Summary
Aims
There is still no effective way to save a surviving healthy mind when there is critical organ failure in the body. The next frontier in CTA is allo‐head and body reconstruction (AHBR), and just as animal models were key in the development of CTA, they will be crucial in establishing the procedures of AHBR for clinical translation.
Methods and results
Our approach, pioneered in mice, involves retaining the donor brain stem and transplanting the recipient head. Our preliminary data in mice support that this allows for retention of breathing and circulatory function. Critical aspects of the current protocol include avoiding cerebral ischemia through cross‐circulation (donor to recipient) and retaining the donor brain stem. Successful clinical translation of AHBR will become a milestone of medical history and potentially could save millions of people.
Conclusions
This experimental study has confirmed a method to avoid cerebral ischemia during the surgery and solved an important part of the problem of how to accomplish long‐term survival after transplantation and preservation of the donor brain stem.
Keywords: Body reconstruction, Composite tissues allo‐transplantation, Head transplantation
Introduction
Composite tissue allo‐transplantation (CTA) involves the grafting of limbs or other complex tissues from an unrelated donor and recipient. In the 1990s, animal studies helped to pave the way for the first successful human hand transplantation in United States, which was performed at the University of Louisville and Christine M. Kleinert Institute for Hand and Microsurgery in 1999 1, 2, 3, 4, 5. This patient recovered fully, and continues to work and lead a normal social life 6, 7, 8. Studies in small animals and a porcine model allowed for optimization of the immunosuppressive regimen, as well as a system for characterizing the degree of immune rejection of transplanted tissues. Facial tissue transplantation has also become a clinical reality 9, 10, 11, 12, 13, and worldwide there have been more than 100 completed cases of the CTA operation 14, 15, 16, 17. However, there is no effective way in which to save a survival healthy mind when there is critical organ failure in the body, such as complete cervical spinal cord injury with paraplegia, tumors metastatic disease, hereditary body muscle atrophy, and others.
If a part of the body as important and complex as a hand can be transplanted successfully, it is natural to ask whether the most important part of the body, the head, could also be transplanted. This is the next frontier in composite tissue transplantation. A number of animal models of head transplantation have been developed over the last century, beginning with that of dogs in the former Soviet Union in the 1950s by Dr. Demichow 18, 19, 20. More clinically applicable studies followed in the 1970s, when Dr. Robert White and colleagues successfully transplanted the head of a rhesus monkey between the 3rd and 4th cervical vertebrae. While this represented important progress in understanding anatomical and physiological aspects of head transplantation 21, 22, 23, these models did not evaluate any measures to prevent immune rejection and did not have an effective strategy for central nervous system recovery. More recently, the Italian neurosurgeon Dr. Sergio Canavero has proposed a surgical strategy referred to as the HEAVEN procedure, which preserves brain function through hypothermia during the transplantation procedure, which is performed at cervical level C5/6. He presents a possible way to connect the recipient and donor spinal cords using inorganic polymers to allow the cell membranes of the donor and recipient axons to fuse 24.
Another new strategy known as allogeneic head and body reconstruction (AHBR) has also recently been proposed 25, 26. In this technique, which is based on the practice of CTA, the donor body is the transplanted composite tissue. In this procedure, brain ischemia is avoided not by deep hypothermia, but by maintaining adequate blood pressure and circulation between the recipient and the donor body, through anastomosis of the donor and recipient carotid arteries and jugular veins during surgery. Furthermore, this procedure preserves the donor body's brain stem, which allows for spontaneous postoperative respiratory and cardiovascular function. This research describes how the AHBR model is being implemented and tested in mice, in preparation for the development of a primate model that will help to evaluate the clinical applicability of AHBR.
Methods
Animals
The experiments were approved by The Animal Care and Use Committee of The Second Affiliated Hospital of Harbin Medical University. Forty Kunming mice and forty C57 wild type mice, all male, age 10–15 weeks, weight 30 g ± 5, were obtained from the animal center of Harbin Medical University.
Experimental Drugs and Equipment
The following drugs were employed as follows: 3% sodium pentobarbital (90 mg/kg), heparin sodium injection (1.0 mL/100 mg), and norepinephrine (5 mg/kg). During surgery, respiration was maintained using the Minivent Mouse Ventilator Type 845 (Hugo Sachs Elektronik Harvard Apparatus Gmbh D‐79232 March, Germany). An Operating Microscope (SXP‐1C) from Shanghai Medical Optical Instruments CO, Ltd., Shanghai, China, was used along with a sixteen‐channel physiological recorder PHY‐001 (BIOPAC MP‐150, Goleta, CA, USA), the intelligent noninvasive blood pressure measurement analysis system (BP‐98A, Softron, Tokyo, Japan), and an electrosurgical generator (HV0300A), Beijing Heng Wei Technology Development CO, Ltd., Beijing, China. Micro‐ and neurosurgical instruments and surgical supplies were purchased from the Shanghai Instrument Company (Shanghai, China).
Monitoring of Vital Signs
Perioperative and postoperative EEG and ECG were monitored. Blood pressure and body temperature were monitored to regulate the body's metabolic condition. The ventilation was set to the rate of 100 ± 5 respirations per minute with a tidal volume of 2.2 mL. After surgery, special attention was given to breathing regularity and frequency as well as ECG indicators because the donor brain stem may have limited physiological function. Nerve reflexes and basic sense of movement and muscle tension were regularly checked and recorded.
Preparation of the Donor Body
A ventral circular incision was made at cervical level C3–C4. The skin and subcutaneous tissue were incised, and muscle, nerves, blood vessels, trachea, and esophagus were dissociated. Tracheal intubation was performed. Next, the distal ends of the carotid and jugular vessels were ligated and the proximal ends on one side of the body were catheterized using a 0.30 mm (I.D.) × 0.64 mm (O.D.) silicone tube to create an anastomosis with the ipsilateral carotid and jugular vessels from the head of the recipient (Figure 1), and flushed with heparin sodium injection (1.0 mL: 100 mg) mixed with sodium chloride injection. The proximal ends of the carotid and jugular vessels on the other side of the body were ligated.
Figure 1.
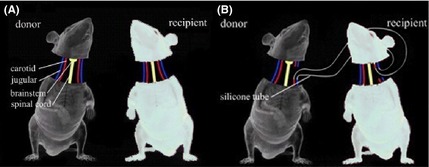
Schematic representation of the AHBR mouse model. (A) Two mice with different coat colors were selected. All tissues were separated including carotid artery, jugular vein, spinal cord, and so on. (B) To ensure transplanted brain tissue undergoes continuous blood circulation to avoid cerebral ischemia and hypoxia, crosscirculation is established by the silicone tubes.
Next, a circular incision was made on dorsal aspect of the head at the level of the midbrain. The scalp and galea aponeurotica were incised to expose the skull. To preserve the integrity of the midbrain and avoid the circle of Willis, a location was selected 3 mm distal to the anterior lambdoid suture, perpendicular to the sagittal suture. After drilling using a dental drill, a sharp cut was made at the level of the midbrain (Figure 2) 27. Importantly, placing the incision at this level preserves the donor brain stem, permitting postoperative spontaneous, independent breathing and circulation. All surgical procedures were completed under the microscope and bleeding was stopped by bipolar coagulation.
Figure 2.
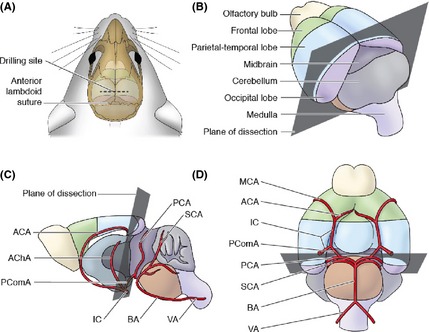
Location of the cutting plane in donor mice. (A) Drill 3 mm distal to the anterior lambdoid suture, (B) superolateral aspect of the brain showing the cutting plane, (C) sagittal section, and (D) inferior aspect of the brain showing the cutting plane and vasculature. The cutting plane passes from the caudal edge of the parietal–temporal lobes, along the rostral aspect of the midbrain, and through the PcomA. This preserves blood flow to the regions of the donor's brain that are retained. ACA, anterior cerebral artery; AChA, anterior choroidal artery; PcomA, posterior communicating artery; IC, internal carotid; MCA, middle cerebral artery; PCA, posterior cerebral artery; SCA, superior cerebellar artery; BA, basilar artery; and VA, vertebral artery.
Preparation of the Recipient Head
Ventral and dorsal incisions weremade at the cervical level C3–C4. The skin and subcutaneous tissue were incised, and muscle, nerves, blood vessels, trachea, and esophagus were dissociated. The vertebrae were exposed, and the arteries and veins around the spinal cord were ligated at level of C3–C4. The spinal cord was cut sharply. The proximal ends of the carotid and jugular vessels were ligated. The distal carotid artery and jugular vein were catheterized as before to connect with the ipsilateral carotid and jugular from the donor body. Likewise, they were flushed with heparin sodium injection (1.0 mL: 100 mg) mixed with sodium chloride injection. The distal ends of the carotid and jugular on the other side were ligated.
With the completion of osseous separation, the Cephalon was associated with the isolated body only through bilateral carotid–jugular vascular loops, which provided cerebral blood flow. Finally, surgical stitching was used to appose donor and recipient tissues including spinal cord membrane, bilateral carotid arteries, bilateral jugular veins, trachea, esophagus, muscles, subcutaneous tissue, and skin.
Results
Forty Kunming mice and forty C57 wild type underwent the AHBR procedure. After transplantation, 18 mice survived for 3 h after the ventilator was disconnected. As anticipated, they were capable of breathing spontaneously at a normal rate, because the donor brain stem remained intact. The rate of respiration was 140 ± 15/min. During these 3 h, the mice awakened and displayed normal cranial nerve function and characteristic responsiveness (blinking, whiskers moving, etc.). After the mice awoke, electroencephalogram (EEG) recordings were made directly from the cortex of the transplanted Cephalons (Figure 3). Because blood supply was maintained at an adequate level during surgery by anastomosing the donor and recipient carotid and jugular vessels, the intra‐ and postoperative EEG and ECG show electrophysiological activity. The systolic blood pressure was maintained above 50 mm/Hg (measured by tail artery). Although ECG monitoring shows an unstable curve postoperatively, there was neither significant tachycardia nor bradycardia, and no lethal arrhythmia. The heart rate was 300 ± 20/min. Interestingly, the mice did not show postoperative decerebrate rigidity (Figure 4).
Figure 3.
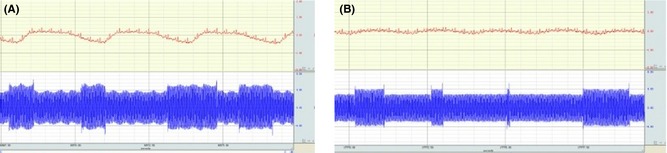
AHBR mouse model (electroencephalographic and electrocardiography recording). (A) The record of ECG and EEG preoperation and (B) postoperation.
Figure 4.
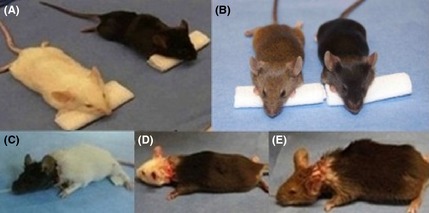
Three pairs of mice before and after surgery. (A and B) Black, white, and brown mice; (C–E) mice after transplantation (black head with white body, white head with black body, and brown head with black body).
Discussion
The brain stem is home to many reflex centers that are required for basic life functions, including cardiovascular and respiratory. As seen in Figure 1, after transplantation, the donor body continues to have a regular heart rate, which supports the hypothesis that retaining the donor's brain stem allows the body to regulate its own cardiovascular function. In animal models where the brain stem is not preserved, that is, in models using C3/4‐level transection, independent breathing and circulation are lost, and survival must depend on life‐support machines. Our short‐term results so far show that retaining the donor brain stem allows the animal to recover both independent breathing and cardiovascular function.
Furthermore, the recipient's brain has continued electrical activity in the postsurgical EEG, which suggests that the donor's heart was able to provide sufficient blood flow to the recipient's brain via the cannulae placed during surgery. White's research described the need for maintaining blood pressure of at least 40 mmHg to support the metabolic needs of brain tissue. In the animal model described herein, the postoperative blood pressure is up to 50 mmHg. This is important because the brain is the most metabolically active organ in the body, making it very intolerant of ischemia. The preoperative temperature of mice is 31 ± 2°C, which is sufficient to meet the body's metabolic needs. As such, this body temperature was maintained during surgery.
Future studies will use electromyography (EMG) to test the action potential of muscles and extend the survival of the mice for a longer time 28. This will allow signs of immune rejection to be monitored while using immunotherapy. Once the mouse model has been validated, we will build upon it to establish a primate model; these models will be crucial for making translation of this technique to the clinic possible 29, 30.
In summary, our short‐term results have been promising. We have confirmed a method to avoid cerebral ischemia during the surgery and solved an important part of the problem of how to accomplish long‐term survival after transplantation and preservation of the donor brain stem. If AHBR can be successfully translated into clinical practice, it has the potential to save countless critically ill or injured patients' lives, which will be a monumental achievement in the history of medicine.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81470425), the funds for Wu Liande foundation of HMU (Wld‐qn1414), and the funds for Harbin Science and Technology Bureau (2014RFXYJ023).
References
- 1. Ren X, Shirbacheh MV, Ustuner ET, et al. Osteomyocutaneous flap as a preclinical composite tissue allograft: Swine model. Microsurgery 2000;20:143–149. [DOI] [PubMed] [Google Scholar]
- 2. Ustuner ET, Zdichavsky M, Ren X, et al. Long‐term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation 1998;66:1581–1587. [DOI] [PubMed] [Google Scholar]
- 3. Shirbacheh MV, Ren X, Jones JW, et al. Pharmacokinetic advantage of intra‐arterial cyclosporin A delivery to vascularly isolated rabbit forelimb. I. Model development. J Pharmacol Exp Ther 1999;289:1185–1190. [PubMed] [Google Scholar]
- 4. Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: Comparisons and observations of the first four clinical cases. Microsurgery 2000;20:360–371. [DOI] [PubMed] [Google Scholar]
- 5. Jones JW Jr, Ustuner ET, Zdichavsky M, et al. Long‐term survival of an extremity composite tissue allograft with FK506‐mycophenolate mofetil therapy. Surgery 1999;126:384–388. [PubMed] [Google Scholar]
- 6. Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One‐year follow‐up. Louisville Hand Transplant Team. N Engl J Med 2000;343:468–473. [DOI] [PubMed] [Google Scholar]
- 7. Lanzetta M, Petruzzo P, Margreiter R, et al. The international registry on hand and composite tissue transplantation. Transplantation 2005;79:1210–1214. [DOI] [PubMed] [Google Scholar]
- 8. Pei G, Xiang D, Gu L, et al. A report of 15 hand allotransplantations in 12 patients and their outcomes in China. Transplantation 2012;94:1052–1059. [DOI] [PubMed] [Google Scholar]
- 9. Gordon CR, Zor F, Cetrulo C Jr, Brandacher G, Sacks J, Lee WP. Concomitant face and hand transplantation: Perfect solution or perfect storm? Ann Plast Surg 2011;67:309–314. [DOI] [PubMed] [Google Scholar]
- 10. Sacks JM, Keith JD, Fisher C, Lee WP. The surgeon's role and responsibility in facial tissue allograft transplantation. Ann Plast Surg 2007;58:595–601. [DOI] [PubMed] [Google Scholar]
- 11. Butler PE, Hettiaratchy S, Clarke A. Facial transplantation: A new gold standard in facial reconstruction? J Plast Reconstr Aesthet Surg 2006;59:211–212. [DOI] [PubMed] [Google Scholar]
- 12. Pomahac B, Aflaki P, Chandraker A, Pribaz JJ. Facial transplantation and immunosuppressed patients: A new frontier in reconstructive surgery. Transplantation 2008;85:1693–1697. [DOI] [PubMed] [Google Scholar]
- 13. Siernionow M, Papay F, Alam D, Bernard S, Fung J. Near‐total human face transplantation for a severely disfigured patient in the USA. Lancet 2009;374:203–209. [DOI] [PubMed] [Google Scholar]
- 14. Hautz T, Engelhardt TO, Weissenbacher A, et al. World experience after more than a decade of clinical hand transplantation: Update on the Innsbruck program. Hand Clin 2011;27:423–431. [DOI] [PubMed] [Google Scholar]
- 15. Gander B, Brown CS, Vasilic D, et al. Composite tissue allotransplantation of the hand and face: A new frontier in transplant and reconstructive surgery. Transpl Int 2006;19:868–880. [DOI] [PubMed] [Google Scholar]
- 16. Hautz T, Brandacher G, Engelhardt TO, et al. How reconstructive transplantation is different from organ transplantation—and how it is not. Transplant Proc 2011;43:3504–3511. [DOI] [PubMed] [Google Scholar]
- 17. Foroohar A, Elliott RM, Kim TW, Breidenbach W, Shaked A, Levin LS. The history and evolution of hand transplantation. Hand Clin 2011;27:405–409. [DOI] [PubMed] [Google Scholar]
- 18. Demikhov VP. Experimental transplantation of vital organs. New York: Consultants Bureau, 1962. [Google Scholar]
- 19. Nava BE, Gutiérrez JA, Arce M, Carbonell J, Obrador S. Head implants in dogs. I. Surgical problems. Rev Clin Esp 1973;129:443–448. [PubMed] [Google Scholar]
- 20. White RK, Albin MS, Locke GE, Davidson E. Brain transplantation: Prolonged survival of brain after carotid‐jugular interposition. Science 1965;150:779–781. [DOI] [PubMed] [Google Scholar]
- 21. White RJ, Wolin LR, Massopust LC Jr, Taslitz N, Verdura J. Primate cephalic transplantation: Neurogenic separation, vascular association. Transplant Proc 1971;3:602–604. [PubMed] [Google Scholar]
- 22. White RJ, Wolin LR, Massopust LC Jr, Taslitz N, Verdura J. Cephalic exchange transplantation in the monkey. Surgery 1971;70:135–139. [PubMed] [Google Scholar]
- 23. White RJ, Albin MS, Verdura J, et al. The isolation and transplantation of the brain. An historical perspective emphasizing the surgical solutions to the design of these classical models. Neurol Res 1996;18:194–203. [DOI] [PubMed] [Google Scholar]
- 24. Canavero S. HEAVEN: The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int 2013;4:S335–S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren X, Laugel MC. The next frontier in composite tissue allotransplantation. CNS Neurosci Ther 2013;19:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren X, Luther C, Haar L, et al. Concepts, challenges and opportunities in AHBR. CNS Neurosci Ther 2014;20:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dorr A, Sled JG, Kabani N. Three‐dimensional cerebral vasculature of the CBA mouse brain: A magnetic resonance imaging and micro computed tomography study. NeuroImage 2007;35:1409–1423. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Jahng G, Choe I, Choi C, Kim D, Kim H. Neural pathway interference by retained acupuncture: A functional MRI study of a dog model of Parkinson's disease. CNS Neurosci Ther 2013;19:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dutta S, Singh G, Sreejith S, et al. Cell therapy: The final frontier for treatment of neurological diseases. CNS Neurosci Ther 2013;19:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lei XH, Zhao D, Li YL, et al. Pifithrin‐α enhances the survival of transplanted neural stem cells in stroke rats by inhibiting p53 nuclear translocation. CNS Neurosci Ther 2013;19:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]


