Summary
Aim
To study whether endogenous endothelial progenitor cells (EPCs) are involved in neovascularization after stroke.
Materials and methods
Animal stroke models were established by subjecting male SD rats to permanent middle cerebral artery occlusion (pMCAO). Vessels in ischemic boundary zone (IBZ) were stained with antibody against laminin at 1 to 21 days after pMCAO. EPCs and newly formed vessels were identified by staining with special markers. After inhibiting recruitment of EPCs with AMD3100, a CXCR4 antagonist, endogenous EPCs, capillary density, cerebral blood flow (CBF) in IBZ, and neurobehavioral functions were assessed by staining, FITC‐dextran, laser‐Doppler perfusion monitor, and neurologic severity score.
Results
After pMCAO, vessels were found in IBZ at day 3, reaching a peak at day 14. The change in number of laminin‐positive cells showed a similar pattern with that of vessels. Apart from few endothelial cells, most of laminin‐positive cells were endogenous EPCs. After treatment with AMD3100, the number of endogenous EPCs, capillary density, and CBF in IBZ were significantly reduced, and neurobehavioral functions were worse as compared with the normal saline group.
Conclusions
Our findings suggested that endogenous EPCs participated in the neovascularization via CXCR4/SDF‐1 axis after pMCAO and mobilizing endogenous EPCs could be a treatment alternative for stroke.
Keywords: CXCR4, EPCs, Neovascularization, Stroke
Introduction
Mounting evidence showed that neovascularization is an important event in the regeneration of ischemic tissues 1. The newly formed blood vessels stimulate neurogenesis, synaptogenesis and enhance neuronal and synaptic plasticity 2. Nevertheless, the exact mechanism of the post‐stroke neovascularization, an important target of stroke intervention, remains elusive.
Recently, endothelial progenitor cells (EPCs) transplantation has been clinically used for treatment for ischemia 3. EPCs are immature hematopoietic cells circulating in peripheral blood and capable of differentiating into mature endothelial cells, taking part in neovascularization and promoting recovery after ischemic events, such as cardiovascular diseases (CVD), limb ischemia, and stroke 4, 5, 6, 7. In stroke patients, the level of circulating EPCs increases and is negatively correlated with lesion volume and outcome of stroke 8. However, little is known about of role of endogenous EPCs in the neovascularization after stroke.
Stromal cell‐derived factor 1 (SDF‐1) and its cellular receptor CXC‐chemokine receptor 4 (CXCR4) are key regulators of EPCs mobilization and recruitment 9. Most studies showed that EPCs expressed CXCR4 receptor 4, 6, 9. In most ischemic models, SDF‐1 expression was upregulated in ischemic area, and contributed to recruitment of transplanted EPCs to ischemic area 4, 6, 10. In this study, we found neovascularization of ischemic boundary zone (IBZ) after stroke. A question naturally presents itself as to whether endogenous EPCs take part in neovascularization of IBZ via CXCR4/SDF‐1 axis after stroke.
In the present study, we used a rat permanent middle cerebral artery occlusion (pMCAO) model to examine (i) the change in the number of vessels in IBZ of brain; (ii) possible involvement of endogenous EPCs in the neovascularization in IBZ; and (iii) recruitment of endogenous EPCs to IBZ by CXCR4/SDF‐1 axis after pMCAO.
Materials and methods
Establishment of pMCAO Model
Adult male Sprague Dawley rats weighing 190–240 g, 6–7 weeks old, were used in this study. Rats were obtained from and maintained in the Animal Care and Use Committee of Tongji Medical College at Huazhong University of Science and Technology, Wuhan, China. All procedures involving animal treatment were approved by the institutional committee of animal care and use. Rats were randomly divided into different groups. Rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate (300 mg/kg). Rectal temperature was maintained at 37.3 ± 0.5°C with a feedback‐regulated heating pad during the procedures. Permanent middle cerebral artery occlusion (pMCAO) was created according to a previous report 11. In brief, the right common carotid artery, right external carotid artery, and right internal carotid artery were isolated via a midline incision. The right external carotid artery was ligated with a 6–0 nylon suture. Then, a poly‐L‐lysine‐coated 4–0 monofilament nylon suture (Sunbio Biotech Co., Ltd., Beijing, China) was inserted from the right internal carotid artery and advanced for about 18 mm to occlude the origin of right MCA. Neurobehavioral functions tests and TTC staining were used to verify the success of surgery 1 day after pMCAO. Sham‐operated rats underwent identical procedures but without filament insertion.
Administration of AMD3100
AMD3100 (Abcam, Cambridge, MA, USA), a specific CXCR4 antagonist, was dissolved with normal saline solution to an injection concentration of 1 mg/ml. AMD3100 (1 mg/kg/day) was injected intraperitoneally 1 h after pMCAO for seven consecutive days. The same amount of normal saline solution was used in the saline‐treated group.
Immunofluorescence Examination
Immunofluorescence staining was performed on 4‐μm paraffin‐embedding sections. The following primary antibodies were used: chicken anti‐Laminin (1:100, Abcam), mouse anti‐Iba1(1:100, Abcam), rabbit anti‐CD163 (1:100, Abcam), rabbit anti‐GFAP (1:100, Abcam), mouse anti‐vWF (1:100; Thermo Fisher Scientific, Hudson, NH, USA), rabbit anti‐Ki67 (1:100, Abcam), mouse anti‐CD34 (1:100; Novus Biologicals, Littleton, CO, USA), goat anti‐VEGFR2 (1:100, Abcam), and rabbit anti‐CXCR4 (1:100, Abcam). For double or triple immunostaining, various combinations of primary antibodies were used. All sections were examined under a TCS SP5 multiphoton laser scanning confocal microscope (Nikon, Tokyo, Japan). Images were processed by using Adobe Photoshop CS (Adobe Systems, Mountain View, CA). Positive cells or vessels were counted with Image J (NIH, Bethesda, MD, USA). The means were calculated from five randomly selected microscopic fields in IBZ, and three consecutive sections were analyzed for each brain by a person blind to the grouping. Data were expressed as mean numbers of cells or vessels per square millimeter as a previously report 12.
Western Blotting
The rats were sacrificed at 3, 6, 12 h, 1, 3, and 7 days after pMCAO. Then, the tissue samples from the ischemic boundary zone (IBZ) were taken, put into the lysis buffer containing protease inhibitor, homogenized, and centrifuged. Protein (30 μg) was added to 16.5% SDS‐polyacrylamide gels and electrophoretically transferred to polyvinylidene fluoride membrane. The membrane was incubated with primary antibodies: rabbit anti‐SDF‐1 (1:300, Novus). Horseradish peroxidase‐conjugated goat anti‐rabbit immunoglobulin G was used as secondary antibody (1: 2000; Santa Cruz). Proteins were visualized using a Super Signal West Pico chemiluminescence kit (Thermo Scientific, USA). β‐actin (1:500, Santa Cruz, Dallas, TX, USA) served as internal control. The protein bands were scanned using Chemi Imager 5500 V2.03 software package, and integrated density values (IDV) were calculated using Image J (NIH Shareware) program, with β‐actin serving as internal control.
Measurement of Capillary Density
Capillaries were identified, as described previously, by intravenous injection of 0.2 ml FITC‐dextran (average mol wt 70,000, 50 mg/ml, Sigma, St. Louis, MO, USA) 10 min before animals were sacrificed13. Three coronal cryosections (20 μm) from each rat at bregma−0.2, −0.8, and −2.8 mm were analyzed using a TCS SP5 multiphoton laser scanning confocal microscope (Nikon). Briefly, ten fields of view from each coronal section were acquired in the IBZ. A threshold was applied to each digitized image to ensure that the numbers of FITC pixels reflected the original FITC‐dextran‐perfused patterns. Data were presented as the numbers of FITC pixels divided by the total numbers of pixels within the field of view, expressed as a percentage.
Neurobehavioral Functions Tests
Modified neurologic severity score was used for assessing neurobehavioral function after pMCAO as described previously 14. Neurologic severity score examination consists of the motor, sensory and reflex tests. According to the method, the severity of injury was graded on a scale of 0 to 14 (with normal score being 0 and maximal deficit score 14). One point was awarded either for the inability to perform, or for abnormal task performance or for the lack of a tested reflex. Neurobehavioral functions were evaluated independently by two person blind to the grouping.
Measurement of Cerebral Blood Flow
A laser‐Doppler perfusion monitor (PF‐5010; Periflux system, Anyang‐si, Korea) was used for the measurement of cerebral blood flow (CBF). The relative CBF in the IBZ was determined as described previously 15. After rats were anesthetized, an incision was made on the scalp to expose the skull. The CBF at the IBZ of ischemic side (1.5 mm posterior, 5 mm lateral to bregma) and the contralateral area (1.5 mm posterior, 5 mm contralateral to bregma) was continuously monitored in all animals for at least 5 min, and the average value was recorded. The relative CBF was calculated using the formula: relative CBF = CBF of ischemic side/CBF of contralateral side × 100%. CBF was measured by a person blind to the grouping.
Statistical Analyses
All values were presented as mean ± standard deviation (SD). Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS 13.0, USA) software. The two‐tailed Student's t‐test or one‐way anova followed by post hoc Fisher's LSD multiple comparison test was used for significance assessment. P < 0.05 was considered to be statistically significant.
Results
The Features of Neovascularization in IBZ of Brain after pMCAO
New vessels were found in the ischemic area of rat brain after pMCAO, with most of them at IBZ (Figure 1A). The vessels arose at day 3 after pMCAO, and the number of the vessels increased gradually, arriving at a peak at day 14. Then, the vessels merged into larger vessels at day 21 (Figure 1B,C). Simultaneously, plenty of laminin‐positive cells were found in IBZ. The number of laminin‐positive cells in IBZ increased gradually from day 1, reaching a peak at day 14, and then decreased (Figure 1B, 1D). The change in the number of laminin‐positive cells showed a pattern similar with that of the new vessels.
Figure 1.
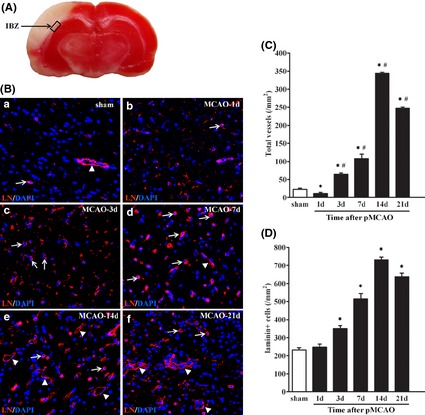
The neovascularization in the IBZ after pMCAO. (A) The box illustrates ischemic boundary zone (IBZ) in brain of rat after pMCAO. (B) Immunofluorescence staining for vessels and laminin (LN)‐positive cells (red) in IBZ from 1 to 21 days after pMCAO. Sections were counterstained with DAPI (blue) to visualize nuclei. The arrows indicate the laminin‐positive cells, and the arrowheads indicate the vessels. (C) Quantification of vessels in IBZ from 1 to 21 days after pMCAO. (D) Quantification of LN‐positive cells in IBZ from 1 to 21 days after pMCAO. LN, laminin. The vessels and cells were counted from at least five randomly selected 40×fields. Data are presented as mean ± SD. Scale bar = 50 μm. n = 7–8/group. *P < 0.05, versus. sham‐operated group, # P < 0.05, versus. 1 day group after pMCAO.
Involvement of Endogenous EPCs in the Neovascularization of IBZ after pMCAO
To identify the laminin‐positive cells in IBZ (Figure 2A), we first stained the cells with the antibodies against the markers of inflammatory cells, such as anti‐Iba1 (a marker of microglial cells) and anti‐CD163 (a marker of macrophages). Our results showed that these laminin‐positive cells were neither microglial cells (Figure 2B,C) nor macrophages (Figure 2D,E). Then, we stained these laminin‐positive cells with antibodies against the markers of glial cells (glial fibrillary acidic protein, GFAP). And we found that the GFAP‐positive cells did not merge with laminin‐positive cells, suggesting that laminin‐positive cells were not glial cells (Figure 2F,G).
Figure 2.
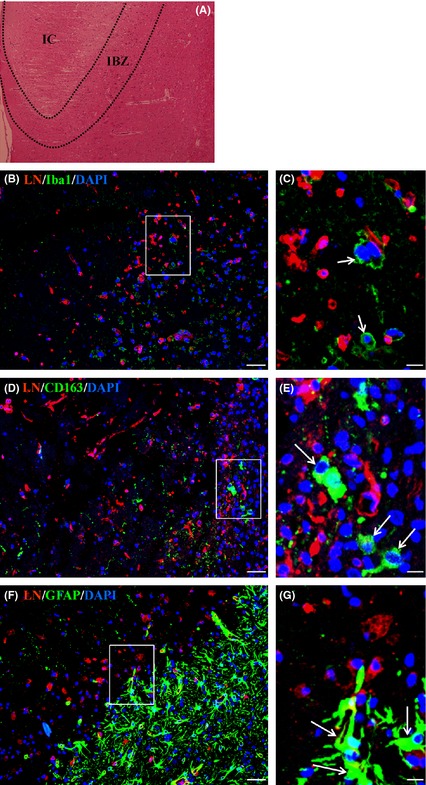
The laminin‐positive cells were neither glial cells nor inflammation cells in the IBZ after pMCAO. (A) HE staining showed the ischemic boundary zone (IBZ) and ischemic core (IC) in brain of rat at day 7 after pMCAO. (B‐G) The laminin (LN)‐positive cells in IBZ at day 7 after pMCAO were stained with antibodies against special cell markers. The results showed that the LN‐positive cells were neither inflammation cells (arrows in C and E) nor glial cells (arrows in G). (B)‐(C). LN/Iba1 (a marker of microglial cell). (D)‐(E). LN/CD163 (a marker of macrophage). (F)‐(G). LN/GFAP (a glial cell marker). Sections were counterstained with DAPI (blue) to visualize nuclei. (C), (E), and (G) are magnified images of boxes in (B), (D), and (F). Scale bar = 20 μm in B, D, and F; Scale bar = 5 μm in (C), (E), and (G).
Finally, we stained the cells with the antibody of endothelial marker (von Willebrand factor, vWF) and found that some of laminin‐positive cells integrated with vWF‐positive cells (Figure 3A). Moreover, we also found that some endothelial cells formed new vessels in IBZ after staining with both anti‐vWF antibody and an anti‐Ki67 antibody (Figure 3B,C). Compared with the contralateral side, the laminin‐positive endothelial cells and new vessels that endothelial cells formed in IBZ were significantly more (Figure 3D,E).
Figure 3.
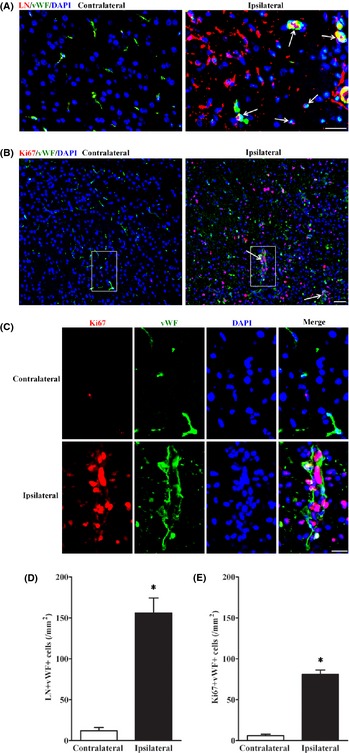
Some of the laminin‐positive cells were endothelial cells in the IBZ after pMCAO. (A) LN/vWF double staining showed part of laminin (LN)‐positive cells in IBZ at day 7 after pMCAO were endothelial cells (arrows). Scale bar = 20 μm. (B) Combination staining of antibodies against an endothelial cell marker (vWF) with a proliferation marker (Ki67) showed that endothelial cells formed new vessels in IBZ at day 7 after pMCAO. Scale bar = 20 μm. (C) is magnified images of boxes in (B). Scale bar = 10 μm. (D) Quantification of LN/vWF dual positive cells in IBZ 7 days after pMCAO. (E) Quantification of Ki67/vWF dual positive cells in IBZ 7 days after pMCAO. n = 7–8/group. *P < 0.05, versus. contralateral side.
To identify the remaining laminin‐positive cells, we detected specific EPC surface markers (CD34 and VEGFR2) 16 on the cells. Our results exhibited that most of laminin‐positive cells in IBZ co‐expressed VEGFR2 and CD34 (Figure 4A), verifying that the cells were EPCs. Moreover, combination staining with both Ki67 and EPC antibodies showed that EPCs also formed new vessels (Figure 4B). Compared with the contralateral side, EPCs and vessels that EPCs formed in IBZ were significantly more (Figure 4C,D). These findings suggested that, apart from endothelial cells, endogenous EPCs also took part in neovascularization in the IBZ of brain after pMCAO.
Figure 4.
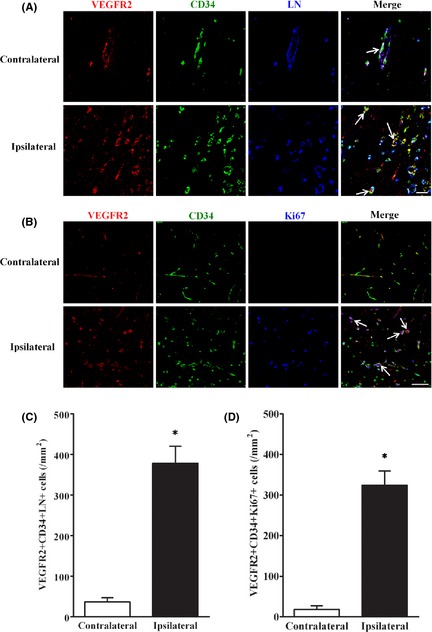
Most of the laminin‐positive cells were endogenous EPCs in the IBZ after pMCAO. (A) LN/VEGFR2/CD34 triple staining showed that most of the laminin (LN)‐positive cells in IBZ 7 days after pMCAO were EPCs (arrows). (B) Combination staining of antibodies against EPCs marker (VEGFR2/CD34) with a proliferation marker (Ki67) showed EPCs formed new vessels in IBZ 7 days after pMCAO. (C) Quantification of LN/VEGFR2/CD34 triple positive cells in IBZ 7 days after pMCAO. (D) Quantification of VEGFR2/CD34/Ki67 triple positive cells in IBZ 7 days after pMCAO. Scale bar = 20 μm in A‐B. n = 7–8/group. *P < 0.05, versus. contralateral side.
Involvement of Endogenous EPCs in the Neovascularization of IBZ via CXCR4/SDF‐1 axis after pMCAO
To clarify whether CXCR4/SDF‐1 axis is involved in EPC recruitment after pMCAO, we first determined the SDF‐1 protein level of IBZ in the brain of pMCAO rats. Western blotting showed that SDF‐1 expression in IBZ was increased 3 h after pMCAO as compared with the sham‐operated group and was further elevated at 6 h. The expression stayed at a higher level for 1 day and was then decreased slowly at day 3 and day 7 (Figure 5A). To further examine the role of CXCR4/SDF‐1 axis in EPC recruitment, we used CXCR4 antagonist AMD3100 to block the axis. The results exhibited that the CXCR4‐positive EPCs in IBZ after pMCAO were significantly decreased in AMD3100 group as compared with normal saline group (Figure 5B). Together, these data indicated that endogenous EPCs were recruited to the IBZ via CXCR4/SDF‐1 axis after pMCAO.
Figure 5.
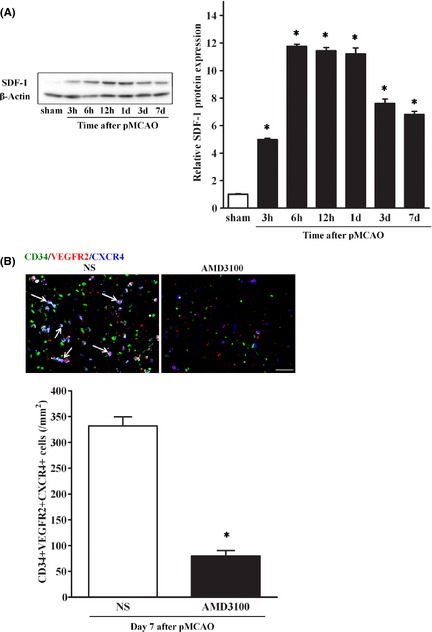
Recruitment of endogenous EPCs via CXCR4/SDF‐1 axis after pMCAO. (A) Western blotting showed SDF‐1 expression in IBZ after pMCAO, with β‐actin serving as an internal control. SDF‐1 expression was significantly increased 3 h after pMCAO, further elevated at 6 h, lasting for 1 day, and then decreased slowly at day 3 and day 7. n = 3–4/group. Data are expressed mean ± SD, *P < 0.05, versus. sham‐operated group. (B) After treatment with AMD3100, combination staining showed CXCR4‐positive EPCs in IBZ at day 7 after pMCAO were significantly less in AMD3100 group than in normal saline group. Scale bar = 20 μm. n = 5–6/group. *P < 0.05, versus. normal saline group.
Effects of Endogenous EPCs on Capillary Density, CBF, and outcome after pMCAO
After FITC‐dextran injection via tail vein, we examined the capillary density in IBZ after pMCAO. Consistent with the EPC recruitment, continuous AMD3100 treatment decreased the capillary density in IBZ, when compared with the normal saline‐treated rats after pMCAO (Figure 6A). Using a laser‐Doppler perfusion monitor to measure CBF, we found that the relative CBF in IBZ was significantly less in AMD3100 treatment group than in saline‐treated group (Figure 6B). We also evaluated the neurobehavioral functions after pMCAO, the result revealed that continuous AMD3100 treatment worsened the performance 9 days after pMCAO, as compared with the saline‐treated rats (Fig 6C). Collectively, these results indicated recruitment of EPCs could increase capillary density and CBF in IBZ and improve outcome after pMCAO.
Figure 6.
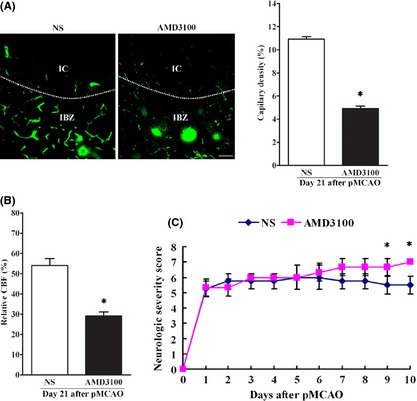
The effect of endogenous EPCs on capillary density, CBF in IBZ and neurobehavioral function after pMCAO. (A) After FITC‐dextran tail vein injection, capillary density was calculated by dividing the number of FITC pixels by the total number of pixels within the field of view in IBZ. IC, ischemic core. IBZ, ischemic boundary zone. The results showed that capillary density in IBZ was significantly less in AMD3100 group than in normal saline group after pMCAO. Scale bar = 20 μm. n = 7–8/group. *P < 0.05, versus. normal saline group. (B) The relative CBF in IBZ in each group. n = 7–8/group. *P < 0.05, versus. normal saline group. (C) Neurobehavioral function assessment revealed that continuous AMD3100 treatment worsened the performance 9 days after pMCAO, as compared with the saline‐treated rats. n = 7–8/group. *P < 0.05, versus. normal saline group.
Discussion
In the present study, we demonstrated that (i) neovascularization took place in IBZ of brain, and the change in the number of vessels followed a pattern mimicking that of laminin‐positive cells after pMCAO; (ii) apart from few endothelial cells, most of the laminin‐positive cells were endogenous EPCs, which were responsible for the neovascularization; (iii) endogenous EPCs were recruited to the IBZ of brain via CXCR4/SDF‐1 axis after pMCAO; (iv) recruited EPCs increased capillary density and CBF in IBZ and improved outcome after pMCAO. These findings indicated that the endogenous EPCs take part in neovascularization after pMCAO and have potential to be used as a target for the treatment for stroke.
Neovascularization plays an important role in the functional recovery of ischemic tissues 4, 17. An early autopsy study revealed angiogenesis in the IBZ of human brain 18. Angiogenesis genes and proteins such as VEGF, ang‐1, ang‐2, and so on, were found to be increased in ischemic areas for days to weeks 19, 20. However, the exact mechanism of the post‐stroke neovascularization is unclear. In the present study, we investigated the change in neovascularization over time in IBZ after pMCAO. The results showed that the vessels arose at day 3 after pMCAO and gradually increased, reaching a peak at day 14, and then merging into larger vessels. The change in the number of vessels paralleled that of laminin‐positive cells in the IBZ after pMCAO. Laminin is an extracellular matrix protein and combines with adhesion receptor of endothelial cells and glial cells to form the basement membrane 21. By combination staining, we found that laminin‐positive cells were neither glial cells, nor inflammatory cells, but endothelial cells and EPCs. Bone marrow‐derived endothelial progenitor cell (EPC) is angioblast that is believed to take part in the formation of the new blood vessels in CAD 4. Intravenously transplanted hEPC was capable of homing into ischemic areas of brain, promoting angiogenesis, and improving neurobehavioral outcome in MCAO mice 6. In the present study, we found that endogenous EPCs were involved in neovascularization of IBZ after pMCAO rats.
EPCs are immature hematopoietic cells circulating in peripheral blood. Studies showed that the number of circulating EPCs is reduced in various stroke risk factors, such as diabetes 15, hypertension 16. However, the level of cEPCs is increased after stroke and is negatively correlated with lesion volume and severity of the outcome 8, 22. These results demonstrated that endogenous EPCs are involved in occurrence and recovery of stroke. In this study, we found that endogenous EPCs were incorporated into newly formed vessels of IBZ after pMCAO. On the other hand, previous studies showed that recruited EPCs could also secret various growth factors and cytokines, such as VEGF, SDF‐1 16, and MMP‐9 4, to improve recruitment of EPCs and neovasularization. Apart from EPCs, we found that endothelial cells in IBZ, which may be from differentiated EPCs, and also from in situ endothelial cells, were also important component of neovascularization in IBZ. Thus, endothelial cells could be stimulated to serve therapeutic purpose after stroke.
Chemokines are important factors that regulate cellular migration. Stromal cell‐derived factor 1 (SDF‐1) is a key regulator of various CXCR4‐positive cells 9. CXCR4/SDF‐1 axis mediates the migration of endogenous neuroblasts and exogenous (transplanted) bone marrow‐derived cells to the ischemic area after stroke 23. After stroke, plasma SDF‐1 was increased and was strongly correlated with the number of cEPCs 8. Meanwhile, we found that SDF‐1 expression and EPCs in the IBZ were also increased. Previous studies documented that most of EPCs expressed CXCR4 receptors 4, 6, 9. After treatment with AMD3100, a CXCR4 antagonist 4, 24, recruitment of EPCs in IBZ was significantly decreased. Collectively, our data suggested that EPCs were mobilized and recruited to IBZ via CXCR4/SDF‐1 axis after stroke. We also showed that capillary density and CBF were significantly reduced in IBZ and neurobehavioral functions were significantly impaired in AMD3100 group as compared with normal saline group. Moreover, Chen et al. 15 found that recruited EPCs increased neurogenesis after stroke, which also contributed to improved outcome. These findings suggested that endogenous EPCs recruited to IBZ through SDF‐1/CXCR4 axis and improved outcome after pMCAO.
However, CXCR4 antagonist AMD3100 not only inhibited EPCs, but also might affect other cells expressing receptor CXCR4, such as inflammatory cells 25, neural progenitors 26. Huang et al. 25 showed that AMD3100 reduced leukocyte infiltration, attenuated brain edema, and improved outcome of stroke. The discrepancy between their finding and our result might be due to the fact that different phase was targeted after stroke. Huang et al. focused on the early phase after stroke and found that inflammatory cytokines and leukocytes were significantly increased within 3 day, and AMD3100 attenuated brain edema by inhibiting acute inflammatory response. In our study, we targeted the neovascularization in the subacute stage of stroke. Circulating EPCs were significantly increased at day 7 after ischemia 4. AMD3100 inhibited neovasularization and aggravated outcome by inhibiting the recruitment of EPCs. Therefore, determination of period for the treatment is of great importance.
To sum up, this study showed that endogenous EPCs partook in neovascularization via CXCR4/SDF‐1 axis after pMCAO, and mobilizing endogenous EPCs could be a treatment alternative for stroke.
However, our study also had some limitations. First, although endogenous EPCs were found in IBZ after pMCAO, we could not reach the conclusion that endogenous EPCs in IBZ are from circulating EPCs, as they might come from brain itself. Second, AMD3100 might affect other CXCR4‐expressing cells, so except EPCs, and neurobehavioral functions might be impaired by other mechanisms in AMD3100‐treated rats. Further studies are warranted to resolve these limitations.
Disclosures
The authors certify that this manuscript have not been published or submitted elsewhere.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81070938 to BH and No. 81101905 to LM), New Century Excellent Talents in University (NCET‐10‐0406 to BH), and the Fundamental Research Funds for the Central Universities, HUST (No. 2010JC028 to BH and No. 2011JC069 to LM).
The first two authors contributed equally to this work.
References
- 1. Liman TG, Endres M. New vessels after stroke: Postischemic neovascularization and regeneration. Cerebrovasc Dis 2012;33:492–499. [DOI] [PubMed] [Google Scholar]
- 2. Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 3. Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: From basic science to clinical applications. J Cereb Blood Flow Metab 2012;32:1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jujo K, Hamada H, Iwakura A, et al. Cxcr4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A 2010;107:11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oh IY, Yoon CH, Hur J, et al. Involvement of e‐selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 2007;110:3891–3899. [DOI] [PubMed] [Google Scholar]
- 6. Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long‐term stroke outcome in mice. Ann Neurol 2009;67:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Xiao X, Chen S, et al. Angiotensin‐converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy novelty and significance. Hypertension 2013;61:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bogoslovsky T, Spatz M, Chaudhry A, et al. Stromal‐derived factor‐1α correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 2011;42:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walter DH, Haendeler J, Reinhold J, et al. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res 2005;97:1142–1151. [DOI] [PubMed] [Google Scholar]
- 10. Jujo K, Ii M, Sekiguchi H, et al. Cxc‐chemokine receptor 4 antagonist amd3100 promotes cardiac functional recovery after ischemia/reperfusion injury via endothelial nitric oxide synthase–dependent mechanism clinical perspective. Circulation 2013;127:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Y, Hua Y, Liu W, Hu H, Keep RF, Xi G. Effects of cerebral ischemia on neuronal hemoglobin. J Cereb Blood Flow Metab 2009;29:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012;43:3063–3070. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Zhang ZG, Zhang R, et al. Adjuvant treatment with a glycoprotein iib/iiia receptor inhibitor increases the therapeutic window for low‐dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation 2003;107:2837–2843. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 2000;20:1311–1319. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Chen J, Chen S, et al. Transfusion of cxcr4‐primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice. PLoS ONE 2012;7:e50105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: Therapeutic perspective for ischemic stroke. CNS Neurosci Ther 2013;19:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ergul A, Alhusban A, Fagan SC. Angiogenesis a harmonized target for recovery after stroke. Stroke 2012;43:2270–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krupinski J, Kaluza J, Kumar P, Wang M, Kumar S. Prognostic value of blood vessel density in ischaemic stroke. Lancet 1993;342:742. [DOI] [PubMed] [Google Scholar]
- 19. Krupinski J, Issa R, Bujny T, et al. A putative role for platelet‐derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke 1997;28:564–573. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Xia Y, Wang Y, et al. Sonic hedgehog (shh) regulates the expression of angiogenic growth factors in oxygen–glucose‐deprived astrocytes by mediating the nuclear receptor nr2f2. Mol Neurobiol 2013;47:967–975. [DOI] [PubMed] [Google Scholar]
- 21. Simon‐Assmann P, Orend G, Mammadova‐Bach E, Spenlé C, Lefebvre O. Role of laminins in physiological and pathological angiogenesis. Int J Dev Biol 2011;55:455–465. [DOI] [PubMed] [Google Scholar]
- 22. Bogoslovsky T, Chaudhry A, Latour L, et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology 2010;75:2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez‐Ramos JC. The chemokine sdf‐1 is a chemoattractant for human cd34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of cd34+ progenitors to peripheral blood. J Exp Med 1997;185:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theiss HD, Vallaster M, Rischpler C, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the sdf‐1/cxcr4 axis. Stem Cell Res 2011;7:244–255. [DOI] [PubMed] [Google Scholar]
- 25. Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y. Cxcr4 antagonist amd3100 protects blood–brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke 2013;44:190–197. [DOI] [PubMed] [Google Scholar]
- 26. Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res 2004;76:20–34. [DOI] [PubMed] [Google Scholar]


