Summary
Aim
The objective of the study was to develop regenerative therapy by transplanting varied populations of dopaminergic neurons, differentiated from mouse embryonic stem cells (mES) in the striatum for correcting experimental parkinsonism in rats.
Methods
mES differentiated by default for 7 days in serum‐free media (7D), or by enhanced differentiation of 7D in retinoic acid (7R), or dopaminergic neurons enriched by manual magnetic sorting from 7D (SSEA−) were characterized and transplanted in the ipsilateral striatum of 6‐hydroxydopamine‐induced hemiparkinsonian rats. Neurochemical, neuronal, glial and neurobehavioral recoveries were examined.
Results
7R and SSEA− contained significantly reduced NANOG and high MAP2 mRNA and protein levels as revealed, respectively, by reverse transcriptase‐PCR and immunocytochemistry, compared with 7D. Striatal engraftment of 7D resulted in a significantly better behavioral and neurochemical recovery, as compared to the animals that received either 7R or SSEA−. The 7R transplanted animals showed improvement neither in behavior nor in striatal dopamine level. The grafted striatum revealed increased GFAP staining intensity in 7D and SSEA−, but not in 7R cells transplanted group, suggesting a vital role played by glial cells in the recovery. Substantia nigra ipsilateral to the side of the striatum, which received transplants showed more tyrosine hydroxylase immunostained neurons, as compared to 6‐hydroxydopamine‐infused animals.
Conclusion
These results demonstrate that default differentiated mixed population of cells are better than sorted, enriched dopaminergic cells, or cells containing more mature neurons for transplantation recovery in hemiparkinsonian rats.
Keywords: 6‐OHDA‐induced hemiparkinsonism, Default differentiation, Embryonic stem cells, Intrastriatal transplantation, Manual magnetic cell sorting, Nigral neuronal loss, Retinoic acid
Introduction
Parkinson's disease (PD) is a neurodegenerative disease, sporadic in nature, exhibiting severe movement dysfunctions by affecting the extrapyramidal system in the brain. The behavioral dysfunctions result from the death of dopamine synthesizing neurons from the A9, substantia nigra (SN) region of the basal ganglia that causes a substantial loss in the levels of dopamine in the terminal region, striatum. The most accepted mode of treatment is dopamine replacement by supplementing L‐3,4‐dihydroxyphenylalanine (L‐DOPA), the precursor of the neurotransmitter. Long‐term treatment of L‐DOPA causes severe drug‐induced dyskinesias prompting withdrawal of medication. Although there are a number of other medications available for the treatment of the disease, yet all of these are based on increasing the level of dopamine in the brain and are symptomatic in nature. Regenerative therapy is an option that aims at transplanting dopamine producing cells in the striatum for substantiating the availability of dopamine in this region. Although clinical trials using autologous transplantation of adrenal medullary chromaffin cells 1, 2 or fetal mesencephalic human tissue 3, 4 showed amazing positive results, yet there is no dopaminergic cell therapy existing for PD. A recent review pointed out several reasons for this state of affairs, such as lack of efficacy in strictly controlled trials, incidences of serious side effects including dyskinesias, inability of the grafted neurons to survive in the grafts, and the inefficacy of the procedure to control nonmotor symptoms of the disease, among several others 5. As certain factors that influence the outcome of transplantation are identified, it is time that dopaminergic cell therapy is given more impetus in defined areas of concerns, such as optimizing differentiation procedures for the generation of sufficient number of dopaminergic neurons from embryonic stem cells (ES) for use in transplantation studies.
Recently, we demonstrated substantial recovery in terms of behavior, striatal dopamine content, and SN dopaminergic neuronal population following intrastriatal transplantation of ES differentiated in the presence of serum‐free media in rotenone‐induced hemiparkinsonian rats 6. Considering the fact that a mixed population of cells are transplanted in that study, which provided astrocytosis and means for rejuvenating dying dopaminergic cells in SN probably by a retrograde transport of trophic factors from the striatum 6, it is a certain possibility that the undifferentiated cells may proliferate inside the host and cause propagation of cells, leading to tumor formation. Therefore, it was thought prudent to assess differentiation procedures that provide population of cells in varying stages of differentiation, or with more matured neurons, or with enriched dopaminergic neurons to examine transplantation recovery in an experimental model of PD.
Embryonic stem cells are primitive, undifferentiated cells derived from the inner cell mass of preimplanted blastocysts. Their unlimited proliferation potential and pluripotency make them an ideal source for generation of dopaminergic (DA‐ergic) neurons. There are many protocols by which these cells can be guided to differentiate toward a neuronal lineage, producing enriched populations of cells with DA‐ergic phenotypes 6, 7, 8, 9. Inconsistent outcomes of transplantation reported in PD models in rats result from limited survival and function of the ES‐derived DA neuronal grafts, which is probably due to mixed population of cells that are at varying stages of differentiation or dividing. With this major issue at the background, search began for strategies to find optimal levels of differentiating, differentiated, and mature neurons contained in a transplant. One of the several means described so far is transplantation of ES cultures matured for a period of 23–42 days, a method that resulted in better graft survival and behavioral recovery 10, 11. In this study, we designed two strategies over the default differentiation procedure to obtain (1) more mature neurons in the cell population and (2) an enriched population of dopaminergic neurons. We compared the recovery in 6‐hydroxydopamine (6‐OHDA)‐induced hemiparkinsonian rats in terms of apomorphine‐ or amphetamine‐induced unilateral rotational behavior, response in elevated body swing test, conditions of tyrosine hydroxylase (TH) immunoreactive neurons in SN and terminal fibers in the striatum, and the status of glial fibrillary acidic protein (GFAP)‐positive glia in the grafts.
Materials and Methods
Cell Maintenance and Differentiation
D3 mES line was purchased from ATCC (Manassas, VA, USA). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco‐Invitrogen, Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 2 mM glutamine (Gibco‐Invitrogen), and 1000 Units of nonessential amino acids (Invitrogen), 100 Units of penicillin and 100 μg streptomycin (Invitrogen), 0.7% β‐mercaptoethanol (MP Biomedicals, Solon, OH, USA) and 1000 Units of leukemia inhibitory factor (Millipore, Billerica, MA, USA). For differentiation, specific number of cells were plated in culture flasks in DMEM supplemented with 10% FBS, other constituents being the same as in routine culture. After 48 h, the medium was changed to Knockout‐DMEM (Gibco‐Invitrogen) supplemented with 10% Knockout serum replacement (Gibco‐Invitrogen) along with all the other components. Cells were maintained for 7 days postplating. For another group, the cells were grown in serum‐free condition and supplemented with retinoic acid (10 μM; Sigma‐Aldrich, St. Louis, MO, USA) every alternate day starting from day 2 and cells were processed on the 7th day of differentiation.
Cell Sorting
Cells differentiated for 7 days in serum‐free media were trypsinized to form a single cell suspension, washed three times with MACS buffer [0.1 M PBS, 2 mM EDTA (Sigma‐Aldrich), 0.05% bovine serum albumin (BSA; SRL, Mumbai, India)] and incubated at 4°C for 15 min with stage‐specific embryonic antigen (SSEA‐1) antibody conjugated with magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were then washed with MACS buffer and loaded onto a magnetic column attached to a strong magnet, MACS (Miltenyi Biotec). Elute collected was labeled as SSEA− and processed for further studies. After three times washing and collection of the elute, buffer was added to the column and it was removed from the magnet and using a piston the entire buffer was forcibly eluted out from the column into a separate tube, labeling the sample as SSEA+. This population was collected only for characterization studies.
Semi‐Quantitative PCR Analysis
Total RNA was isolated using TRI reagent (Sigma‐Aldrich), and 5 μg of the RNA was reverse‐transcribed using Mulv reverse transcriptase enzyme (Genei, Bangalore, India). The cDNA (25 ng) was used for PCR [HGPRT‐ 5′‐ CAGCGTCGTGATTAGTGATG‐3′,5′‐ CAGCAGGTCAGCAAAGAACT‐3′; NANOG‐5′‐ TTGGTTGGTGTCTTGCTCTTT‐3′,5′‐ CCTTGTTCTCCTCCTCCTCA‐3′; NESTIN‐5′‐ GGGAAGAGGGAGAGGAAGAA‐3′, 5′‐AGGGCAGTTACAAGAACATTAGC‐3′; MAP2‐5′‐ ACTTGGGACCTGGACGAGTA‐3′, 5′‐ AGACACAAGCCCATCCTAACA‐3′] for which the conditions were: initial denaturation at 94°C for 5 min, followed by 30 cycles of amplification reaction with denaturation at 94°C, annealing at 60°C and extension at 72°C for 20 s each. This was followed by a final extension at 72°C for 10 min. Equal amount of the product was electrophoresed on ethidium bromide (Sigma‐Aldrich) containing 1.5% agarose (Himedia, Mumbai, India) gel. Bands were visualized in a ChemiDoc XRS (Bio‐Rad, Hercules, CA, USA) gel documentation system, and densitometry was carried out using the software, Bio‐Rad version 4.6.0.
Immunocytochemistry
Cells postsorting were plated on poly‐l‐lysine‐coated coverslips in serum‐free media. In the cases of 7D and 7R groups, differentiated cells were trypsinized, passed through the MACS column, and plated on poly‐L‐lysine‐coated coverslips. Two days postplating, the cells were fixed with 4% paraformaldehyde. These were washed with PBS and permeabilized with 0.1% Triton X 100 for 5 min, washed with PBS, and blocked with 4% BSA for 30 min. This was followed by an overnight incubation with primary antibody (TH, NANOG, PITX3 and NURR1 antibodies were purchased from Abcam; MAP2 antibody was purchased from SantaCruz) at 4°C. The cells were extensively washed with PBS and incubated with fluorescence tagged secondary antibody (Alexa Fluor‐488,564; Invitrogen) for 1 h in the dark. The coverslips containing the cells were mounted on slides using an antifade mounting medium (Prolong Gold antifade reagent, Invitrogen), containing DAPI that stains nuclei, and were examined using an Epi‐fluorescence inverted microscope (Leica Microsystems, Solms, Germany) and photographed.
Animals
Adult male Sprague‐Dawley rats (250–300 g), obtained from the institute's animal facility, were used for this study. Animals were maintained under standard conditions of 12 h light/dark cycles, 22 ± 1°C temperature and 60 ± 5% humidity. Food and water were provided ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) that is appointed and authorized by the Committee for the Purpose of Control and Supervision of Experimentation in Animals (CPCSEA) of the Division of Animal Welfare, under the Ministry of Environment and Forests, Govt. of India.
Experimental paradigm
Animals were intracranially infused with 6‐OHDA (MP Biomedicals) to create hemiparkinsonian rat models. Only those animals showing more than three rotations per min following amphetamine (Sigma‐Aldrich) administration (2.5 mg/kg, intraperitoneally) on the 14th day postinfusion of 6‐OHDA were selected for the study. The animals were divided into four groups: control group with Hank's balanced salt solution (Gibco‐Invitrogen) infused into the striatum, mES differentiated for 7 days transplanted (7D) group, 7D cells free of the dividing cells (SSEA−) transplanted group, and mES differentiated with serum‐free media and retinoic acid (7R) transplanted group. Transplantation surgery was performed on the 18th day after 6‐OHDA infusion. The animals were then maintained for 2 weeks and analyzed for amphetamine‐ or apomorphine‐induced rotation studies 12, 13 elevated body swing test 6, 14 immunocytochemistry of glia and nigrostriatal dopaminergic neurons 15 and striatal DA levels 16.
6‐OHDA Infusion into the Median Forebrain Bundle
Rats were anesthetised with ketamine (80 mg/kg) and xylazine (8 mg/kg) administered intraperitoneally. They were placed in a flat skull position on a stereotaxic frame (Stoelting, Wooddale, IL, USA) with incisor bar fixed at 3.5 mm below the interaural line. 6‐OHDA (6 μg in 1 μL) dissolved in 0.01% ascorbic acid (SRL) was infused into the right Median Forebrain Bundle (MFB) at a flow rate 0.2 μL/min by employing a microinfusion pump consisting of a Worker Bee and Syringe pump (BAS, West Lafayette, IN, USA). After stopping the pump, the probe was kept in the same position for 5 min for complete diffusion of the drug, and then slowly retracted. Stereotaxic coordinates of antero‐posterior = −0.20 cm; lateral = 0.18 cm; and dorsoventral = −0.82 cm from the Bregma point were followed for reaching MFB in rats 17.
Cell Transplantation
Cells of three groups, 7D, SSEA−, and 7R were counted to make aliquots of 5 × 105 cells in 2 μL of Hank's balanced salt solution. These cells were then transplanted into the ipsilateral striatum of the 6‐OHDA‐lesioned animals, with rat stereotaxic coordinates, antero‐posterior = +0.02 cm; lateral = 0.26 cm; and dorsoventral = −0.50 cm from the Bregma point 17.
Amphetamine‐Induced Rotations
Rats were injected with 2.5 mg/kg dose of amphetamine intraperitoneally and placed in Perspex, transparent cylindrical cages (45 cm diameter and height). Ipsilateral rotations were recorded for a period of 2 h 12, 13. These rotations were carried out twice during the course of the experiment, first to screen the animals prior to transplantation on 14th day following 6‐OHDA infusion and second to assess recovery 2 weeks posttransplantation.
Apomorphine‐Induced Rotations
Similar to the amphetamine‐induced rotations, apomorphine‐induced (Sigma‐Aldrich) rotations were carried out on these animals 16th day posttransplantation. Animals were injected with apomorphine (0.5 mg/kg, subcutaneously), and the resulting rotations were counted for a period of 1 h 12, 13.
Elevated Body Swing Test
Animals were held 2 cm from the base of their tail and elevated to 4 cm above the surface of the table. Animals were held at 90° to the surface of the table, and this position was defined as no deviation, deviation of about 10° or less to either side was still considered as no deviation. A swing was recorded when the animals moved their head away from the vertical axis (angle >10°). Swings were recorded for 45 s 14. After each completed swing, the animals were made to return to the vertical position by letting their forelimbs touch the surface of the table. Results are expressed as the ratio of total number of contralateral swings: ipsilateral swings recorded for 45 s 6. This experiment was performed on the 36th day post 6‐OHDA infusion.
DA Measurement
Animals were sacrificed on the 37th day postlesioning, and each striatum was dissected out and collected in separate labeled tubes. The tissues were sonicated in ice‐cold HClO4 (0.1 M) containing 0.01% EDTA and kept on ice for 30 min, centrifuged at 18,000 × g for 10 min at 4°C, and 10 μL of the supernatant was injected into the chromatographic system (BAS). The sensitivity of the system was set at 200 nA range. The flow rate was set at 0.7 mL/min, and electrochemical detection was carried out at 0.74 V 16.
Immunohistochemistry
Animals were transcardially perfused with 4% paraformaldehyde (Sigma‐Aldrich) on the 37th day post 6‐OHDA lesioning of the brain. Whole brain was dissected out and kept in the fixative overnight and then transferred to 30% sucrose (SRL). Brain sections passing through the striatal graft were cut and rinsed in cold phosphate buffered saline (PBS; 0.1 M and pH 7.4) three times for 5 min each. The free‐floating sections were then permeabilized with 0.4% Triton X 100 (MP Biomedicals) for 30 min, blocked with 8% BSA and 0.1% Triton X 100, and incubated overnight with primary antibody (Tyrosine hydroxylase [1:500]; Glial fibrillary acidic protein [1:250]) at 4°C. After washing, horseradish peroxidase‐conjugated secondary antibody (1:500) was added and incubated for 1 h. Following PBS wash, these sections were developed with 3,3‐diaminobenzidine (MP Biomedicals) and taken onto gelatin (Sigma‐Aldrich) coated slides. These slides containing the stained sections were then rinsed in water, and then dehydrated in increasing concentrations of alcohol (50%–70%‐absolute), cleared in xylene and mounted in DPX (SRL). The permanent slides were then viewed under a microscope (Axiovert 200, Carl Zeiss, Oberkochen, Germany), and the photographs were taken.
Intensity of Staining and Cell Counting
Phase‐contrast images of the mounted slides were captured using the epi‐fluorescence inverted microscope. Intensity per unit area was calculated for immunostained cells in each group using ImageJ software (For reference see, http://www.unige.ch/medicine/ bioimaging/tricks / imagejtutorials/Quantification.pdf). Cells in the ipsilateral and contralateral substantia nigra were counted using ImageJ software (For reference see, http://www.unige.ch/medecine/bioimaging/tricks/imagejtutorials/CellCounting.pdf).
Statistical Measures
The data were evaluated for statistical significance employing Student's ‘t’ test. Results are given as Mean ± SEM. Value of P ≤ 0.05 was considered significant.
Results
Manually Magnetic‐Sorted Cells Contain Low Stem Cell, but Higher Levels of Neuronal Markers
The serum‐free media‐induced differentiated cells (7D) were sorted into two groups, the SSEA+ cell population and the SSEA− cell population. The former contained cells that possessed the SSEA‐1 antigen and hence were bound to the magnetic column. The latter consisted of all the cells that did not possess this antigen and hence were eluted out first, but contained very few dividing cells. To test the efficiency of the system, we did a semi‐quantitative PCR for stem cell marker, NANOG, neural progenitor cell marker, NESTIN and mature neuronal marker, microtubule‐associated protein2 (MAP2) (Figure 1). The 7R cells were also characterized for the same markers.
Figure 1.
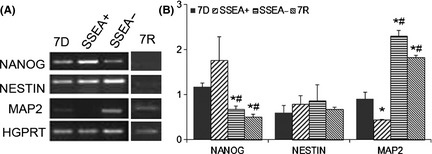
Characterization of serum‐free media‐induced differentiated cells; postsorting. (A) Representative images of agarose gels, showing stem cell marker, NANOG; neuronal markers NESTIN and microtubule‐associated protein (MAP2), with hypoxanthine guanine phosphoribosyl transferase (HGPRT) as a housekeeping gene. (B) Band intensity ratio for the candidate cDNA bands to that of HGPRT bands for the respective samples. 7D represents the serum‐free media‐induced differentiated murine embryonic stem (mES) cells, stage‐specific embryonic antigen positive (SSEA+) population represents the sorted out dividing cells from 7D cultures, and the SSEA negative (SSEA−) population is the elute, with very few dividing cells. 7R group consists of mES differentiated in serum‐free medium supplemented with retinoic acid. Results are given as Mean ± SEM, *P ≤ 0.05 versus 7D; # P ≤ 0.05 versus SSEA+.
Agarose gel images for NANOG, NESTIN, and MAP2 cDNA products from the 7D, SSEA+, SSEA−, and 7R groups are provided in Figure 1A. The band intensities of each gene, as normalized with the intensity of respective HGPRT bands, are provided in Figure 1B. The SSEA+ population contained cells with higher expression levels of NANOG, but was not significant statistically from 7D group (Figure 1B). The cells in SSEA− and 7R groups exhibited significantly less NANOG expression as compared to 7D and SSEA+ groups (Figure 1B). NESTIN did not show any change in either of the groups, but MAP2 expression was significantly high in SSEA− and 7R population of cells and low in SSEA+ population (Figure 1B), as compared to 7D cultures. MAP2 expression of the SSEA− and 7R population was significantly greater than SSEA+ and 7D groups (Figure 1B).
Immunocytochemistry for NANOG, NESTIN, and MAP2 proteins in these different groups showed similar expression as was seen for the mRNA levels. NANOG expression was absent in the 7R group, but in all the other groups, it was distinct (Figure 2A–D). In the 7D and the SSEA+ groups, the NANOG‐positive cells were seen to be present in rounded colonies (Figure 2A,B). All the groups showed Nestin expression; however, in the 7R group, it was less compared to the others (Figure 2A–D). SSEA− and the 7R groups showed increased expression for MAP2 as compared to the other two groups (Figure 2E–H).
Figure 2.
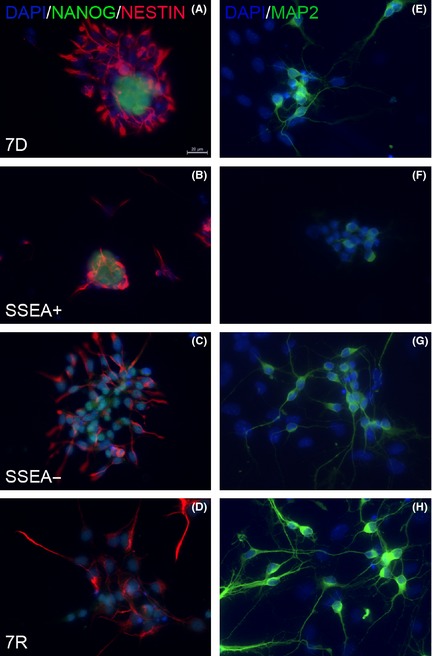
Immunostaining for stem cell marker NANOG, neuronal progenitor cell marker NESTIN, and neuronal marker MAP2. Co‐immunolabeling for NANOG and NESTIN revealed the presence of both population of cells in 7D (A), SSEA+ (B), SSEA− (C) and 7R (D). NANOG expression was least in the 7R group, while NESTIN was least in the SSEA+ group. MAP2 staining was high in the SSEA− (G) and the 7R (H) group as compared to the 7D (E) and SSEA+ (F) groups. Scale bar 20 μm given in A applies to every other image.
Transplantation of Mixed Cells, but not Enriched Dopaminergic Cells Protects against Amphetamine‐Induced Ipsilateral Rotations in Unilateral Parkinsonian Rats
The 6‐OHDA‐lesioned animals without any cell grafts showed high, mean ipsilateral rotations (457 ± 175) following amphetamine administration due to release of DA in the contralateral striatum, which is known in literature 12, 13, 18. Transplantation of 7D cells was able to bring down the number of ipsilateral rotations caused by amphetamine administration to 97 ± 56 rotations, which was significantly less compared with the 6‐OHDA control group. The SSEA− cell‐transplanted group was also able to significantly reduce the number of ipsilateral rotations following the treatment of amphetamine compared with 6‐OHDA‐lesioned animals with the mean values being 67 ± 35. The 7R group, however, did not show any significant change in the number of amphetamine‐induced rotations (Figure 3A).
Figure 3.
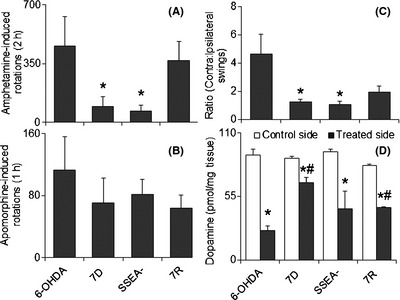
Posttransplantation effects on behaviors and striatal DA levels. (A) Amphetamine‐induced ipsilateral rotations were significantly brought down by 7D and SSEA cell transplantation. (B) Apomorphine‐induced contralateral rotations were not affected in any of the groups posttransplantation, compared to 6‐OHDA control. (C) Elevated body swing test showed a higher contralateral:ipsilateral swing ratio, which was significantly brought down, close to 1, by the 7D and SSEA cell‐grafted groups. Results are shown as mean ± SEM, *P ≤ 0.05 versus 6‐OHDA group. (D) Transplantation of 7D and 7R cells in the ipsilateral striatum of 6‐OHDA‐infused rats increased striatal DA levels significantly as compared to 6‐OHDA control group. Results are shown as Mean ± SEM, *P ≤ 0.05 versus control side, # P ≤ 0.05 versus ipsilateral side of 6‐OHDA group. Abbreviations are as in legends to Figure 1.
Apomorphine, a DA receptor agonist, acts on the postsynaptic receptors. Due to fall in striatal DA levels in the 6‐OHDA‐lesioned animals, a severe supersensitivity of the postsynaptic receptors is developed with time 12, 18, 19. Therefore, these hemiparkinsonian animals respond with contralateral rotations in response to apomorhine administration in them. In our study, vehicle‐infused control animals showed a higher number of contralateral rotations. None of the cell‐transplanted groups were able to bring down the number of contralateral rotations significantly (Figure 3B).
Transplantation of Mixed Cells and Enriched Dopaminergic Cells Protect Against Unilateral Bias in Elevated Swings in Experimental Parkinsonism in Rats
Elevated body swing test is a nondrug‐induced behavioral parameter. A high contralateral to ipsilateral swing, 4.6 ± 1.4, in the 6‐OHDA‐lesioned animals was observed, corresponding to earlier reports 6, 14. In the transplanted groups, the results were similar to that of the amphetamine‐induced behavior; that is, while 7D and SSEA− population showed a ratio close to one (1.3 ± 0.2 and 1.1 ± 0.3, respectively), 7R group showed a little higher value (2.0 ± 0.4). Elevated body swing ratio in 7D and SSEA− cell‐transplanted groups were significantly less compared to vehicle infused, 6‐OHDA‐lesioned animals (Figure 3C).
Default Differentiated Mixed Cells, but not Enriched Dopaminergic Cells Protect Against 6‐OHDA‐Induced Striatal Dopamine loss
The animals were sacrificed on the 18th day posttransplantation. 6‐OHDA‐infused animals displayed a significant loss of DA levels in the ipsilateral striatum, as compared to the contralateral side. There was a reduction of about 72% in the striatal DA content in the treated side of vehicle‐infused striatum, compared with the contralateral side (Figure 3D). Cell transplantation was able to bring down the percent reduction values to about 23%, 52%, and 44% in 7D, SSEA− and 7R groups, respectively. Increase in striatal DA content was significant in 7D and 7R cell‐transplanted groups with respect to the vehicle‐infused control (6‐OHDA group), while SSEA− population though showed a high DA level, yet it was not statistically different from the control (Figure 3D).
Mature Neurons Fail, but Default Differentiated Mixed Cells and Enriched Dopaminergic Cells Transplantation in to Striatum Protect Dopaminergic Terminals
Infusion of 6‐OHDA into MFB showed complete loss of TH immunoreactivity in the ipsilateral striatum of the animals (Figure 4A). Faint TH staining was seen in the ipsilateral dorsal striatum of 7D grafted animals (Figure 4C) as is seen in the magnified image (Figure 4D). SSEA− cells transplanted group also did not show any TH‐positive cells in the striatal graft, however here also there was some TH immunoreactivity observed in the ipsilateral striatum, but outside the graft (Figure 4E,F). No staining for TH was observed in the 7R cell grafts in the striatum (Figure 4G,H).
Figure 4.
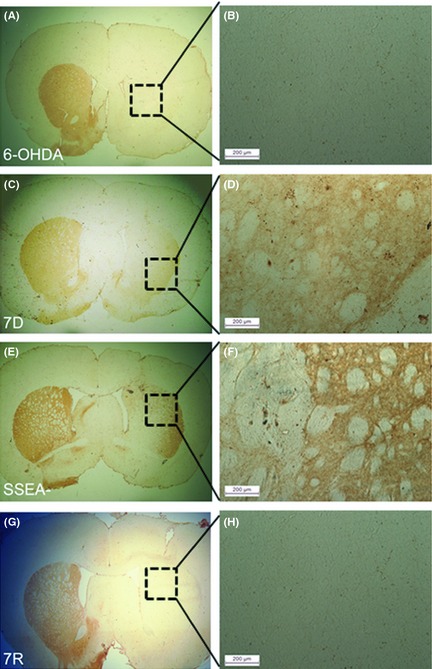
Tyrosine hydroxylase (TH) immunoreactivity in the striatum. (A) Ipsilateral side of the 6‐OHDA control group showed no TH staining in the dorsolateral striatum, and (B) shows the magnified image of the dotted square in the image, A. Striatal sections from rat brains transplanted with cells from mES differentiated for 7 days (7D) (C); 7D cells free of the dividing cells (SSEA−) (E). mES differentiated with serum‐free media and retinoic acid (7R) (G) did not show the presence of TH neurons in the graft. Magnification 4×. Magnified images of the area within the dotted squares of A, C, E and G, that is, B, D, F and H, respectively, showed the presence TH‐stained fibers in the ipsilateral striatum of 7D (D) and SSEA− (F) cell‐transplanted groups (Scale bars: 200 μm).
Striatal Transplantation of Differentiated mES Causes Glial Cell Proliferation in the Graft Site
Immunohistochemical analyses for an astrocyte marker, glial fibrillary acidic protein (GFAP) in the ipsilateral striatum of the transplanted groups was carried out (Figure 5). 7D (Figure 5A–D), SSEA− (Figure 5E–H) and 7R (Figure 5I–L) cell‐transplanted groups showed definite and sharp staining for GFAP. In the 7D and SSEA− cell‐transplanted animals, GFAP staining was seen around the graft in the former group (Figure 5A,B), whereas in the latter group the staining was visible in the grafts too (Figure 5E,F). The 7R group exhibited intense GFAP throughout the ipsilateral striatum (Figure 5I,J). The intensity of GFAP staining for each of the treatment group is measured using ImageJ software. The average staining per unit area was found to be 106 ± 0.13, 112 ± 0.09, and 69 ± 0.17 for 7D, SSEA−, and 7R cell‐transplanted groups, respectively (Figure 5). SSEA− exhibited a significantly higher intensity staining compared with 7D, but 7R was with significantly lower staining intensity than 7D or SSEA−. The value for each group is found to be statistically significant as compared to the other two groups (Figure 6).
Figure 5.
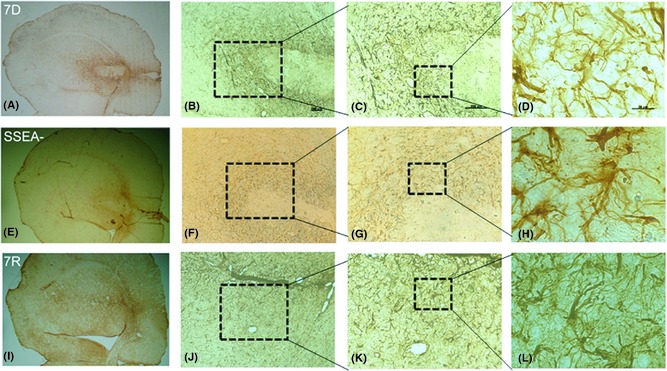
Immunolabeling for glial fibrillary acidic protein (GFAP), a marker for astrocytes. Striata of mES differentiated for 7 days transplanted (7D) group (A–D), 7D cells free of the dividing cells (SSEA−) transplanted group (E–H) and mES differentiated with serum‐free media and retinoic acid (7R) group (I–L) stained intensely for GFAP implying astrocytosis. Scale bar for each column is provided in the first image of the same column in B, C, D, which, respectively, are 200 μm, 200 μm, and 20 μm. Images A, E and I are 4× magnification.
Figure 6.
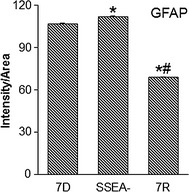
Glial fibrillary acidic protein (GFAP), staining intensity in the grafted striatum. The average GFAP staining intensity was calculated per stained cells and structures for a given area under magnification as shown in Figure 5C, G, and K. The results are represented as intensity per unit area. The three bars represent: mES differentiated for 7 days transplanted group (7D), 7D cells free of the dividing cells transplanted group (SSEA−) and mES differentiated with serum‐free media and retinoic acid transplanted group (7R). Results are given as Mean ± SEM, *P ≤ 0.05 versus 7D; # P ≤ 0.05 versus SSEA‐.
Differentiated mES Transplantation in the Striatum Saves DA‐ergic Neurons of the Substantia Nigra
The ipsilateral SN of 6‐OHDA‐infused control rats showed complete loss of TH‐positive cells (Figure 7A). In the transplanted groups that received 7D, SSEA− and the 7R cells, distinct TH immunostaining was visible (Figure 7B,C,D). The staining, however, was more in case of 7D and SSEA− cell‐implanted groups (Figure 7B,C). The contralateral side of all the groups stained intensely for TH. The percentage cell loss in the ipsilateral SN as compared to the contralateral SN was 88% in the 6‐OHDA control rats, 52% in the 7D cell‐transplanted group, 47% in the SSEA− cell‐transplanted group, and 60% in the 7R cell‐transplanted group (Figure 7E). The difference was not significant between the cell‐transplanted groups, but each was significantly less from the 6‐OHDA control group.
Figure 7.
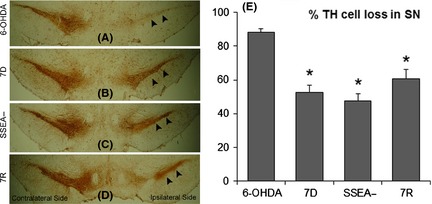
Tyrosine hydroxylase (TH) immunohistochemistry images of midbrain coronal sections, passing through substantia nigra (SN). Significant loss of TH immunoreactivity of the dopaminergic neurons in SN, ipsilateral to the side of 6‐OHDA infusion (A; arrowheads) is seen as compared to the contralateral side. 7D (B: arrowheads), SSEA− (C: arrowheads) and 7R (D: arrowheads) cells transplanted into the ipsilateral striatum, caused retention of substantial immunoreactive cells within the SN, ipsilateral to the side of 6‐OHDA infusion into the median forebrain bundle. Contralateral side showed distinct staining SN neurons for all the groups (A–D). Magnification 4×. There appeared a significant percentage of improvement in the TH‐positive neurons in the ipsilateral SN relative to the contralateral side (E) in the 7D, SSEA‐ and 7R cell‐transplanted groups. Results are presented as Mean ± SEM. *P ≤ 0.05, as compared to 6‐OHDA control group. Sections from three different brain samples were considered for each group.
Discussion
The major outcomes of the present study are: (i) successful, but partial segregation of the proliferating stem cells from the differentiated neurons, as clearly indicated by lowered presence of NANOG, and higher levels of MAP2 in the MACS separated cells, (ii) the failure of SSEA− neuronal transplantation group to provide any better protection to the 6‐OHDA‐lesioned animals than the rats transplanted in the striatum with mixed population of differentiated cells (7D), and (iii) the complete failure to make any recovery in behavioral or neurochemical parameters following transplantation with mature cells differentiated with default process and by retinoic acid treatment (7R).
SSEA‐1 is expressed on the surface of early mouse embryos, murine embryonic carcinoma cells, mES, as well as in mouse and human germ cells 20, 21. The expression of this antigen is down‐regulated following differentiation of cells 21. Sorting out the SSEA+ cell population from the mixed cell cultures of 7D serum‐free media‐induced differentiated cells ensured a reduction in the expression of NANOG in the SSEA free cultures, labeled as SSEA− cells. NANOG is a transcription factor required for the formation and/or maintenance of the inner cell mass during mouse preimplantation development and for self‐renewal of pluripotent ES 22, 23 and is found to be down‐regulated following default differentiation 6. Hence, decreased expression of NANOG in the SSEA− cultures denotes a reduction in the population of undifferentiated cells. An increased expression of MAP2 in the SSEA− population suggested that sorting out the undifferentiated cell population increased the percentage of neuronal population in the remaining cells. However, some NANOG expression in this group was still discernable; the reason for which could be that some dividing cells remained unbound to beads during the labeling procedure and hence could not be retained in the column. As SSEA− population contained only a few ES cells, we thought it prudent to examine transplantation recovery following grafting of these cells in a rodent model of PD.
6‐Hydroxydopamine is a specific neurotoxin for DA‐ergic neurons 24 and is commonly used for modeling experimental parkinsonism 12, 13, 18, and in transplantation studies in PD 25, 26, 27, 28, 29. Infusion of this toxin into MFB causes lesioning of the nigrostriatal tract, damaging both the neuronal perikarya in the SN and their terminals in the striatum 30 resulting in a severe and dose‐dependent loss of striatal DA levels, more than caused by rotenone or MPP+ unilateral infusion in rats 12. In our study, we found that infusion of 6‐OHDA unilaterally into MFB followed by infusion of HBSS in the ipsilateral striatum, displayed the reported unlilateral bias in behaviors, both drug‐induced and otherwise. The ipsilateral striatum contained only about 25% of DA of the contralateral side. Analysis of the midbrain A9 substantia nigra and the striatum revealed the loss of TH‐positive cell bodies and DA‐ergic terminals in the ipsilateral side as shown by TH immunoreactivity. This confirmed the efficiency of our experimental animal model system and also showed that the vehicle used in suspending the cells for transplantation did not have any effect on the outcome of the study.
In our previous study, characterization of serum‐free media‐induced differentiated ES cell (7D) showed the presence of TH‐positive cells in vitro; and transplantation of these cells into the unilateral rotenone‐induced parkinsonian rats showed improvement in amphetamine‐induced rotations, recovery in nigral neuronal population, and striatal DA levels 6. In the present study, the effect of transplantation of these cells in 6‐OHDA‐infused animal model of parkinsonism is examined. The results demonstrate that 7D cells graft is able to significantly reduce amphetamine‐induced rotations and increase the striatal DA content significantly in the grafted animals. Reduction in the rotations could be attributed to the release of DA by the grafted cells, although there is a lack of extensive immunoreactivity for TH in the graft. TH staining was seen only in some fibers of the ipsilateral striatum, and therefore, it is suggested that the improvement in behavior could be resulting from protection of some of the DA‐ergic neurons of the nigra, similar to our previous study 6. It can also be speculated that 7D transplantation results in the sprouting of DA terminals in the striatum by increasing the synthesis and release of glial cell line derived neurotrophic factor (GDNF), as demonstrated by us earlier 6. This speculation is supported by the immunostaining for GFAP in the transplant as well as in the host tissues of the striata. High intensity of GFAP staining in 7D suggests a probable increase in the release of GDNF and cytokines that result in neuronal sprouting. In human PD brain, long‐term, continuous intraputaminal infusions of GDNF results in sprouting of neurites in the DA terminal region 31, 32, 33. Moreover, neural stem/progenitor cells are known to protect against experimental parkinsonism through secretion of neurotrophic factors 34, 35, 36. Similar recovery was also seen for the SSEA− cell‐transplanted group. Transplantation of these cells significantly reduced the number of amphetamine‐induced rotations. The ratio of contralateral to ipsilateral swings, in elevated body swing test was close to one. Although there was an increase in the DA content of the striatum, there was a lot of variation in this group due to which the values were not statistically different from the sham group. As varying the ratio of different cell types in the graft is known to change the effects of transplantation observed in animals 37, we anticipate that this difference in striatal DA content could be due to the difference in the ratio of undifferentiated to differentiated neurons in the grafted population.
Surprisingly, the last group that contained the mES differentiated in serum‐free media along with retinoic acid supplementation (7R) did not show recovery in any of the parameters studied. The amphetamine‐induced rotations, although lesser compared to sham treated animals, was not significantly different from the same. 7R cell‐transplanted group showed a reduction in the contralateral to ipsilateral swing ratio, in elevated body swing test; the ratio here was close to one (1.28) but the difference was not statistically different from the sham control transplanted animals. TH immunohistochemistry showed no staining either in the grafted cells or in the striatal DA‐ergic terminals. Lack of staining of the terminals indicates want of sprouting and is indicated by low intensity staining for GFAP for this group. Previous report by Brederlau et al. 11 showed that mature neurons survive transplantation poorly than do immature neurons. In another study, a similar finding was reported, wherein mid‐stage neurons (i.e., the ones that are not terminally differentiated) are shown to be the most ideal stage of differentiation for transplantation studies, as their survival rate is high 38.
None of the groups could significantly reduce the apomorphine‐induced rotations in rats. We consider this to be due to our experimental paradigm wherein we conduct the behavioral studies 2 weeks posttransplantation. This, we suppose is a short time to revert the super‐sensitivity in the postsynaptic neurons, due to which the dopamine agonist causes contralateral rotations in the hemiparkinsonian animals 12, 19. Two weeks posttransplantation is a very short period to determine whether the mode of recovery is due to rebuilding of neuronal network or due to survival of neurons. However, reduced loss of SN TH‐positive cells indicates that cell transplantation has played some role in the process.
Conclusions
This study demonstrates beyond doubt that a mixed population of differentiated cells is more ideal for transplantation recovery in PD animals. It may also be concluded that SSEA− population is also favorable for engraftment studies, as these are capable of showing behavioral revival in 6‐OHDA‐lesioned animals. However, further study is warranted to examine the potential of these cells to develop tumor in the host brain. This study also confirms the earlier findings that population of differentiated neurons containing a high percentage of mature neurons are not ideal for transplantation‐induced recovery in animal models of PD. The results of this study suggest that mixed population of differentiated neurons with moderate population of dopaminergic progenitors provide neuroprotection and behavior revival in experimental parkinsonism in rats. When the percentage of mature neurons is high in the transplant, the transplantation recovery is found to be slim. These findings have direct significance on dopamine cell replacement therapy in Parkinson's disease, for which no cure is currently available.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
D.T. and P.V. received research fellowships from the Council of Scientific and Industrial Research (CSIR), Govt. of India. M. B. received fellowship from University Grants Commission, Govt. of India. D.N.N‐N. received fellowship from TWAS‐DBT (The Third World Academy of Sciences and Department of Biotechnology, Ministry of Science and Technology, Govt. of India). The study was funded by the project ‘Neurodegenerative disorders: Causes and corrections’ (miND) under CSIR's 12th five year plan program. D.T. also worked in this project as a project fellow.
References
- 1. Backlund EO, Granberg PO, Hamberger B, et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg 1985;62:169–173. [DOI] [PubMed] [Google Scholar]
- 2. Goetz CG, Olanow CW, Koller WC, et al. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson's disease. N Engl J Med 1989;320:337–341. [DOI] [PubMed] [Google Scholar]
- 3. Spencer DD, Robbins RJ, Naftolin F, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson's disease. N Engl J Med 1992;327:1541–1548. [DOI] [PubMed] [Google Scholar]
- 4. Kordower JH, Freeman TB, Snow BJ, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med 1995;332:1118–1124. [DOI] [PubMed] [Google Scholar]
- 5. Lindvall O. Developing dopaminergic cell therapy for Parkinson's disease – give up or move forward? Mov Disord 2013;28:268–273. [DOI] [PubMed] [Google Scholar]
- 6. Tripathy D, Haobam R, Nair R, Mohanakumar KP. Engraftment of mouse embryonic stem cells differentiated by default leads to neuroprotection, behaviour revival and astrogliosis in parkinsonian rats. PLoS ONE 2013;8:e72501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ko JY, Park CH, Koh HC, et al. Human embryonic stem cell‐derived neural precursors as a continuous, stable, and on demand source for human dopamine neurons. J Neurochem 2007;103:1417–1429. [DOI] [PubMed] [Google Scholar]
- 8. Park CH, Minn YK, Lee JY, et al. In vitro and in vivo analyses of human embryonic stem cell‐derived dopamine neurons. J Neurochem 2005;92:1265–1276. [DOI] [PubMed] [Google Scholar]
- 9. Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 2004;101:12543–12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC‐derived neural cells in a primate model of Parkinson's disease. Stem Cells 2012;30:935–945. [DOI] [PubMed] [Google Scholar]
- 11. Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell‐derived cells to a rat model of Parkinson's disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006;24:1433–1440. [DOI] [PubMed] [Google Scholar]
- 12. Sindhu KM, Banerjee R, Senthilkumar KS, et al. Rats with unilateral median forebrain bundle, but not striatal or nigral, lesions by the neurotoxins MPP+ or rotenone display differential sensitivity to amphetamine and apomorphine. Pharmacol Biochem Behav 2006;84:321–329. [DOI] [PubMed] [Google Scholar]
- 13. Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6‐hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 1970;24:485–493. [DOI] [PubMed] [Google Scholar]
- 14. Borlongan CV, Sanberg PR. Elevated body swing test: A new behavioural parameter for rats with 6‐OHDA induced parkinsonism. J Neurosci 1995;15:5372–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and micraglia, and reduces serotonin metabolism in 3‐Nitropropionic acid‐induced rat model of Huntington's disease. CNS Neurosci Ther 2014;20:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muralikrishnan D, Mohanakumar KP. Neuroprotection by bromocriptine against 1‐methyl‐4‐ phenyl‐1,2,3,6‐tetrahydropyridine‐induced neurotoxicity in mice. FASEB J 1998;12:905–912. [DOI] [PubMed] [Google Scholar]
- 17. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th edn New York: Academic Press, 1982. [Google Scholar]
- 18. Hudson JL, van Horne CG, Stromberg I, et al. Correlation of apomorphine‐ and amphetamine‐induced turning with nigrostriatal dopamine content in unilateral 6‐hydroxydopamine lesioned rats. Brain Res 1993;626:167–174. [DOI] [PubMed] [Google Scholar]
- 19. Ungerstedt U. Postsynaptic supersensitivity after 6‐hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol Scand Suppl 1971;367:69–93. [DOI] [PubMed] [Google Scholar]
- 20. Damjanov I, Fox N, Knowles BB, Solter D, Lange PH, Fraley EE. Immunohistochemical localization of murine stage‐specific embryonic antigens in human testicular germ cell tumors. Am J Pathol 1982;108:225–230. [PMC free article] [PubMed] [Google Scholar]
- 21. Solter D, Knowles BB. Monoclonal antibody defining a stage‐specific mouse embryonic antigen (SSEA‐1). Proc Natl Acad Sci USA 1978;75:5565–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003;113:643–655. [DOI] [PubMed] [Google Scholar]
- 23. Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379–391. [DOI] [PubMed] [Google Scholar]
- 24. Mendez JS, Finn BW. Use of 6‐hydroxydopamine to create lesions in catecholamine neurons in rats. J Neurosurg 1975;42:166–173. [DOI] [PubMed] [Google Scholar]
- 25. Blandini F, Cova L, Armentero MT, et al. Transplantation of undifferentiated human mesenchymal stem cells protects against 6‐hydroxydopamine neurotoxicity in the rat. Cell Transplant 2010;19:203–217. [DOI] [PubMed] [Google Scholar]
- 26. Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6‐OHDA‐lesioned rats. Stem Cells Dev 2010;19:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martínez‐Cerdeño V, Noctor SC, Espinosa A, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6‐OHDA‐lesioned rats. Cell Stem Cell 2010;6:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line‐derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci 2001;21:8108–8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakao N, Yokote H, Nakai K, Itakura T. Promotion of survival and regeneration of nigral dopamine neurons in a rat model of Parkinson's disease after implantation of embryonal carcinoma‐derived neurons genetically engineered to produce glial cell line‐derived neurotrophic factor. J Neurosurg 2000;92:659–660. [DOI] [PubMed] [Google Scholar]
- 30. Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6‐hydroxydopamine‐treated rats: Possible role of GDNF. J Neurochem 2003;85:299–305. [DOI] [PubMed] [Google Scholar]
- 31. Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line–derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 2005;11:703–704. [DOI] [PubMed] [Google Scholar]
- 32. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line‐derived neurotrophic factor. J Neurosurg 2005;102:216–222. [DOI] [PubMed] [Google Scholar]
- 33. Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell linederived neurotrophic factor in Parkinson disease. Nat Med 2003;9:589–595. [DOI] [PubMed] [Google Scholar]
- 34. Goldman S. Stem and progenitor cell‐based therapy of the human central nervous system. Nat Biotechnol 2005;23:862–871. [DOI] [PubMed] [Google Scholar]
- 35. Rafuse VF, Soundararajan P, Leopold C, Robertson HA. Neuroprotective properties of cultured neural progenitor cells are associated with the production of sonic hedgehog. Neuroscience 2005;131:899–916. [DOI] [PubMed] [Google Scholar]
- 36. Jung CG, Hida H, Nakahira K, Ikenaka K, Kim HJ, Nishino H. Pleiotrophin mRNA is highly expressed in neural stem (progenitor) cells of mouse ventral mesencephalon and the product promotes production of dopaminergic neurons from embryonic stem cell‐derived nestinpositive cells. FASEB J 2004;18:1237–1239. [DOI] [PubMed] [Google Scholar]
- 37. Carlsson T, Carta M, Muñoz A, et al. Impact of grafted serotonin and dopamine neurons on development of L‐DOPA‐induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain 2009;132:319–335. [DOI] [PubMed] [Google Scholar]
- 38. Ganat YM, Calder EL, Kriks S, et al. Identification of embryonic stem cell‐derived midbrain dopaminergic neurons for engraftment. J Clin Invest 2012;122:2928–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]


