbic forebrain area are critical in mouse sensitization induced by intermittent i.p. administration of morphine.
Dopamine and glutamate receptors are both densely expressed in the NAc. Active interactions between these receptors occur at the receptor level. The glutamatergic system is involved in addiction, which is directly or indirectly related to interaction with the dopaminergic system 32. The regulatory effect of D3R on NR2B expression after chronic morphine treatment was tested. Coadministration of ifenprodil or nafadotride with morphine inhibited the expression of pNR2B and NR2B subunit‐containing NMDA receptors in the NAc and the development of behavioral sensitization. This result suggests that D3R can partly adjust morphine behavioral sensitization by reversing the overexpression of NR2B receptors in mouse NAc.
The dopamine–glutamate interaction is a key factor to contribute to the development of various forms of synaptic and behavioral plasticity in response to stimulants. Spatial learning is modulated by the dopamine–glutamate interplay in the ventral striatum 33. Different dopaminergic projections may be altered differentially in the addicted state, resulting in an altered dopamine–glutamate interaction that ultimately leads to aberrant control over behavior and compulsive drug‐taking behavior 13, 22. Recent studies have focused on the molecular mechanisms underlying the interaction. Liu 13 found that D3Rs interact with the NR2B anchor protein CaMKII and change the function of receptors. Other studies found that regulated D2R–NR2B interaction is essential in constructing behavioral responsiveness to cocaine 12. The phosphorylation state of dopamine‐ and cAMP‐regulated phosphoprotein with a molecular weight of 32 kDa in striatal neurons is regulated by both dopamine and glutamate 34. The present study found that D3R‐regulated NR2B in the NAc may critically contribute to the development of morphine‐induced behavioral sensitization. The mechanism underlying this phenomenon needs further study.
It is reported that the NAc can be further divided into two subregions termed the core and the shell, which play different functional roles in motivated behavior 35, 36, 37, 38, 39. The in vivo neurochemical evidence shows that the NAc shell is the critical site of DA reward. NAc shell DA acting on D1 receptors is also involved in learning and behavioral 38. Our electrophysiological observation showed that NR2B receptors underwent prolonged upregulation in NAc shell neurons after chronic morphine administration, and those NMDA receptor‐mediated responses were enhanced in in vitro brain slices. Inhibition of D3R receptors by administering nafadotride notably reversed the enhancement of NMDA receptor‐mediated synaptic transmission in the morphine‐sensitized mice and inhibited morphine behavioral sensitization. These results suggest that nafadotride can partially downregulate the enhancement of excitatory synaptic transmission in the NAc by chronic morphine administration. Thus, the downregulated NR2B subunit expression by nafadotride can explain the reduced NMDA receptor‐mediated EPSCs in NAc synapses. The results of the present study suggest a new role for NMDA receptors in long‐term behavioral sensitization to morphine.
In summary, D3Rs are involved in behavioral locomotor sensitization in mice by downregulating NR2B subunits of NMDA receptors in the NAc. This linkage is critically involved in the development of morphine‐induced behavioral sensitization. Drugs are important in modulating the expression of drug‐related behaviors. Therefore, agents that block D3R signaling and NR2B activation may be useful approaches for the treatment of drug craving and relapse in humans.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Professor Ming‐Gao Zhao (Fourth Military Medical University, China) for valuable discussions and critical revision of this manuscript. This project was supported by National Natural Science Foundation of China No. 81373253 and 81273351, the Fundamental Research Funds for the Central Universities, Shaanxi Key Project on Science and Technology (2013K12‐01‐09).
The first two authors contributed equally to this work.
References
- 1. Neve KA, Seamans JK, Trantham‐Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 2004; 24: 165–205. [DOI] [PubMed] [Google Scholar]
- 2. Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ. Novel insights in dopamine receptor physiology. Eur J Endocrinol 2007;156(Suppl 1):S13–S21. [DOI] [PubMed] [Google Scholar]
- 3. Sokoloff P, Schwartz JC. Novel dopamine receptors half a decade later. Trends Pharmacol Sci 1995;16:270–275. [DOI] [PubMed] [Google Scholar]
- 4. Heidbreder CA, Gardner EL, Xi ZX, et al. The role of central dopamine D3 receptors in drug addiction: A review of pharmacological evidence. Brain Res Brain Res Rev 2005;49:77–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 2006;5:25–43. [DOI] [PubMed] [Google Scholar]
- 6. Li T, Hou Y, Cao W, Yan CX, Chen T, Li SB. Role of dopamine D3 receptors in basal nociception regulation and in morphine‐induced tolerance and withdrawal. Brain Res 2012;1433:80–84. [DOI] [PubMed] [Google Scholar]
- 7. Li T, Hou Y, Yan CX, Chen T, Zhao Y, Li SB. Dopamine D3 receptor knock‐out mice display deficits in locomotor sensitization after chronic morphine administration. Neurosci Lett 2010;485:256–260. [DOI] [PubMed] [Google Scholar]
- 8. Ma YY, Yu P, Guo CY, Cui CL. Effects of ifenprodil on morphine‐induced conditioned place preference and spatial learning and memory in rats. Neurochem Res 2011;36:383–391. [DOI] [PubMed] [Google Scholar]
- 9. Johansson T, Elfverson M, Zhou Q, Nyberg F. Allosteric modulation of the NMDA receptor by neurosteroids in rat brain and the impact of long term morphine administration. Biochem Biophys Res Commun 2010;401: 504–508. [DOI] [PubMed] [Google Scholar]
- 10. Liu SB, Ma L, Guo HJ, et al. Gentiopicroside attenuates morphine rewarding effect through downregulation of GluN2B receptors in nucleus accumbens. CNS Neurosci Ther 2012;18:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kao JH, Huang EY, Tao PL. NR2B subunit of NMDA receptor at nucleus accumbens is involved in morphine rewarding effect by siRNA study. Drug Alcohol Depend 2011;118:366–374. [DOI] [PubMed] [Google Scholar]
- 12. Liu XY, Chu XP, Mao LM, et al. Modulation of D2R‐NR2B interactions in response to cocaine. Neuron 2006;52:897–909. [DOI] [PubMed] [Google Scholar]
- 13. Liu XY, Mao LM, Zhang GC, et al. Activity‐dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron 2009;61:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li T, Yan CX, Hou Y, et al. Cue‐elicited drug craving represses ERK activation in mice prefrontal association cortex. Neurosci Lett 2008;448:99–104. [DOI] [PubMed] [Google Scholar]
- 15. Jezova D, Mlynarik M, Zelena D, Makara GB. Behavioral sensitization to intermittent morphine in mice is accompanied by reduced adrenocorticotropine but not corticosterone responses. Brain Res 2004;1021:63–68. [DOI] [PubMed] [Google Scholar]
- 16. Liang J, Zheng X, Chen J, et al. Roles of BDNF, dopamine D(3) receptors, and their interactions in the expression of morphine‐induced context‐specific locomotor sensitization. Eur Neuropsychopharmacol 2011;21: 825–834. [DOI] [PubMed] [Google Scholar]
- 17. Chiang YC, Chen PC, Chen JC. D(3) dopamine receptors are down‐regulated in amphetamine sensitized rats and their putative antagonists modulate the locomotor sensitization to amphetamine. Brain Res 2003; 972:159–167. [DOI] [PubMed] [Google Scholar]
- 18. Richtand NM, Logue AD, Welge JA, et al. The dopamine D3 receptor antagonist nafadotride inhibits development of locomotor sensitization to amphetamine. Brain Res 2000;867:239–242. [DOI] [PubMed] [Google Scholar]
- 19. Cook CD, Beardsley PM. The modulatory actions of dopamine D2/3 agonists and antagonists on the locomotor‐activating effects of morphine and caffeine in mice. Pharmacol Biochem Behav 2003;75:363–371. [DOI] [PubMed] [Google Scholar]
- 20. Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav 2011;100: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology 2009; 56(Suppl 1):169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry 2003; 8:373–382. [DOI] [PubMed] [Google Scholar]
- 23. Narita M, Aoki T, Suzuki T. Molecular evidence for the involvement of NR2B subunit containing N‐methyl‐d‐aspartate receptors in the development of morphine‐induced place preference. Neuroscience 2000; 101:601–606. [DOI] [PubMed] [Google Scholar]
- 24. Schenk S, Valadez A, McNamara C, et al. Development and expression of sensitization to cocaine's reinforcing properties: Role of NMDA receptors. Psychopharmacology 1993;111:332–338. [DOI] [PubMed] [Google Scholar]
- 25. Stewart J, Druhan JP. Development of both conditioning and sensitization of the behavioral activating effects of amphetamine is blocked by the non‐competitive NMDA receptor antagonist, MK‐801. Psychopharmacology 1993; 110:125–132. [DOI] [PubMed] [Google Scholar]
- 26. Dunn JM, Inderwies BR, Licata SC, Pierce RC. Repeated administration of AMPA or a metabotropic glutamate receptor agonist into the rat ventral tegmental area augments the subsequent behavioral hyperactivity induced by cocaine. Psychopharmacology 2005; 179: 172–180. [DOI] [PubMed] [Google Scholar]
- 27. Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 2005;25:9144–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology 2001;157:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 2005;25:11107–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou SH, Zarrindast MR. Morphine‐induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacology 2008;81:333–343. [DOI] [PubMed] [Google Scholar]
- 31. Bajo M, Crawford EF, Roberto M, Madamba SG, Siggins GR. Chronic morphine treatment alters expression of N‐methyl‐d‐aspartate receptor subunits in the extended amygdala. J Neurosci Res 2006;83:532–537. [DOI] [PubMed] [Google Scholar]
- 32. Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 2001;63:241–320. [DOI] [PubMed] [Google Scholar]
- 33. Coccurello R, Oliverio A, Mele A. Dopamine‐glutamate interplay in the ventral striatum modulates spatial learning in a receptor subtype‐dependent manner. Neuropsychopharmacology 2012;37:1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Svenningsson P, Nairn AC, Greengard P. DARPP‐32 mediates the actions of multiple drugs of abuse. Aaps J 2005; 7: E353–E360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA 1995;92:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996;382:255–257. [DOI] [PubMed] [Google Scholar]
- 37. Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 1997;276:2048–2050. [DOI] [PubMed] [Google Scholar]
- 38. Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 2004;47(Suppl 1):227–241. [DOI] [PubMed] [Google Scholar]
- 39. Giorgi O, Piras G, Lecca D, Corda MG. Differential activation of dopamine release in the nucleus accumbens core and shell after acute or repeated amphetamine injections: A comparative study in the Roman high‐ and low‐avoidance rat lines. Neuroscience 2005;135: 987–998. [DOI] [PubMed] [Google Scholar]


