Summary
Aims
We tested the hypothesis that endothelial progenitor cell (EPC)‐mediated functional recovery after stroke may be associated with the endothelial nitric oxide synthase (eNOS)/brain‐derived neurotrophic factor (BDNF) signaling pathway.
Methods
Mice were infused with either EPCs or saline after being subjected to middle cerebral artery occlusion. The EPC‐treated mice also received intravenous injections of either Nω‐nitro‐l‐arginine methyl ester (L‐NAME, the NOS inhibitor) or saline.
Results
The activation of eNOS and the expression of BDNF were significantly increased in ischemic brain of the EPC‐treated mice, along with increased angiogenesis and neurogenesis. On diffusion tensor imaging (DTI), significant increases in fractional anisotropy and fiber count were observed in white matter, indicating axonal growth stimulated by EPCs. However, the EPC‐treated mice that were received an L‐NAME injection failed to exhibit the observed increases in angiogenesis, neurogenesis, and axonal growth. In addition, the neurons cocultured with EPCs in vitro exhibited the increased expression of BDNF and decreased apoptosis after oxygen–glucose deprivation compared with the control group. This EPC‐induced protective effect was virtually absent in the L‐NAME treatment group.
Conclusion
The eNOS/BDNF pathway may be involved in the EPC‐mediated functional recovery of stroke mice. DTI is feasible for dynamically tracking the orientation of axonal projections after EPC treatment.
Keywords: Brain‐derived neurotrophic factor, Diffusion tensor imaging, Endothelial nitric oxide synthase, Endothelial progenitor cells, Ischemic stroke
Introduction
Despite scientific advances, stroke remains a leading cause of disability and death worldwide 1. Accordingly, a novel treatment that may be effective beyond the acute 4.5‐h time window after stroke onset will benefit patients in the clinical setting 2, 3. Cell‐based therapy for ischemic stroke has recently been introduced in the clinical setting 4, 5, 6, 7. Endothelial progenitor cells (EPCs) may be used as markers of stroke outcomes 8. EPCs isolated from the bone marrow 9, peripheral blood 10, and cord blood 11, 12 have exhibited potential for neovascularization, which improving functional recovery from ischemic stroke in animal models.
To date, no magnetic resonance imaging (MRI) studies have assessed structural changes after EPC transplantation in animal models of cerebral ischemia. Fractional anisotropy (FA) generated from diffusion tensor imaging (DTI) has been widely applied to noninvasively detect the architectural organization of white matter 13, 14. The diffusion anisotropy of water may be observed before the onset of myelination 15. Moreover, the density and orientation of axonal fibers may also be visualized by fiber tractography. In this study, we used FA and fiber tractography to assess structural changes in the white matter of stroke mice after EPC transplantation.
The mechanism of EPC‐mediated brain regeneration remains unclear. It has been reported that EPC transplantation improves cardiac function after myocardial infarction, a process mediated by the activation of endothelial nitric oxide synthase (eNOS) 16. eNOS plays important physiological and pathophysiological roles in the cerebrovascular and cardiovascular systems. Nitric oxide produced by eNOS plays a critical role in the regulation of cerebral blood flow, angiogenesis, and vascular remodeling 17, 18. Moreover, previous studies have also demonstrated that the activation of eNOS in the brain tissue influences the migration of neurons, the outgrowth of neurites, the proliferation of progenitor cells, and neurological outcome after stroke via the upregulation of brain‐derived neurotrophic factor (BDNF) expression 19, 20, 21. Based on these observations, eNOS may be an important regulator of BDNF expression in the ischemic brain when stimulated by EPC transplantation.
In this study, we used MR‐DTI to assess changes in white matter integrity after EPC transplantation and to investigate whether the regulation of eNOS is a crucial determinant in the EPC‐associated induction of BDNF and the functional recovery of mice after ischemic stroke using an inhibitor of NOS, Nω‐nitro‐l‐arginine methyl ester (L‐NAME).
Materials and Methods
Cell Cultures
Endothelial progenitor cells isolated from the bone marrow mononuclear cells of 5‐week‐old male C57BL/6 mice (Academy of Military Medical Science, Beijing, China) were cultured in growth factor‐supplemented Endothelial Basal Media‐2 (EBM‐2; Lonza, Basel, Switzerland) via Ficoll density gradient centrifugation. The cultured cells were characterized using immunofluorescence staining of EPC‐specific markers (CD133, CD34, and VEGFR2; Abcam, Hong Kong, China) and double staining with dil‐acetylated low‐density lipoprotein (Dil‐acLDL; Life Technologies, Grand Island, Grand Island, NY, USA) and FITC‐Ulex europaeus agglutinin (FITC‐UEA; Life Technologies) on day 14 (Figure S1A,B). The purity of EPCs isolated from BM was approximately 80% 22.
The neurons were dissected from D1 mouse hippocampi (C57BL/6; Academy of Military Medical Science) as previously reported 22, 23, 24. The cells were grown in neurobasal media (Gibco, Burlington, ON, Canada) and characterized using a tubulin stain (Sigma‐Aldrich, St Louis, MO, USA) (Figure S1C). On day 5, the neurons were either cocultured with or cultured without EPCs for 24 h (100,000 EPCs per well with 1,000,000 neurons) after being subjected to a 2‐h oxygen–glucose deprivation (OGD) as previously described 24. L‐NAME (1 μM; Sigma‐Aldrich) and BDNF (100 ng/mL; Peprotech, Princeton, NJ, USA) were also added to the culture media when the neurons were co‐cultured with EPCs.
Animal Model and Experimental Protocol
All animal experiments were performed in accordance with the Guidelines for Animal Care and Use established by Medical School of Southeast University Institutional Animal Care and Use Committee. The protocol was approved by the Committee on the Ethics of Animal Experiments of Medical School of Southeast University. Male C57BL/6 mice (8 weeks old; Academy of Military Medical Science) were anesthetized with 1% isoflurane (KeYuan, Shandong, China) in an oxygen/air mixture using an anesthetic mask. The animals’ body temperature and respiratory rate were monitored using a physiological monitoring unit (Model 1025; SA Instruments Inc., Stony Brook, NY, USA) throughout the experiments, including during the surgery and the MRI scans.
Right middle cerebral artery occlusion (MCAO) was induced via a photochemical reaction 25. Under an operating microscope, an incision was made between the orbit and the ear. The right lateral aspect of the skull was exposed after separating the temporalis muscle. The middle cerebral artery was visualized under the skull. A laser beam (Shanghai Laser & Optics Century Co., Ltd., Shanghai, China) with a diameter of 0.1 mm and a wavelength of 532 nm was stereotactically focused onto the middle cerebral artery for 2 min after rose bengal injection (iv., 50 mg/kg; Sigma, St Louis, MO, USA). Thereafter, the skin was sutured and ischemia was verified via T2‐weighted imaging 24 h after surgery.
The in vivo experimental groups included the following: group 1, sham controls subjected to laser irradiation but not administered rose bengal (n = 23); group 2, mice subjected to MCAO and saline infusion (n = 22); group 3, mice subjected to MCAO and EPC infusion (n = 22); and group 4, mice subjected to MCAO, EPC infusion, and L‐NAME injection (n = 22). Either pure saline or 1,000,000 EPCs in 100 μL of saline was randomly infused into the mice from groups 2, 3, and 4 at 24 h postischemia via the ipsilateral internal carotid artery. A dose of 30 mg/kg of L‐NAME 26 was intravenously injected 30 min before the start of the EPC infusion into the mice from group 4.
MRI Measurements
MRI scans were performed using a 7‐T small animal MR scanner (Bruker PharmaScan, Ettlingen, Germany). T2‐weighted imaging was acquired using a fast spin echo sequence (repetition time/echo time, 3000/36 msecond, 4 average). A total of 12 axial slices with a field of view of 20 × 20 mm, a matrix of 256 × 256, and a slice thickness of 1 mm were positioned over the brain. The infarct volume measured from the T2‐weighted images using the program NIH ImageJ was divided by the volume of the contralateral hemisphere.
In vivo DTI was performed on day 21 postischemia. An echo planar imaging sequence was used to acquire 30 distinct diffusion directions and five reference images (repetition time/echo time of 5,000/32.2 msecond, b‐value of 1000 s/mm2, field of view of 16 × 16 mm, matrix of 128 × 128, slice thickness of 0.6 mm, 20 slices). The ParaVision 5.0 software was used (Bruker PharmaScan MRI); the FA was derived from the tensor map. Regions of interest were delimited on direction‐encoded color maps in the internal capsule (IC) of the brain. Fiber tracking was performed using the TrackVis (version 0.5.2.1; Massachusetts General Hospital, Boston, MA, USA) and Diffusion Toolkit (version 0.6.2.1; Massachusetts General Hospital) software.
Behavioral Tests
Behavioral tests including the modified neurological severity score (mNSS) and the foot‐fault test were used. These tests were performed prior to MCAO and on days 1, 7, 14, and 21 after MCAO by an investigator blinded to the experimental groups 22, 27.
Histology
On days 7 and 21 after MCAO, the animal brains were embedded in paraffin or optimum cutting temperature compound (OCT). Immunohistochemical staining was carried out for doublecortin (Dcx), NeuN, and myelin basic protein (MBP) as previously described 19. Optical density was measured using the Image‐Pro Plus software.
A terminal transferase‐mediated dUTP nick‐end labeling (TUNEL) assay was performed using an In Situ Cell Death Detection Kit Fluorescein (Roche, Indianapolis, IN, USA) to measure neuronal cell death. The brain sections and cultured neurons were also incubated with primary antibodies (rabbit anti‐BDNF, mouse anti‐NeuN, goat anti‐GFAP; Abcam), followed by staining with secondary antibodies (Alexa Fluor 546 goat anti‐rabbit IgG, Alexa Fluor 488 donkey anti‐mouse IgG, Alexa Fluor 488 donkey anti‐goat IgG; Life Technologies).
Fluorescein‐labeled lycopersicon esculentum lectin (Vector Labs, Burlingame, CA, USA) was diluted 1:1 with saline, and 200 μL was intravenously injected into the mouse models 5 min before death 28. To obtain sections for histology, the brains were harvested and cut into 20‐μm sections on a freezing microtome. Three random brain coronal sections from each mouse were chosen for microvessel counting. The numbers of microvessels within the peri‐infarct region were obtained from six mice per group. Only vessels with a clearly defined lumen or a well‐defined linear vessel shape were considered 29. This quantification was performed by an investigator blinded to the experimental groups.
Western Blotting
Equal amounts of protein obtained from the right hemisphere of mice from various groups on day 7 were used for the Western blotting analysis 22. The following primary antibodies were used: anti‐eNOS (1:1,000; Abcam), anti‐p‐eNOS (1:1,000; Abcam), anti‐BDNF (1:1000; Abcam), and anti‐β‐actin (1:2000; Abcam).
Statistical Analysis
The data are presented as means ± standard deviations (SDs). All statistical analyses were performed using the SPSS software, version 18.0. The data were tested for distribution normality and variance homogeneity. The MRI data and histology results were analyzed using one‐way ANOVA with post hoc Bonferroni tests, and the behavioral data were analyzed using repeated‐measures ANOVA with post hoc least significant difference (LSD) tests. P values <0.05 were considered statistically significant.
Results
Neurological Deficits and Lesion Volume
The mortality rate of the mice subjected to MCAO was approximately 20%, and the dead animals were excluded from the experiments. Neurological functional deficits were significantly attenuated in the EPC‐treated mice according to the mNSS (Figure 1A) and the foot‐fault test (Figure 1B), beginning on day 7 after MCAO, compared with the saline‐treated mice. Moreover, the functional improvement induced by EPCs was blocked by pretreatment with L‐NAME, an inhibitor of NOS (Figure 1A,B).
Figure 1.
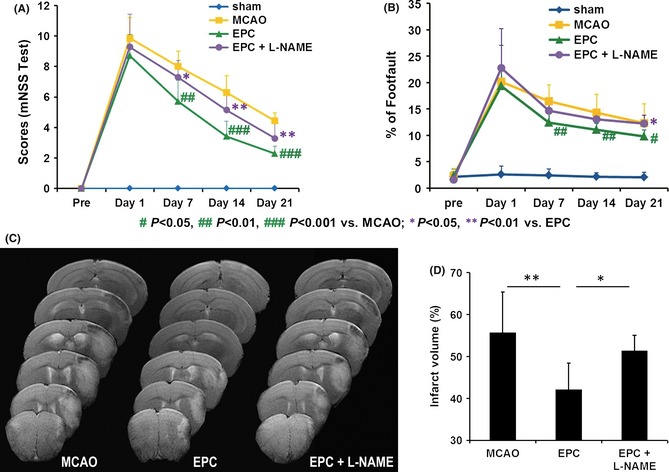
Endothelial progenitor cell (EPC) treatment of stroke improves functional outcomes in mice but fails to improve functional outcomes after pretreatment with Nω‐nitro‐l‐arginine methyl ester (L‐NAME). (A) The modified neurological severity score (mNSS) test and (B) the foot‐fault test were performed before middle cerebral artery occlusion (MCAO) and on days 1, 7, 14, and 21 after MCAO (n = 7 or 8 per group). (C) Representative T2‐weighted images of mice in various groups on day 7 poststroke. (D) Quantification of the stroke volume is depicted in the panel (n = 9/group). *P < 0.05 and **P < 0.01.
T2‐weighted imaging was used to examine the effect of EPCs on the infarct volume of the ipsilesional hemisphere. EPC transplantation reduced the ischemia‐induced loss of ipsilesional hemispheric volume, whereas EPC infusion with L‐NAME pretreatment did not cause significant infarct volume decreases compared with the saline infusion (Figure 1C,D).
Decreased Angiogenesis After Injection of L‐NAME
The treatment with EPCs initiated 24 h after MCAO significantly increased the perfused capillary density (Figure 2) in the peri‐infarct region (Figure 2A) on day 7 after MCAO compared with saline treatment. However, this EPC‐induced angiogenesis was decreased by an injection of L‐NAME (Figure 2).
Figure 2.
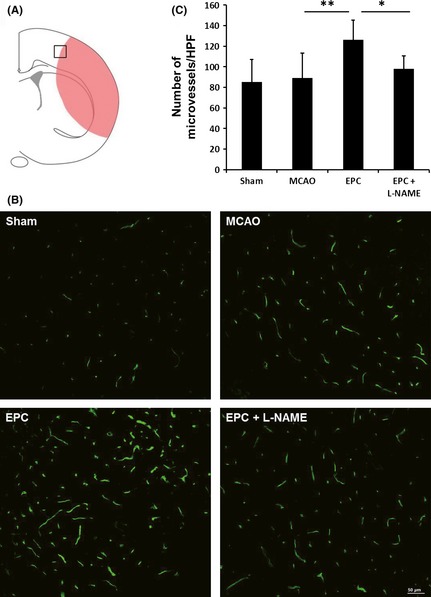
Endothelial progenitor cell (EPC) transplantation promotes angiogenesis in the ischemic brain on day 7 but fails to promote angiogenesis after pretreatment with Nω‐nitro‐l‐arginine methyl ester (L‐NAME). Lectin staining of microvessels (B) and quantitative vascular density (C) data in the peri‐infarct region (A) of mice (n = 6/group). Scale bar = 50 μm, *P < 0.05 and **P < 0.01.
Decreased Neurogenesis and Neuronal Survival After Injection of L‐NAME
Neurogenesis was assessed by counting the number of Dcx+ cells (indicating migrating neuroblasts) in the subventricular zone (SVZ) on day 7 after injury. The EPC infusion significantly increased the migration of endogenous SVZ neural progenitor cells compared with the saline infusion (Figure 3A,B). Meanwhile, the EPC infusion significantly increased the number of neurons (Figure 3C) and reduced the number of TUNEL+ apoptotic cells (Figure 3D) in the peri‐infarct region of mice on day 7 compared with saline treatment, suggesting that EPC transplantation improves neuron survival. To determine whether eNOS regulates EPC‐mediated neurogenesis and neuronal survival, L‐NAME was administered. Our data demonstrated that the additional injection of L‐NAME significantly decreased the migration of neural progenitor cells in the SVZ and the survival of neurons in the peri‐infarct region of the mice infused with EPCs (Figure 3).
Figure 3.
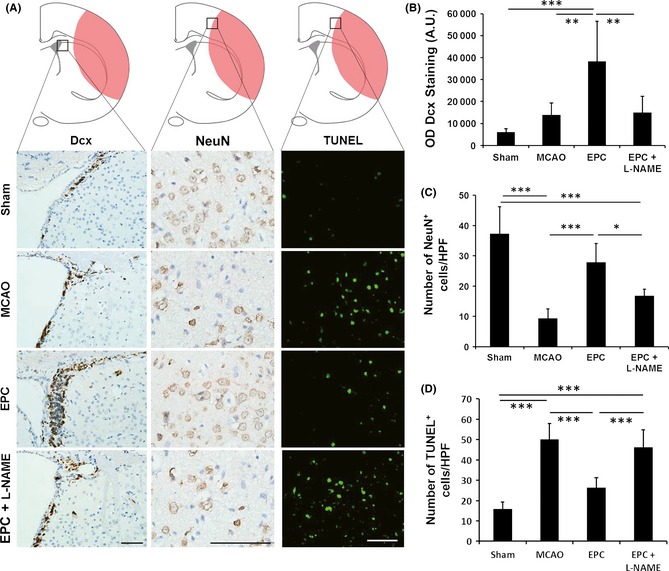
Endothelial progenitor cell (EPC) transplantation increases neurogenesis and neuronal survival in the ischemic brain on day 7 but fails to maintain these effects after pretreatment with Nω‐nitro‐l‐arginine methyl ester (L‐NAME). (A) Dcx immunostaining, NeuN immunostaining, and terminal transferase‐mediated dUTP nick‐end labeling (TUNEL) immunofluorescence staining in the ischemic brains of mice. Quantitative results of (B) Dcx staining, (C) NeuN‐positive cells, and (D) TUNEL‐positive cells (n = 6/group). Scale bar = 50 μm, *P < 0.05, **P < 0.01, and ***P < 0.001.
Decreased Axon Remodeling After Injection of L‐NAME
An in vivo DTI analysis demonstrated that EPC treatment increased FA in the ipsilesional IC on day 21 compared with saline treatment (Figure 4A,B). The fiber count in the IC of the EPC‐treated mice was significantly higher than that of saline‐treated mice (Figure 4C,D). MBP+ fiber coherence was significantly increased within regions of the IC in the EPC‐treated mice compared with the saline‐treated controls on day 21 (Figures 4E,F). These results indicate that EPC treatment increases ischemia‐induced axonal rewiring. However, the FA, fiber counts, and myelination in the IC of EPC‐treated mice were significantly reduced when pretreatment with L‐NAME was performed (Figure 4), suggesting that EPC‐induced axon remodeling may be mediated by eNOS.
Figure 4.
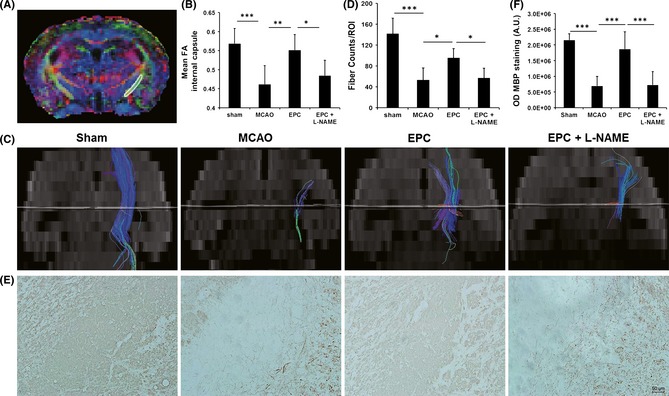
Endothelial progenitor cell (EPC) transplantation increases axonal growth in the internal capsule (IC) on day 21 after middle cerebral artery occlusion (MCAO) but exerts limited effects with an additional injection of Nω‐nitro‐l‐arginine methyl ester (L‐NAME). (A) The region of interest in the ipsilesional IC is outlined on the tensor map. (B) Quantitative data of the fractional anisotropy (FA) values in the IC are presented in the panel (n = 7 or 8 per group). Fiber tracking using diffusion tensor imaging (C) and quantitative data (D) in the IC of mice from various groups indicate that the nerve fibers were reorganized after stroke in both the treated and the control mice (n = 7 or 8 per group). Myelin basic protein (MBP)‐immunostaining in the IC region (E) and the quantitative data (F) were in accordance with the results of fiber tracking (n = 6–8 per group). Scale bar = 50 μm, *P < 0.05, **P < 0.01, and ***P < 0.001.
Decreased Expression of BDNF in the Ischemic Brain After Injection of L‐NAME
BDNF is critical to the survival of neurons, neurogenesis, and functional recovery after brain injury 30. To confirm the inhibition of eNOS and to investigate whether the functional recovery promoted by the EPCs was mediated by eNOS/BDNF, a Western blotting analysis was performed. The results indicated that the EPC infusion significantly increased both the activation of eNOS (Figure 5A) and the expression of BDNF and VEGF (Figure 5B) in right hemisphere of mice. Furthermore, the increased expression of BDNF but not VEGF was reversed by an additional injection of L‐NAME (Figure 5A,B). Moreover, BDNF was observed to be primarily secreted by neurons rather than astrocytes in the peri‐infarct region stimulated by EPC transplantation (Figure 5C). This EPC‐induced secretion of BDNF was reduced by the administration of L‐NAME (Figure 5C).
Figure 5.
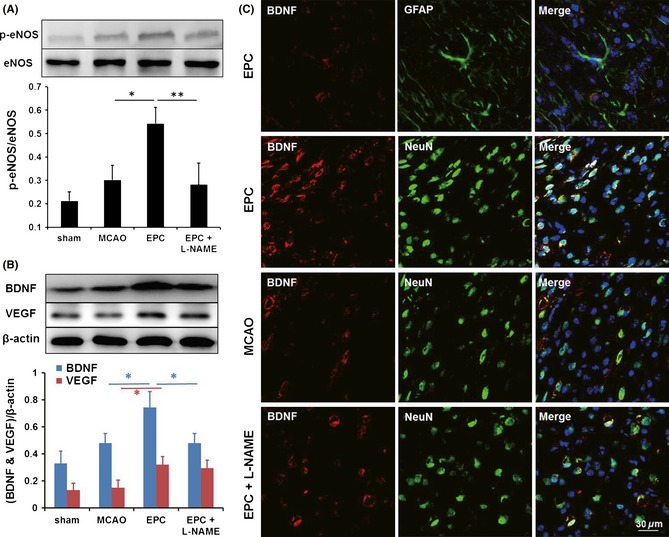
Endothelial progenitor cell (EPC) treatment promotes the activation of endothelial nitric oxide synthase (eNOS) and expression of brain‐derived neurotrophic factor (BDNF) on day 7 in the ischemic brain; however, these effects are not maintained after pretreatment with Nω‐nitro‐l‐arginine methyl ester (L‐NAME). Western blot analyses of the activation of eNOS (A) and the expression of BDNF and VEGF (B) in the ischemic brains of mice in various groups (n = 3/group). (C) Immunofluorescence staining revealed that only a few BDNF‐positive cells (red) were colabeled with GFAP (green) and that most of the BDNF‐positive cells (red) were colabeled with NeuN (green) in the peri‐infarct region of the mice treated with EPCs. Scale bar = 30 μm, *P < 0.05, **P < 0.01.
In vitro Coculture of Neurons with EPCs
The neurons exposed to OGD and subsequently cocultured with EPCs exhibited a significant increase in the secretion of BDNF (Figure 6A,B) and a significant reduction in apoptosis compared with the neurons without EPCs after OGD (Figure 6C,D). This protective action of EPCs was attenuated by the addition of L‐NAME but then restored by the addition of BDNF to the media (Figure 6).
Figure 6.
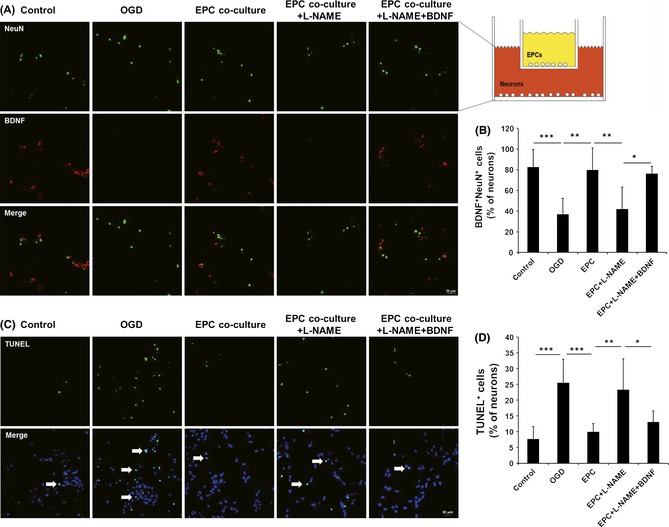
Neurons cocultured with endothelial progenitor cells (EPCs) in vitro exhibited increased brain‐derived neurotrophic factor (BDNF) expression and decreased apoptosis after being exposed to oxygen–glucose deprivation (OGD). BDNF and NeuN double staining (A) and quantitative data (B) revealed that the BDNF expression induced by the EPCs was attenuated by adding Nω‐nitro‐l‐arginine methyl ester (L‐NAME, n = 6/group). Terminal transferase‐mediated dUTP nick‐end labeling (TUNEL) staining (C) and quantitative data (D) also showed that the reduced apoptosis induced by EPC failed to persist when L‐NAME was also administered, whereas protection was restored if additional BDNF was added to the media (n = 8/group). Scale bar = 30 μm, *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
We observed that the delivered EPCs reduced infarct volume and neurobehavioral scores and increased FA values in the ipsilateral IC as measured by DTI. EPC transplantation upregulated both the activation of eNOS and the expression of BDNF. Our data demonstrated that blocking eNOS largely abolished the BDNF‐induced neuroprotection stimulated by the EPCs, and the complementary in vitro data demonstrated the crucial role of eNOS/BDNF in EPC‐induced brain protection.
Diffusion tensor imaging has been used to evaluate white matter damage in patients and animals with ischemic stroke 31, 32. However, no studies have used DTI to monitor the therapeutic reorganization of white matter after EPC transplantation. IC is the most important type of white matter in the brain 33. Motor axons form a tract running from the ipsilateral cortex, passing the ipsilateral IC and progressing toward the contralateral spinal cord. FA is related directly to histological markers of myelination 34, 35. Increases in FA correlate with white matter tract integrity, whereas reductions in FA correlate with functional deficits 32, 34, 36. Based on our DTI data, FA values were decreased in the ipsilateral IC after ischemia, indicating a loss of axonal integrity. EPC treatment increased FA values in the ipsilateral IC of ischemic mice, which were subsequently decreased by an additional injection of L‐NAME. DTI fiber tracking exhibited higher fiber counts and revealed that the orientation of the axonal projections in the EPC‐treated mouse brain was reorganized 3 weeks after stroke. Therefore, we demonstrated that DTI can be used to track the orientation of axonal projections dynamically and to evaluate the density of the fiber tracts in mice after stroke.
The current therapy for ischemic stroke targets macrovascular recanalization to restore the cerebral blood flow (such as thrombolytic therapy) and has a narrow treatment window. However, endogenous angiogenesis that is microvessels from preexisting vessels contributes to the formation of collateral circulation. After the 4.5‐h time window of stroke onset, angiogenesis can create a microenviroment in which successful recovery may ensue. EPC‐based therapy may amplify these endogenous restorative effects. Previous studies have demonstrated the therapeutic potential of EPCs in numerous vascular diseases 9, 10, 16, 37. Although endogenous EPCs may be recruited into ischemic tissue, the decreased number of EPCs in the peripheral blood of stroke patients may be insufficient for tissue repair 8. Therefore, transplanting exogenous EPCs is an effective method for providing sufficient EPCs to participate in the repair of brain tissue. Previous studies have demonstrated that injected EPCs could home to local injured brain tissue and facilitate angiogenesis after stroke 9, 10, 12. We further investigated the mechanisms underlying the EPC‐induced protective effects in the current study using the NOS inhibitor L‐NAME. Endothelial nitric oxide synthase, which was demonstrated to induce vascular protection after EPC transplantation 38, exerts multiple effects on the vasodilation of cerebral vessels and blood flow 18. The endothelium‐derived nitric oxide produced by eNOS plays a crucial role in the regulation of vascular function and is a downstream mediator of the responses to several growth factors. The inhibition of eNOS blocks the migration and proliferation of endothelial cells 19. In the present study, the increase in VEGF after EPC transplantation was not affected by the additional injection of L‐NAME; however, angiogenesis in the peri‐infarct region was inhibited by L‐NAME, indicating that the activation of eNOS is the intermediate process between the release of pro‐angiogenic factors from EPCs and the induction of angiogenesis (Figure S2).
Previous studies have also shown that administering EPCs after stroke enhances endogenous neurogenesis and improves neurological outcome via the acceleration of neovessel formation 11. The mechanisms underlying this relationship might include making the ischemic tissue more suitable for neuronal regeneration by facilitating the removal of toxic products and enhancing the production of chemokines and trophic agents by the neovasculature. BDNF, which may be one of the factors involved, promotes the proliferation, differentiation, and recruitment of neural progenitor cells and amplifies axonal growth and synaptic plasticity after stroke 39, 40. In the current study, BDNF was demonstrated to be highly expressed in the right hemisphere of the mice treated with EPCs and was associated with improved functional recovery. Furthermore, the BDNF stimulated by EPC treatment was secreted primarily by neurons in this study. Combined with the results of previous studies performed by other groups 16, 19, 20, 21, we have inferred that neovascularization in response to the administration of EPCs induces neurogenesis and axonal growth by providing the necessary supportive environment, including BDNF (Figure S2).
The expression of BDNF would be expected to be downregulated when EPC‐induced angiogenesis is blocked by an additional injection of L‐NAME. This hypothesis was supported by the findings of the present study. The mice treated with EPCs in combination with L‐NAME exhibited decreased BDNF expression and reduced neurogenesis and axonal growth after stroke compared with the mice treated with only EPCs. Moreover, the neurons treated with OGD were used to study the in vitro simulation of the ischemic environment. These in vitro results further confirmed the hypothesis. The findings of this study imply that in patients with dysfunctional eNOS, treatment with EPCs may not have beneficial effects.
There were several limitations to this study. L‐NAME is a nonspecific NOS inhibitor. Accumulating evidence suggests that both induced NOS and neuronal NOS exert detrimental effects on neurons, whereas only eNOS exerts beneficial effects in the setting of cerebral ischemia 41. The inhibition of induced NOS and neuronal NOS may exert neuroprotective effects, which may interfere with the negative effects exerted by the inhibition of eNOS. Despite the nonspecific inhibition, we obtained fairly satisfactory results. In the future, we will use eNOS−/− mouse models to uncover additional evidence of eNOS‐mediated neuroprotection after EPC transplantation.
Conclusions
In summary, our data provide evidence that eNOS/BDNF plays a crucial role in processes underlying microvessel angiogenesis, neurogenesis, and axonal remodeling. We have demonstrated that EPC therapy potentiates the secretion of BDNF from neurons in ischemic brain tissue, which may partially explain their ability to promote functional recovery. These data strongly suggest that EPC treatment may be an effective means of enhancing neuronal regeneration and functional recovery after stroke. DTI may be used to track the orientation of axonal projections dynamically and to evaluate the density of the fiber tracts in mice with cerebral ischemia after EPC transplantation.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. The characterization of endothelial progenitor cells (EPCs) and neurons.
Figure S2. A graphical illustration of transplanted endothelial progenitor cells (EPCs) may in action.
Acknowledgments
This project was supported by the National Nature Science Foundation of China (NSFC, No. 81071125, No. 81371538 and No. 81230034), the Major State Basic Research Development Program of China (973 Program) (No. 2013CB733802), the Fundamental Research Funds for the Central Universities (No. 3290005424), and the Jiangsu Provincial Special Program of Medical Science (BL2013029).
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chimowitz MI. Endovascular treatment for acute ischemic stroke–still unproven. N Engl J Med 2013;368:952–955. [DOI] [PubMed] [Google Scholar]
- 3. Grossman AW, Broderick JP. Advances and challenges in treatment and prevention of ischemic stroke. Ann Neurol 2013;74:363–372. [DOI] [PubMed] [Google Scholar]
- 4. Stem Cell Therapies as an Emerging Paradigm in Stroke Participants . Stem cell therapies as an emerging paradigm in stroke (steps): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009;40:510–515. [DOI] [PubMed] [Google Scholar]
- 5. Chopp M, Steinberg GK, Kondziolka D, et al. Who's in favor of translational cell therapy for stroke: Steps forward please? Cell Transplant 2009;18:691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borlongan CV, Chopp M, Steinberg GK, et al. Potential of stem/progenitor cells in treating stroke: The missing steps in translating cell therapy from laboratory to clinic. Regen Med 2008;3:249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000;55:565–569. [DOI] [PubMed] [Google Scholar]
- 8. Chu K, Jung KH, Lee ST, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 2008;39:1441–1447. [DOI] [PubMed] [Google Scholar]
- 9. Zhang ZG, Zhang L, Jiang Q, et al. Bone marrow‐derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res 2002;90:284–288. [DOI] [PubMed] [Google Scholar]
- 10. Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long‐term stroke outcome in mice. Ann Neurol 2010;67:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taguchi A, Soma T, Tanaka H, et al. Administration of cd34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004;114:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001;32:2682–2688. [DOI] [PubMed] [Google Scholar]
- 13. Zhang B, Xu Y, Zhu B, et al. The role of diffusion tensor imaging in detecting microstructural changes in prodromal alzheimer's disease. CNS Neurosci Ther 2014;20:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen F, Zhang X, Li M, et al. Axial diffusivity and tensor shape as early markers to assess cerebral white matter damage caused by brain tumors using quantitative diffusion tensor tractography. CNS Neurosci Ther 2012;18:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stone BS, Zhang J, Mack DW, et al. Delayed neural network degeneration after neonatal hypoxia‐ischemia. Ann Neurol 2008;64:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng Y, Jiang S, Hu R, et al. Potential mechanism for endothelial progenitor cell therapy in acute myocardial infarction: Activation of vegf‐ pi3k/akte‐nos pathway. Ann Clin Lab Sci 2013;43:395–401. [PubMed] [Google Scholar]
- 17. Chen J, Cui X, Zacharek A, et al. eNOS mediates to90317 treatment‐induced angiogenesis and functional outcome after stroke in mice. Stroke 2009;40:2532–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Endres M, Gertz K, Lindauer U, et al. Mechanisms of stroke protection by physical activity. Ann Neurol 2003;54:582–590. [DOI] [PubMed] [Google Scholar]
- 19. Chen J, Zacharek A, Zhang C, et al. Endothelial nitric oxide synthase regulates brain‐derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci 2005;25:2366–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canossa M, Giordano E, Cappello S, et al. Nitric oxide down‐regulates brain‐derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci USA 2002;99:3282–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng A, Wang S, Cai J, et al. Nitric oxide acts in a positive feedback loop with bdnf to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 2003;258:319–333. [DOI] [PubMed] [Google Scholar]
- 22. Bai YY, Gao X, Wang YC, et al. Image‐guided pro‐angiogenic therapy in diabetic stroke mouse models using a multi‐modal nanoprobe. Theranostics 2014;4:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen R, Yu H, Jia ZY, et al. Efficient nano iron particle‐labeling and noninvasive mr imaging of mouse bone marrow‐derived endothelial progenitor cells. Int J Nanomedicine 2011;6:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park KJ, Park E, Liu E, et al. Bone marrow‐derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab 2014;34:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saiki R, Nishimura K, Ishii I, et al. Intense correlation between brain infarction and protein‐conjugated acrolein. Stroke 2009;40:3356–3361. [DOI] [PubMed] [Google Scholar]
- 26. Horinaka N, Artz N, Jehle J, et al. Examination of potential mechanisms in the enhancement of cerebral blood flow by hypoglycemia and pharmacological doses of deoxyglucose. J Cereb Blood Flow Metab 1997;17:54–63. [DOI] [PubMed] [Google Scholar]
- 27. Cui X, Chopp M, Zacharek A, et al. The neurorestorative benefit of gw3965 treatment of stroke in mice. Stroke 2013;44:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y, Lee C, Shen F, et al. Angiopoietin‐2 facilitates vascular endothelial growth factor‐induced angiogenesis in the mature mouse brain. Stroke 2005;36:1533–1537. [DOI] [PubMed] [Google Scholar]
- 29. Xu B, Wu YQ, Huey M, et al. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab 2004;24:237–244. [DOI] [PubMed] [Google Scholar]
- 30. Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus 2009;26:E3. [DOI] [PubMed] [Google Scholar]
- 31. Borich MR, Wadden KP, Boyd LA. Establishing the reproducibility of two approaches to quantify white matter tract integrity in stroke. NeuroImage 2012;59:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding G, Jiang Q, Li L, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil‐treated rats. J Cereb Blood Flow Metab 2008;28:1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Velthoven CT, van de Looij Y, Kavelaars A, et al. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann Neurol 2012;71:785–796. [DOI] [PubMed] [Google Scholar]
- 34. Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury – a review. NMR Biomed 2002;15:561–569. [DOI] [PubMed] [Google Scholar]
- 35. Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed 2002;15:435–455. [DOI] [PubMed] [Google Scholar]
- 36. Shereen A, Nemkul N, Yang D, et al. Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia‐ischemia‐induced thrombotic stroke. J Cereb Blood Flow Metab 2011;31:1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ryu JC, Davidson BP, Xie A, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation 2013;127:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Nigris F, Balestrieri ML, Williams‐Ignarro S, et al. Therapeutic effects of autologous bone marrow cells and metabolic intervention in the ischemic hindlimb of spontaneously hypertensive rats involve reduced cell senescence and cxcr4/akt/enos pathways. J Cardiovasc Pharmacol 2007;50:424–433. [DOI] [PubMed] [Google Scholar]
- 39. Leventhal C, Rafii S, Rafii D, et al. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 1999;13:450–464. [DOI] [PubMed] [Google Scholar]
- 40. Cui X, Chopp M, Shehadah A, et al. Therapeutic benefit of treatment of stroke with simvastatin and human umbilical cord blood cells: Neurogenesis, synaptic plasticity, and axon growth. Cell Transplant 2012;21:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci 1997;20:132–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The characterization of endothelial progenitor cells (EPCs) and neurons.
Figure S2. A graphical illustration of transplanted endothelial progenitor cells (EPCs) may in action.


