Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease with high morbidity and mortality. Cerebral vasospasm, which develops in 3–12 days after aneurysm rupture, has traditionally been considered the major cause of poor outcome post‐aSAH. However, clinical trials demonstrated that antivasospastic drugs, such as clazosentan, failed to improve the neurofunctional outcomes of patients with aSAH 1, indicating that a complex etiology is involved in the pathogenesis of delayed ischemic neurological deficits (DINDs). Therefore, further elucidation of the pathogenic mechanisms and development of novel effective drugs is of great importance for aSAH treatment.
Recently, extensive research has focused on early brain injury (EBI), which occurs within the first 72 h after aSAH. In addition to mechanical damage, hypoxia, and molecular alterations (e.g., NO pathway dysfunction, oxidative stress), EBI can result from inflammation. Importantly, cerebral edema is a major risk factor for early death and bad outcome following aSAH. Additionally, disruption of blood brain barrier (BBB) and inflammation exacerbates the development of EBI by promoting cerebral edema formation. Matrix metalloproteinase 9 (MMP‐9) is involved in the degradation of tight junction proteins and microvascular basal lamina proteins, leading to BBB breakdown and brain edema. Thus, suppressing the inflammatory response and MMP‐9 activity is vital for the alleviation of EBI in aSAH.
Resveratrol (RVS) (3,4′,5‐trihydroxystilbene), a natural polyphenol, exerts protective effects against multiple neurological injuries in animal models such as stroke, traumatic brain injury, and spinal cord injury 2, 3, 4. The beneficial effects of resveratrol in central nervous system injuries are associated with its anti‐oxidant, antiinflammatory, and antiapoptotic properties 3. In particular, the inhibitory action of resveratrol on NF‐κB has been demonstrated in experimental diabetic neuropathy 5. NF‐κB inhibition can result in downregulation of MMP‐9 expression and suppression of the inflammatory response post‐SAH 6. However, although the antivasospastic effect of resveratrol in experimental SAH has been reported 7, no experimental studies to date have focused on the effect of resveratrol on BBB disruption and inflammation after SAH. In our study, we aimed to evaluate whether resveratrol could improve neurological outcomes through inhibition of the NF‐κB‐dependent inflammatory/MMP‐9 pathway after SAH.
In this study, aSAH model was induced using a perforation method in sixty Sprague–Dawley rats. Then, resveratrol (30 mL/kg) or normal saline (30 mL/kg) was administered intraperitoneally immediately and again at 6 h after SAH. We found that resveratrol reduced brain edema and promoted functional recovery by preventing BBB disruption after SAH. Further, post‐SAH resveratrol treatment inhibited the activation of NF‐κB, which leads to the downregulation of MMP‐9 expression and inhibition of inflammation, contributing to maintenance of BBB integrity.
The BBB is a highly specialized structure of the brain microvascular system, and the integrity of BBB is essential for maintaining cerebral microenvironment homeostasis and neural function. Matrix metalloproteases (MMPs), a family of zinc endopeptidases, can degrade interendothelial tight junction proteins and basal lamina proteins of the BBB. Accumulating evidence has demonstrated that MMP‐9 participates in the early BBB disruption and cerebral edema formation after aSAH 8. Increased activity of NF‐κB has also been observed in both early and late stages of SAH. In addition to orchestrating the inflammatory cascade, NF‐κB is known to directly regulate MMP‐9 transcription. Thus, inhibition of NF‐κB activity results in decreased MMP‐9 expression and suppressed inflammatory response, which could attenuate BBB breakdown and brain edema after SAH.
Consistent with a previous study 8, BBB disruption and brain edema were observed in rats subjected to 24 h of SAH in our study (Figure 1 D,F). This process was inhibited by resveratrol treatment following SAH, indicating the protective effects of resveratrol on BBB integrity. Under physiological conditions, the heterodimer NF‐κB, which consists of three subunits (p65, p50, and IκB‐α), exists in the cytoplasm in an inactive state. Inhibitory kappa B (IκB‐α), one subunit of NF‐κB, plays an important role in maintaining the inactivation of NF‐κB. Various stimuli (e.g., ROS, TNF‐α) can induce the activation of inhibitory kappa kinases (IKK), resulting in phosphorylation of IκB‐α and proteasomal degradation of NF‐κB. Then, the p65 subunits of NF‐κB are released from the heterodimer and translocate into nucleus, where they can promote transcription of cytokines (e.g., TNF‐α, IL‐1β, and IL‐6) and MMP‐9. Consistent with the previous research 5, 9, our results also showed that resveratrol inhibited the nuclear translocation of p65 from cytoplasm, suggesting an inhibitory effect of resveratrol on NF‐κB activity post‐SAH (Figure 2 C–F).
Figure 1.
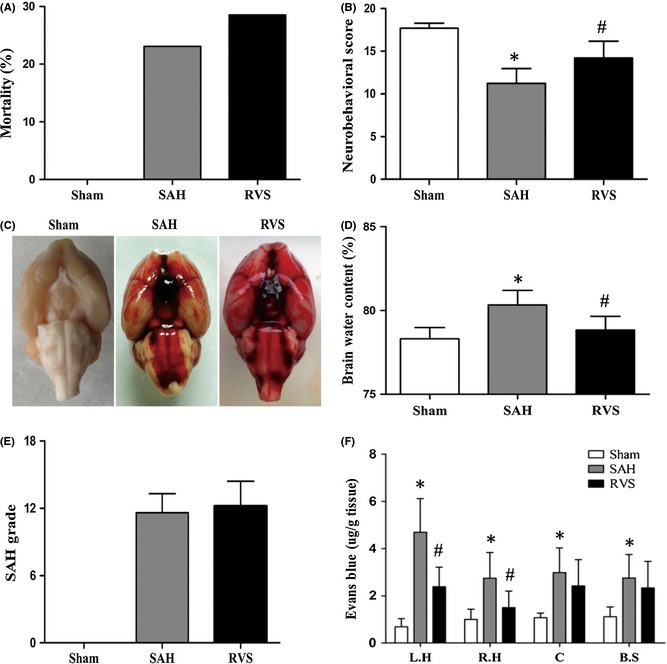
Resveratrol‐treated (30 mg/kg, intraperitoneal) rats exhibited reduced neurological deficits compared with those in the SAH group (B), obviously reduced brain water content (D), and significantly reduced Evans blue dye extravasation in the left hemisphere and right hemisphere (F). There was no difference found in SAH grade or mortality between SAH and RVS groups (A,C,E). Values are expressed as the means ± SD. Statistical significance was analyzed by one‐way analysis of variance (ANOVA) followed by Tukey's test for multiple comparisons. For mortality, Fisher's exact test was used for group comparisons. *P < 0.05 compared with sham group; # P < 0.05 compared with SAH group.
Figure 2.
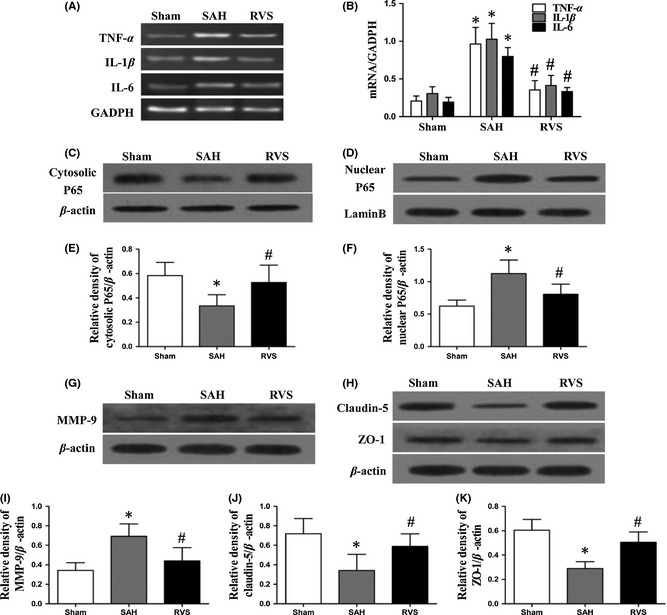
The effect of resveratrol in NF‐κB‐dependent inflammatory/MMP‐9 pathways after SAH induced. The cytoplasm‐to‐nucleus translocation of p65, a subunit of NF‐κB, was induced in the SAH group and inhibited markedly by administration of resveratrol (C–F). The levels of TNF‐a, IL‐1β, and IL‐6 mRNA increased after subarachnoid hemorrhage (SAH), but were suppressed in the resveratrol‐treated group (A,B). Resveratrol increased the expression of MMP‐9 in cortex (G,I) and decreased the level of claudin‐5 and ZO‐1 (H,J,K). Values are expressed as the means ± SD. Statistical significance was analyzed by one‐way analysis of variance (ANOVA) followed by Tukey's test for multiple comparisons. *P < 0.05 compared with sham group; # P < 0.05 compared with SAH group.
Next, we assessed the effect of resveratrol on NF‐κB‐dependent MMP‐9 expression. Resveratrol inhibited the SAH‐induced increase in MMP‐9 and concomitantly restored protein levels of tight junction proteins (including claudin‐5 and zonula occludens‐1 (ZO‐1)) (Figure 2 G–K). These findings suggested that resveratrol can block NF‐κB‐dependent MMP‐9 elevation and tight junction protein downregulation, which lead to BBB protection. However, oxidative stress could upregulate MMP‐9 levels in brain after SAH; as an antioxidant, resveratrol might inhibit NF‐κB activity partially by suppressing oxidative stress injury post‐SAH. Thus, further studies are required to address this question.
Inflammatory pathways are activated in early stages after aSAH and contribute to brain edema formation post‐SAH 3. Proinflammatory cytokines (TNF‐α, IL‐1β, and IL‐6), which are also end products of the NF‐κB activation cascade, play important roles in post‐SAH inflammation. The antiinflammatory properties of resveratrol have been shown both in vitro and in vivo 5, 9. In this study, resveratrol decreased the mRNA levels of TNF‐α, IL‐1β, and IL‐6, indicating the antiinflammatory effect of resveratrol by inhibiting NF‐κB activity (Figure 2 A,B). In contrast, cytokines such as TNF‐α and IL‐1β can enhance their own production by activating toll‐like receptor 4/NF‐κB signaling 10. Therefore, resveratrol‐induced downregulation of TNF‐α and IL‐6 contributes to NF‐κB inhibition in return.
However, this study has limitations. First, we have not used a specific inhibitor of NF‐κB to further verify the inhibitory effect of resveratrol on NF‐κB. Additionally we have not further investigated the dose and time window of resveratrol activity in SAH. Future studies should focus on these issues mentioned earlier.
In conclusion, this study indicated that resveratrol improves functional outcomes after SAH by attenuating inflammation, inhibiting BBB disruption, and alleviating brain edema. Furthermore, we hypothesized that these protective effects might be associated with resveratrol‐mediated deactivation of NF‐κB activity, leading to low expression of MMP‐9 and suppression of inflammatory response.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by Grant No. 81171096 from National Natural Science Foundation of China, Grant No. 20120101120030 from Doctoral Program of the Ministry of Education, and Grant No. 2013KYA088 from Health department of Zhejiang province.
Reference
- 1. Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS‐1): randomized, double‐blind, placebo‐controlled phase 2 dose‐finding trial. Stroke 2008;39:3015–3021. [DOI] [PubMed] [Google Scholar]
- 2. Sakata Y, Zhuang H, Kwansa H, Koehler RC, Doré S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 2010;224:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ates O, Cayli S, Altinoz E, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 2007;294:137–144. [DOI] [PubMed] [Google Scholar]
- 4. Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res 2011;16:100–109. [DOI] [PubMed] [Google Scholar]
- 5. Kumar A, Sharma SS. NF‐kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun 2010;394:360–365. [DOI] [PubMed] [Google Scholar]
- 6. Lee WR, Chung CL, Hsiao CJ, et al. Suppression of matrix metalloproteinase‐9 expression by andrographolide in human monocytic THP‐1 cells via inhibition of NF‐kappaB activation. Phytomedicine 2012;15:270–277. [DOI] [PubMed] [Google Scholar]
- 7. Karaoglan A, Akdemir O, Barut S, et al. The effects of resveratrol on vasospasm after experimental subarachnoidal hemorrhage in rats. Surg Neurol 2008;70:337–343. [DOI] [PubMed] [Google Scholar]
- 8. Feiler S, Plesnila N, Thal SC, Zausinger S, Schöller K. Contribution of matrix metalloproteinase‐9 to cerebral edema and functional outcome following experimental subarachnoid hemorrhage. Cerebrovasc Dis 2011;32:289–295. [DOI] [PubMed] [Google Scholar]
- 9. Zhong LM, Zong Y, Sun L, et al. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS‐stimulated microglial cells. PLoS ONE 2012;7:e32195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potnis PA, Dutta DK, Wood SC. Toll‐like receptor 4 signaling pathway mediates proinflammatory immune response to cobalt‐alloy particles. Cell Immunol 2013;282:53–65. [DOI] [PubMed] [Google Scholar]


