Summary
For around two decades, electrical fastigial nucleus stimulation (FNS) has been demonstrated to induce neuroprotection involving multiple mechanisms. In this review, we summarize the protective effects of FNS against cerebral ischemia through the inhibition of electrical activity around the lesion, excitotoxic damage on neurons, and brain inflammatory response, as well as apoptosis. Moreover, FNS has been reported to promote nerve tissue repair, reconstruction, and neurological rehabilitation and improve stroke‐related complications including poststroke cognitive dysfunction, depression, and abnormal heart rate variability. We thus further discuss the potential of FNS for clinical applications. Given the absence of any risk of inducing sublethal damage, FNS may offer a new approach to preconditioned neuroprotection against cerebral ischemia.
Keywords: Cerebral ischemia, Fastigial nucleus, Neuroprotection, Stroke
Abbreviations
- FN
fastigial nucleus
- FNS
fastigial nucleus stimulation
- MCAO
middle cerebral artery occlusion
- FPR
cerebral blood flow
- AVP
arginine vasopressin
- FDR
fastigial depressor response
- LDF
laser Doppler flowmetry
- DN
dentate nucleus
- IBO
ibotenic acid
- CCNN
conditioned central neurogenic neuroprotection
- PIDs
per‐infarction depolorizing waves
- iNOS
inducible nitric oxide synthase
- IL‐1
interleukin‐1
- ICAM‐1
intercellular adhesion molecule‐1
- NF‐κB
nuclear factor‐κB
- HSP70
heat shock protein 70
- PPARγ
Peroxisome proliferator‐activated receptor‐γ
- PKC
protein kinase C
- UCP4
uncoupling protein 4
- 8‐OHdG
8‐hydroxy‐2' ‐deoxyguanosine
- rOGG1
8‐oxodeoxyguanosine glycosylase
- 5‐HT
5‐hydroxytryptamine
- GAP‐43
Growthassociated protein‐43
- NgR
Nogo receptor
- CVFT
cerebrovascular function therapeutic apparatus
- PSD
poststroke depression
- NE
norepinephrine
- HRV
heart rate variability
- FD
fractal dimension
Introduction
The fastigial nucleus (FN) is located at the top of the fourth ventricle and includes adrenergic intrinsic neurons and nerve fibers. Electrical stimulation excites fibers passing through the FN, leading to increased blood pressure, reflexive vascular expansion, and increased cerebral blood flow, which together are referred to as the fastigial pressor response (FPR) 1. FPR is potentiated by suppressing the baroreceptor reflex, which releases adrenaline and noradrenaline from the adrenal medulla and arginine vasopressin (AVP) from the posterior pituitary 2, 3. The increase in cerebral blood flow (CBF) is global (including the spinal cord) but uneven, with the largest increase in the frontal and parasaggital regions of the cortex 4, 5. FN‐evoked elevation in regional CBF is independent of arterial pressure (AP), and most probably mediated by intrabrain intrinsic circuitry 6. Conversely, microinjection of excitatory amino acids, which excite only FN nerve cells, lower AP, heart rate, and global CBF, together called the fastigial depressor response (FDR) 7, 8. Though selective damage to the FN can eliminate the FDR, the FPR effect from electrical fastigial nucleus stimulation (FNS) is preserved, suggesting that the FPR effect generated by the passing fibers exceeds the sympathetic inhibitory response by FN nerve cells.
Fastigial nucleus stimulation can induce neuroprotection against cerebral ischemia. One hour of FNS immediately after middle cerebral artery occlusion (MCAO) in rats reduces infarction volume 24 h later by 40–50%, with the protected areas confined to the penumbra surrounding the infarction core 9, 10, 11, 12. Additionally, to establish the time course of FN‐evoked neuroprotection, Reis et al. 13 demonstrated that one hour of FNS prior to permanent MCAO in a rat model also reduces infarct volume. The neuroprotective effect is most obvious when MCAO is delayed up to 3 days after FNS, and the infarction volume is reduced by about 60%. Extending the interval between the FNS and MCAO gradually diminishes the protective effect. Seven days after FNS, the infarct volume is reduced by 26%, but the neuroprotection disappears when MCAO is performed 3 weeks after FNS. These data demonstrate that FNS can significantly enhance the tolerance of brain tissue to subsequent cerebral ischemia.
Electrical stimulation of the structures near the FN such as the cerebellar dentate nucleus (DN) or white matter does not have a neuroprotective effect. Additionally, FNS 3 days after selective excitotoxic lesions of FN neurons fails to induce neuroprotection despite the FPR effect is preserved, suggesting that long‐lived protection against cerebral ischemia is produced in intrinsic FN neurons and transmitted over pathways distinct from those regulating rCBF 14.
Laser doppler flowmetry (LDF) and autoradiography during FNS show that CBF increases in the nonischemic areas of the hemisphere ipsilateral to the occlusion and in the contralateral hemisphere. However, CBF does not increase in the penumbral zone (the salvaged area) 4, 12. In addition, the increase in CBF produced by FNS is transient; CBF gradually increases within 30–40 seconds in response to FNS, remains elevated only during stimulation, and returns to baseline thereafter 12. When MCAO is performed 24 h after FNS, although there is no increase in systemic mean AP or regional CBF (rCBF), the infarct volume can still be reduced.
Because the level of metabolism increases in the penumbral area surrounding the infarction core, inhibition of glucose utilization (CGU) may be neuroprotective. However, in animals that did not undergo MCAO, FNS does not significantly affect global cerebral CGU. In animals subjected to MCAO, FNS increases CGU in the nonischemic tissue and contralateral hemisphere, but not in the penumbra 4.
These data demonstrate that the neuroprotective effect of FNS is site‐dependent and not mediated by either CBF increase or CGU decrease. This phenomenon indicates that intrinsic neuroprotective system exists within the brain, which mediates a conditioned central neurogenic neuroprotection (CCNN) 15. It can be initiated by excitation of intrinsic neurons within the brain and activation of a series of neural pathways. The FNS‐induced neuroprotection is a typical CCNN, which renders the brain more tolerant to adverse stimuli when activated 15. Oxygen‐conserving reflex, also known as reflex central neurogenic vasodilation, is another central approach of central neurogenic neuroprotection. Oxygen‐sensitive sympathoexcitatory reticulospinal neurons of the rostral ventrolateral nucleus medulla (RVLM) would be excited within seconds under conditions of ischemia or hypoxia. Systemic blood pressure and rCBF increase profoundly. However, unlike long‐lasting CCNN by FNS, neuroprotection induced by oxygen‐conserving reflex remains only during stimulation and disappears upon cessation of the stimulation 15.
Many studies have sought to explore the mechanisms that might account for the neuroprotection elicited by FNS. We focus on those mechanisms and the clinical applications of FNS in treatment of ischemic stroke.
Mechanisms of Electrical Fastigial Nucleus Stimulation‐Induced Neuroprotection
Inhibition of Electrical Activity around the Lesion
In addition to brain tissue necrosis in the ischemic central area, focal cerebral ischemia can induce neuron membrane potential instability in ischemic penumbra, which causes repeated episodes of irregular depolarization and the formation of periinfarction depolarizing waves (PIDs) around the infarction lesion 16, 17. PIDS can further deplete energy and aggravate ischemic injury of neurons in the penumbra. The volume of the infarct increases stepwise for each PID, and the number and frequency of PIDs is proportional to the infarct volume 18, 19. The transmission mode of PIDs is similar to the cortical spreading depression (CSD), which is mediated by the NMDA receptor and can be inhibited by NMDA receptor antagonist 20.
FNS immediately or 72 h prior to MCAO in rats reduces infarction volumes by approximately 45%, increases PID latency >10‐fold, and decreases the number of PIDs by >50%, suggesting that FNS significantly inhibits PIDs 21. The emergence of PIDs correlates with ATP‐dependent potassium (K‐ATP) channel activity in the neuron membrane 22. The neuroprotective effect of FNS against MCAO is partially abolished when the K‐ATP channel is blocked by glibenclamide before FNS, which indicates that FNS can prolong the opening of the K‐ATP channel and lead to neuronal hyperpolarization and decreased excitability, thereby suppressing the generation and conduction of the PIDs or CSD 22.
Inhibition of Excitotoxic Damage on Neurons
One of the main mechanisms underlying neuronal death in ischemic brain injury is excitotoxicity, which is triggered by excessive activation of glutamate receptors [especially N‐methyl‐D‐aspartate (NMDA) receptors] and subsequent excessive Ca2+ influx and overload 23. The glutamatergic NMDA receptor antagonist MK801 blocks the NMDA receptor channel and reduces excitotoxic damage, effectively inhibiting delayed neuronal death in the hippocampal CA1 region and the penumbra 24.
One hour of FNS 10 days prior to injection of IBO into the striatum reduces the lesion volume by 80%, while lesions in FN (but not DN) abolished the neuroprotective effect 14. Dong 25 found that FNS significantly decreases extracellular glutamate levels in the ischemic penumbra in a rat MCAO/reperfusion model, suggesting that FNS mediates neuroprotective effects by inhibiting the release of glutamate. FNS‐mediated inhibition of excitotoxicity may be achieved by reducing the binding ability of the glutamate receptor, although the exact mechanism remains unclear.
Inhibition of Brain Inflammatory Response
Both global and focal cerebral ischemia trigger the inflammatory response, resulting in secondary brain damage. In the early stages of cerebral ischemia, the increased glutamate release and the activation of NMDA receptor cause Ca2+ overload, which in turn activates nitric oxide synthase (NOS) expression and increases NO synthesis 26. NO mediates neuronal damage through a variety of mechanisms, such as generation of peroxynitrite, DNA damage, and inhibition of ATP‐producing enzymes 27. One hour of FNS 48 hours prior to MCAO reduces induced NOS (iNOS) mRNA and protein expression in the penumbra (but not the core) by more than 90% and decreases iNOS enzyme activity by 44%, suggesting that down‐regulation of iNOS expression may be associated with neuroprotection elicited by FNS 28.
Interleukin‐1 (IL‐1) is one of the most important inflammatory cytokines induced by ischemia. It in turn induces expression of iNOS and intracellular adhesion molecule‐1 (ICAM‐1) through the activation of nuclear factor‐κB (NF‐κB) 29. One hour of FNS 72 h prior to microinjection of IL‐1β into the rat striatal area reduces the number of enlisted leukocytes by 50%. In isolated microvessels, FNS decreases the IL‐1β‐induced up‐regulation of iNOS and ICAM‐1 mRNA, whereas it increases IkappaB‐alpha mRNA expression 29. These data suggest that FNS may render brain microvessels refractory to IL‐1β through overproduction of IkappaB‐alpha. Hsp70 has inflammatory properties due to inhibition of NF‐κB 30. We found that in rats with one hour of FNS 1, 4, or 7 days prior to MCAO/reperfusion, NF‐κB expression is decreased significantly; however, HSP70 expression is up‐regulated. In addition, leukocyte infiltration in the infarct area is reduced by FNS performed before or after MCAO/reperfusion in rats 31. The suppression of microvascular inflammation may contribute to the neuroprotection elicited by FNS.
Peroxisome proliferator‐activated receptor‐γ (PPARγ) is a ligand‐dependent transcription factor that regulates target gene expression through binding to conserved DNA sequences called peroxisome proliferator response elements as heterodimers with another nuclear receptor, the retinoic acid receptor 32. PPARγ participates in cell glucose uptake, lipid metabolism, and energy storage 33. Additionally, the activation of PPARγ transrepresses DNA binding of NF‐κB, thereby reducing the expression of NF‐κB target genes including ICAM‐1, matrix metalloproteinase‐9 (MMP‐9), and cyclooxygenase‐2 (COX‐2), leading to suppression of the inflammatory response 34. The PPARγ antiinflammatory effect has become an important therapeutic target in stroke 35. Our study found that FNS promotes PPARγ expression in neurons and inhibits the inflammatory response induced by cerebral ischemia.
Inhibition of Apoptosis
The main manner of neuronal death in the cerebral ischemic penumbra is apoptosis, which is initiated and induced by the cell death gene, with a variety of intracellular molecules involved in the process 36. Regardless of the means by which apoptosis is induced, ultimately caspase‐3, the final pathway of apoptosis, will be activated 37. Caspase‐3 can degrade a variety of substrate proteins in the nucleus such as poly ADP‐ribose polymerase (PARP, which has a DNA repair function), cell cycle regulatory factors, DNA binding protein, and the Bcl‐2 protein family. Although the loss of these proteins' activity leads to DNA cleavage and ultimately cell death, apoptosis can be inhibited by blocking caspase‐3 activity 38, 39, 40. Zhou et al. 41 found that FNS significantly reduces staurosporine‐induced caspase‐3 activity. We found that FNS reduces by >50% the number of apoptotic neurons in cerebral ischemic penumbra in rats. In addition, fluorescence quantitative RT‐PCR reveals that expression of caspase‐3 mRNA is decreased by 63% and expression of inhibitor of apoptosis (IAPs) mRNA is increased by 133% in ischemic penumbra 25. Those results suggest that FNS inhibits neuronal apoptosis in cerebral ischemic penumbra through blocking apoptosis initiation and execution.
Protein kinase C (PKC) is a Ca2+‐ and phosphatidylserine‐dependent protein kinase that catalyzes phosphorylation of serine or threonine residues of various protein substrates. The PKC family, which comprises at least 10 isoforms with distinct means of regulation and tissue distribution patterns, exerts both inhibitory and stimulatory influences on apoptosis 42. Studies suggest that PKCδ may be the major mediator or modulator of apoptotic in response to excitotoxic insult or brain ischemia 43, 44. Moreover, administering a PKCδ‐selective inhibitor peptide at the onset of reperfusion significantly reduces cerebral tissue damage 45. We found that PKCδ and PKCγ expression is elevated in ischemic brain tissue in rats, and the distribution of PKC expression is consistent with the distribution of neuronal apoptosis. However, one hour of FNS 1 day, 4 days, or 7 days prior to MCAO/reperfusion significantly inhibited PKCδ and PKCγ expression 46. Those results suggest that down‐regulation of PKC expression in cerebral ischemia may contribute to the antiapoptotic effects of FNS.
Mitochondria are critical in determining cell fate. Some data demonstrate that FNS can inhibit apoptosis through stabilizing mitochondrial function. In an in vitro rat brain slice culture model, FNS results in a 56.5% reduction in cytochrome c release upon staurosporine incubation 41. Mitochondria isolated from FN‐stimulated rats exhibit a marked increase in their ability to sequester Ca2+ and an increased resistance to Ca2+ ‐induced membrane depolarization and depression in respiration. Additionally, in brain slices, FNS reduces the staurosporine‐induced insertion of the pro‐apoptotic protein Bax into the mitochondria, a critical step in the mitochondrial mechanisms of apoptosis 47. These results suggest that FNS directly modifies the sensitivity of brain cells to apoptotic stimuli.
Yamamoto et al. 48 found that 72 h after FNS (for 1 h), mRNA levels of uncoupling protein 4 (UCP4), a mitochondrial protein, increase by 160% in the cortex of spontaneously hypertensive rats. Even in MCAO rats, levels of UCP4 mRNA are increased by 150% in the cortex. In FNS rats, the decrease of mitochondrial membrane potential evoked by carbonylyanide‐4‐ (trifluoromethoxy) phenylhydrazone (FCCP, a mitochondrial toxin) was significantly attenuated in the cortex 48. These data indicate that increased UCP4 expression and mitochondrial tolerance following FNS may be components of the endogenous neuroprotective mechanism.
Prohibitin (PHB) is another essential mitochondrial protein well described in many cell types and localized to neuronal mitochondria 49. Recently, in a proteomic screen of rat brains in which ischemic tolerance was induced by FNS, Zhou et al. 49 found that PHB is up‐regulated in mitochondria. In addition, up‐regulation of PHB reduces neuronal death from various injury modalities, whereas its down‐regulation increases neuronal susceptibility to injury, an effect associated with loss of mitochondrial membrane potential and increased mitochondrial production of reactive oxygen species. Those findings suggest that PHB is an endogenous neuroprotective protein involved in ischemic tolerance.
Cerebral ischemia induces massive inflow of Ca2+ into neurons and activation of calpain (a calcium‐dependent protease), which leads to degradation of cytoskeletal protein, culminating in neuronal ulceration and necrosis 50. Studies have demonstrated that calpain can mediate neuronal apoptosis through activating caspase‐3, 6, 7, 12, and cleaving Bax and other apoptosis‐related proteins; however, calpain inhibitor can help prevent apoptosis 51, 52. One of our own studies found that FNS significantly inhibits the activation of the calpain induced by MCAO in rats and reduces the degradation of its substrate spectrin, thereby reducing delayed neuronal death.
DNA repair ability after DNA damage is one of the main determinants of cell survival, and cerebral ischemia can induce oxidative DNA damage 36. Liu et al. 53 found that 8‐hydroxy‐2'‐deoxyguanosine (8‐OHdG) accumulates in the ischemic region of rat brains subjected to MCAO/reperfusion, while the expression of the mRNA of the DNA repair enzyme 8‐oxodeoxyguanosine glycosylase (rOGG1) in the ischemic region is reduced. However, FNS significantly reduces the 8‐OHdG content and up‐regulates rOGG1 mRNA expression in the ischemic region at both 24 and 48 hours after MCAO/reperfusion. In addition, one hour of FNS up‐regulated the activity of Ku‐70, another DNA repair enzyme and reduced neuronal apoptosis in the ischemic region.
Gadd45β is one isoform of the highly homologous Gadd45 protein family, members of which participate in DNA repair, cell survival and apoptosis, cell cycle arrest, and probably DNA demethylation in response to environmental and physiological stress 54, 55. Gadd45β can be transiently induced by neuronal activity and may promote adult neurogenesis through dynamic DNA demethylation of specific gene promoters in the hippocampus 56. Chen et al. 57 and Jin et al. 58 found that Gadd45mRNA and protein are overexpressed in neurons that survive cerebral ischemia, indicating that Gadd45 may play a protective role in the injured brain. Recently, Liu et al. 59 found that FNS promotes Gadd45β expression and motor function recovery after focal cerebral ischemia.
In conclusion, FNS can significantly reduce infarct size in the acute stage of stroke through a variety of mechanisms (Figure 1) and improve neurological outcomes. To identify the endogenous neuroprotective mechanisms of FNS, most of the studies cited above adopt preconditioning strategies. In this context, FNS may be considered an effective preconditioning method to elicit neurogenic neuroprotection against subsequent severe ischemic brain injury. Additionally, transient FNS is superior to ischemic preconditioning because it causes no brain damage. However, preconditioning through FNS seems to be of little value from the standpoint of clinical stroke treatment. Therefore, further experimental evidence is urgently needed to establish the time course of FN‐evoked neuroprotection when FNS is performed after MCAO.
Figure 1.
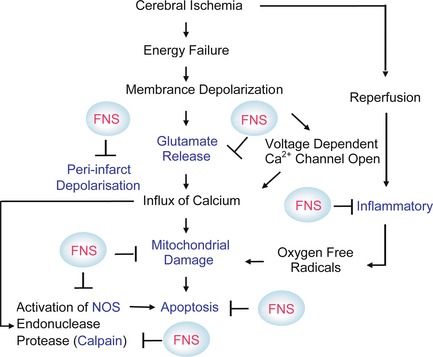
A schematic diagram illustrating the mechanisms by which ischemia injury neurons and some targets (blue mark) through which fastigial nucleus stimulation provides protection on the neurons.
Promotion of Nerve Tissue Repair and Reconstruction
Growth‐associated protein‐43 (GAP‐43) is an axonal membrane protein, a type of neuron‐specific protein involved in nerve cell growth, external growth synapse formation, and nerve cell regeneration. It plays an important role in the ontogenetic process and regeneration after injury 60, 61. One hour of FNS 24 h prior to MCAO increased the expression of GAP‐43 mRNA in neurons around the infarction area during recovery 7 and 14 days after MCAO, suggesting that FNS can promote axonal regeneration and plasticity. Nerve fiber regeneration during the middle and late stages after stroke onset may help to promote recovery of limb motor function.
Nogo receptor (NgR) is a neuron‐specific protein ubiquitous in the central nervous system (CNS) that is capable of inhibiting axonal growth through specific binding with Nogo‐66 molecules 62, 63. Recently, Zhang et al. 64 found that NgR expression in the infarct cortex and hippocampus is significantly up‐regulated and that axons are grossly damaged 24 h after cerebral ischemia‐reperfusion. However, one hour of FNS administered 2 h after ischemia reduces NgR expression and improves axonal growth at both 24 h and 2 weeks after ischemia‐reperfusion. These results suggest that FNS may offer a new strategy to promote CNS axonal regeneration.
Focal ischemia/reperfusion can activate proliferation and migration of neural stem cells (NSCs) in the lateral ventricle and hippocampus area 65. Huang et al. found that FNS promotes the proliferation and migration of NSCs after focal cerebral ischemia/reperfusion, and the underlying mechanisms may be associated with the up‐regulation of basic fibroblast growth factor (bFGF) and brain derived neurotrophic factor (BDNF) expression by FNS. Additionally, Huang et al. 66 found that FNS prolongs the survival of transplanted neural stem cells and improves neurological function score in MCAO rats.
In summary, current experimental data indicate that FNS has both early neuroprotection and late recovery enhancement effects. This potential dual effect could translate into a robust treatment effect on neurological outcome if FNS is initiated early stroke onset.
Clinical Application Prospects
Improvement of Poststroke Neurological Dysfunction
Given that large amounts of experimental data demonstrate the neuroprotective and neural restorative effects of FNS, it is reasonable to speculate that FNS has potential clinical applications. Using a biological bionic current to therapeutically stimulate FN, we have developed the cerebrovascular function therapeutic apparatus (CVFT). Additionally, through animal experiments we have demonstrated that stimulation of FN can be achieved extracranially 31. Some clinical studies have found that FNS through CVFT can be applied safely in stroke treatment and significantly improve neurological outcomes 67, 68, 69. However, due to the small number of cases in these studies, the clinical efficacy of FNS through CVFT in ischemic stroke still lacks sufficient evidence.
Improvement of Poststroke Cognitive Dysfunction
There is increasing evidence that the cerebellum plays a role in cognition, behavior, and psychiatric illness 70, 71, 72, 73. Theoretically, stroke in any part of the brain (including the frontal lobe and hippocampus) can affect cerebellar function and produce vascular dementia (VD). Several studies have found that activation of the cerebellum significantly alleviates VD. Fan et al. found that cognitive function in rats is reduced after 2 months of chronic cerebral hypoperfusion and becomes even worse after 4 months of hypoperfusion; however, it is improved by FNS treatment. Similarly, Tan et al. 74 found that FNS significantly improves learning and memory disorders in rats after 2 weeks or even 12 weeks of recirculation following repetitive global ischemia. These data suggest that FNS may have potential in treating patients with vascular or poststroke cognitive dysfunction.
Improvement of Poststroke Depression
Poststroke depression (PSD) is the most frequent psychiatric complication of stroke and often difficult to treat. The cerebellum plays an important role in the regulation of emotion, and recent data suggest that cerebellar dysfunction may be common in poststroke depression 75. Functional imaging studies found low local regional blood flow and low excitability in the cerebellar cortex of patients with depression 76. Wu et al. found that FNS up‐regulates norepinephrine (NE) and 5‐HT in the frontal lobes of rats with depression. Recent clinical studies have shown that FNS alone or combined with drug therapy contributes to improvements in PSD. Liu et al. found that FNS through CVFT improves emotional disorders in stroke patients. Similarly, Zhu et al. demonstrated that FNS improves PSD symptoms. Taken together, these data indicate that FNS through CVFT may become a new strategy to treat poststroke PSD.
Improvement of Poststroke Heart Rate Variability
Ischemic stroke often causes reduced heart rate variability (HRV), which can last for as long as 6 months 77 and is associated with adverse clinical outcomes 78. We analyzed HRV in rats with right MCAO and found that the power spectral components and chaos of HRV after MCAO are significantly reduced; however, FNS performed two hours after MCAO improves HRV parameters starting 3 days after MCAO 79. Moreover, we found that FNS treatment by CVFT in stroke patients alleviates abnormal HRV, enhances cardiac parasympathetic activity, and increases the chaotic fractal dimension (FD) index, which indicates that FNS can improve cardiac autonomic nerve dysfunction after stroke and reduce the risk of brain‐derived sudden death 80. The mechanisms underlying the improvement in HRV elicited by FNS remain unclear. Electrophysiological studies and anatomic tracing techniques have demonstrated extensive connections between FN and the central autonomic nervous system 81, 82, 83, 84. When the FN is stimulated, projections and related pathways are activated through indirect paths and multiple synapses, which can regulate the expression and release of neurotransmitters including acetylcholine (ACH), neuropeptide Y (NPY), and norepinephrine (NE), thereby allowing sympathetic–parasympathetic activity to reach a new equilibrium 70, 85.
In conclusion, the cerebellum not only maintains balance, integration, and stability in the somatic motor sphere, but also helps to balance, integrate, and stabilize other brain functions including cognition and emotion 75. According to preliminary results and the above clinical observations, FNS not only alleviates neurological dysfunction after stroke, but also helps to improve stroke‐related complications including cognitive dysfunction, depression, and abnormal HRV. These data indicate that FNS by CVFT may be a safe and low‐cost therapy to improve clinical stroke outcomes.
Conclusions
As FNS can induce robust CCNN against cerebral ischemia, and transient FNS runs no risk of inducing sublethal damage, it may offer a new approach to preconditioned neuroprotection for cerebral ischemia and other neurological disorders. It is reasonable to visualize the application of FNS in populations at high risk to develop resistance or tolerance to stroke. Although some clinical studies have shown that FNS by CVFT is safe and effective in stoke treatment, high‐quality clinical studies are urgently needed to confirm its efficacy.
Conflict of Interest
The authors have no conflict of interests.
Acknowledgments
This work was supported by grants from the Bill & Melinda Gates Foundation (to X.W.), the Muscular Dystrophy Association (to X.W.), the ALS Therapy Alliance (to X.W.), and Key Project of the National Natural Science Foundation of China (No. 39730170 to D.W.W.).
References
- 1. Miura M, Reis DJ. Cerebellum: A pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Res 1969;13:595–599. [DOI] [PubMed] [Google Scholar]
- 2. Del Bo A, Sved AF, Reis DJ. Fastigial stimulation releases vasopressin in amounts that elevate arterial pressure. Am J Physiol 1983;244:H687–H694. [DOI] [PubMed] [Google Scholar]
- 3. Del Bo A, Sved AF, Reis DJ. Inhibitory influences from arterial baroreceptors on vasopressin release elicited by fastigial stimulation in rats. Circ Res 1984;54:248–253. [DOI] [PubMed] [Google Scholar]
- 4. Golanov EV, Reis DJ. Cerebral cortical neurons with activity linked to central neurogenic spontaneous and evoked elevations in cerebral blood flow. Neurosci Lett 1996;209:101–104. [DOI] [PubMed] [Google Scholar]
- 5. Nakai M, Iadecola C, Reis DJ. Global cerebral vasodilation by stimulation of rat fastigial cerebellar nucleus. Am J Physiol 1982;243:H226–H235. [DOI] [PubMed] [Google Scholar]
- 6. Golanov EV, Zhou P. Neurogenic Neuroprotection. Cell Mol Neurobiol 2003;23:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chida K, Iadecola C, Underwood MD, Reis DJ. A novel vasodepressor response elicited from the rat cerebellar fastigial nucleus: The fastigial depressor response. Brain Res 1986;370:378–382. [DOI] [PubMed] [Google Scholar]
- 8. Bradley DJ, Pascoe JP, Paton JF, Spyer KM. Cardiovascular and respiratory responses evoked from the posterior cerebellar cortex and fastigial nucleus in the cat. J Physiol 1987;393:107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reis DJ, Underwood MD, Berger SB, Khayata M, Zaiens NI. Fastigial nucleus stimulation reduces the volume of cerebral infarction produced by occlusion of the middle cerebral artery in rat In: Seylaz J, MacKenzie ET, editors. Neurotransmission and Cerbrovascular Function I. New York: Elsevier Science, 1989;401–404. [Google Scholar]
- 10. Reis DJ, Berger SB, Underwood MD, Khayata M. Electrical stimulation of cerebellar fastigial nucleus reduces ischemic infarction elicited by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 1991;11:810–818. [DOI] [PubMed] [Google Scholar]
- 11. Golanov EV, Yamamoto S, Reis DJ. Electrical stimulation of cerebellar fastigial nucleus fails to rematch blood flow and metabolism in focal ischemic infarctions. Neurosci Lett 1996;210:181–184. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto S, Golanov EV, Reis DJ. Reductions in focal ischemic infarctions elicited from cerebellar fastigial nucleus do not result from elevations in cerebral blood flow. J Cereb Blood Flow Metab 1993;13:1020–1024. [DOI] [PubMed] [Google Scholar]
- 13. Reis DJ, Kobylarz KY, Amamoto S, Golanov EV. Brief electrical stimulation of cerebellar fastigial nucleus conditions long‐lasting salvage from focal cerebral ischemia‐conditioned central neurogenic neuroprotection. Brain Res 1998;780:161–165. [PubMed] [Google Scholar]
- 14. Glickstein SB, Golanov EV, Reis DJ. Intrinsic neurons of fastigial nucleus mediate neurogenic neuroprotection against excitotoxic and ischemic neuronal injury in rat. J Neurosci 1999;19:4142–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reis DJ, Golanov EV, Galea E, Feinstein DL. Central neurogenic neuroprotection: Central neural systems that protect the brain from hypoxia and ischemia. Ann N Y Acad Sci 1997;835:168–186. [DOI] [PubMed] [Google Scholar]
- 16. Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab 1993;13:568–574. [DOI] [PubMed] [Google Scholar]
- 17. Strong AJ, Harland SP, Meldrum BS, Whittington DJ. The use of in vivo fluorescence image sequences to indicate the occurrence and propagation of transient focal depolarizations in cerebral ischemia. J Cereb Blood Flow Metab 1996;16:367–377. [DOI] [PubMed] [Google Scholar]
- 18. Mies G, Kohno K, Hossmann KA. Prevention of periinfarct direct current shifts with glutamate antagonist NBQX following occlusion of the middle cerebral artery in the rat. J Cereb Blood Flow Metab 1994;14:802–807. [DOI] [PubMed] [Google Scholar]
- 19. Takano K, Latour LL, Formato JE, et al. The role of spreading depression in focal ischemia evaluated by diffusion mapping. Ann Neurol 1996;39:308–318. [DOI] [PubMed] [Google Scholar]
- 20. Ohta K, Graf R, Rosner G, Heiss WD. Calciumion transients in peri‐infarct depolarizations may deteriorate ion homeostasis and expand infarction in focal cerebral ischemia in cats. Stroke 2001;32:535–543. [DOI] [PubMed] [Google Scholar]
- 21. Golanov EV, Reis DJ. Neuroprotective electrical stimulation of cerebellar fastigial nucleus attenuates expression of periinfarction depolarizing waves (PIDS) and inhibits cortical spreading depression. Brain Res 1999;818:304–315. [DOI] [PubMed] [Google Scholar]
- 22. Golanov EV, Christensen JD, Reis DJ. Role of potassium channels in the central neurogenic neuroprotection elicited by cerebellar stimulation in rat. Brain Res 1999;842:496–500. [DOI] [PubMed] [Google Scholar]
- 23. Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci 1993;16:359–365. [DOI] [PubMed] [Google Scholar]
- 24. Golanov EV, Reis DJ. Contribution of oxygen‐sensitive neurons of the rostral ventrolateral medulla to hypoxic cerebral vasodilatation in the rat. J Physiol 1996;495:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong WW. Fastigial nucleus electrical stimulation and central neurogenic neuroprotection. Eng Sci 2001;3:32–38. [Google Scholar]
- 26. Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PS‐95 protein. Science 1999;284:1845–1848. [DOI] [PubMed] [Google Scholar]
- 27. Murphy MP. Nitric oxide and cell death. Biochim Biophys Acta 1999;1411:401–414. [DOI] [PubMed] [Google Scholar]
- 28. Galea E, Golanov EV, Feinstein DL, Kobylarz KA, Glickstein SB, Reis DJ. Cerebellar stimulation reduces inducible nitric oxide synthase expression and protects brain from ischemia. Am J Physiol 1998;274:H2035–H2045. [DOI] [PubMed] [Google Scholar]
- 29. Galea E, Glickstein SB, Feinstein DL, Golanov EV, Reis DJ. Stimulation of cerebellar fastigial nucleus inhibits interleukin‐1 beta‐induced cerebrovascular inflammation. Am J Physiol 1998;275:H2053–H2063. [DOI] [PubMed] [Google Scholar]
- 30. Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti‐inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 2008;28:53–63. [DOI] [PubMed] [Google Scholar]
- 31. Xia YL, Luo Y, Dong WW. Effect and mechanism of fastigial nucleus stimulation on stroke in rats. J Apoplexy Nerv Dis 1999;16:3–5. [Google Scholar]
- 32. Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int 2006;49:136–144. [DOI] [PubMed] [Google Scholar]
- 33. Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator‐activated receptor gamma in microglia/macrophages. Ann Neurol 2007;61:352–362. [DOI] [PubMed] [Google Scholar]
- 34. Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d‐Prostaglandin J2 activates peroxisome proliferator‐activated receptor‐gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab 2006;26:811–820. [DOI] [PubMed] [Google Scholar]
- 35. Gillespie W, Tyagi N, Tyagi SC. Review Role of PPARgamma, a nuclear hormone receptor in neuroprotection. Indian J Biochem Biophys 2011;48:73–81. [PubMed] [Google Scholar]
- 36. Pandya RS, Mao L, Zhou H, et al. Central nervous system agents for ischemic stroke: Neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem 2011;11:81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Figueroa BE, Stavrovskaya IG, et al. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke 2009;40:1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang WH, Wang H, Wang X, et al. Nortriptyline protects mitochondria and reduces cerebral ischemia/hypoxia injury. Stroke 2008;39:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Wang X, Baranov SV, et al. Dipyrone Inhibits Neuronal Cell Death and Diminishes Hypoxic/Ischemic Brain Injury. Neurosurgery 2011;69:942–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou H, Wang J, Jiang J, et al. N‐Acetyl‐serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J Neurosci 2014;34:2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou P, Qian L, Glickstein SB, Golanov EV, Pickel VM, Reis DJ. Electrical stimulation of cerebellar fastigial nucleus protects rat brain, in vitro, from staurosporine‐induced apoptosis. J Neurochem 2001;79:328–338. [DOI] [PubMed] [Google Scholar]
- 42. Gutcher I, Webb PR, Anderson NG. The isoform‐specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci 2003;60:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaasinen SK, Goldsteins G, Alhonen L, Jänne J, Koistinaho J. Induction and activation of protein kinase C delta in hippocampus and cortex after kainic acid treatment. Exp Neurol 2002;176:203–212. [DOI] [PubMed] [Google Scholar]
- 44. Koponen S, Goldsteins G, Keinänen R, Koistinaho J. Induction of Protein Kinase Cδ Subspecies in Neurons and Microglia After Transient Global Brain Ischemia. J Cereb Blood Flow Metab 2000;20:93–102. [DOI] [PubMed] [Google Scholar]
- 45. Bright R, Raval AP, Dembner JM, et al. Protein kinase C delta mediates cerebral reperfusion injury in vivo . J Neurosci 2004;24:6880–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu G, Dong WW, Luo Y, Peng GG. Effect of fastigial nucleus pre‐stimulation on cerebral ischemia in rats. Mod Rehabil 2001;5:60–61. [Google Scholar]
- 47. Zhou P, Qian L, Zhou T, Iadecola C. Mitochondria are involved in the neurogenic neuroprotection conferred by stimulation of cerebellar fastigial nucleus. J Neurochem 2005;95:221–229. [DOI] [PubMed] [Google Scholar]
- 48. Yamamoto S, Koizumi S, Thura M, Ihara H, Golanov EV. Electrical stimulation of cerebellar fastigial nucleus up‐regulates uncoupling protein 4 and stabilizes mitochondrial membrane potential in the cortex. Neurosci Res 2011;71:e406. [Google Scholar]
- 49. Zhou P, Qian LP, D'Aurelio M, et al. Prohibitin reduces mitochondrial free radical production and protects brain cells from different injury modalities. J Neurosci 2012;32:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokota M, Saido TC, Kamitani H, Tabuchi S, Satokata I, Watanabe T. Calpain induces proteolysis of neuronal cytoskeleton in ischemic gerbil forebrain. Brain Res 2003;984:122–132. [DOI] [PubMed] [Google Scholar]
- 51. Yokota M, Tani E, Tsubuki S, et al. Calpain inhibitor entrapped in liposome rescues ischemic neuronal damage. Brain Res 1999;819:8–14. [DOI] [PubMed] [Google Scholar]
- 52. Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis 2007;12:993–1010. [DOI] [PubMed] [Google Scholar]
- 53. Liu JL, Li JP, Dong WW. Fastigial nudeus electrical stimulation protecting agaimt ischamia‐ reperfusion induced oxidative DNA damage in rat brain. Stroke Nerv Dis 2004;11:147–150. [Google Scholar]
- 54. Gupta M, Gupta SK, Hoffman B, Liebermann DA. Gadd45a and Gadd45b protect hematopoietic cells from UV‐induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem 2006;281:17552–17558. [DOI] [PubMed] [Google Scholar]
- 55. Liebermann DA, Hoffman B. Gadd45 in the response of hematopoietic cells to genotoxic stress. Blood Cells Mol Dis 2007;39:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma DK, Jang MH, Guo JU, et al. Neuronal activity‐induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009;323:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen J, Uchimura K, Stetler RA, et al. Transient global ischemia triggers expression of the DNA damage inducible gene GADD45 in the rat brain. J Cereb Blood Flow Metab 1998;18:646–657. [DOI] [PubMed] [Google Scholar]
- 58. Jin K, Chen J, Kawaguchi K, et al. Focal ischemia induces expression of the DNA damage‐inducible gene GADD45 in the rat brain. NeuroReport 1996;7:1797–1802. [DOI] [PubMed] [Google Scholar]
- 59. Liu B, Li JR, Li LL, Yu LH, Li CQ. Electrical stimulation of cerebellar fastigial nucleus promotes the expression of growth arrest and DNA damage inducible gene and motor function recovery in cerebral ischemia/reperfusion rats. Neurosci Lett 2012;520:110–114. [DOI] [PubMed] [Google Scholar]
- 60. Tagaya M, Matsuyama T, Nakamura H, et al. Increased F1/GAP‐43 mRNA accumulation in gerbil hippocampus after brain ischemia. J Cereb Blood Flow Metab 1995;15:1132–1136. [DOI] [PubMed] [Google Scholar]
- 61. Sommervaille T, Reynolds ML, Woolf CJ. Time‐dependent differences in the increase in GAP‐43 expression in dorsal root ganglion cells after peripheral axotomy. Neuroscience 1991;45:213–220. [DOI] [PubMed] [Google Scholar]
- 62. GrandPré T, Li S, Strittmatter SM. Nogo‐66 receptor antagonist peptide promotes axonal regeneration. Nature 2002;417:547–551. [DOI] [PubMed] [Google Scholar]
- 63. Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo‐A and Nogo‐66 receptor proteins at sites of axon‐myelin and synaptic contact. J Neurosci 2002;22:5505–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang S, Zhang Q, Zhang JH, Qin X. Electro‐stimulation of cerebellar fastigial nucleus (FNS) improves axonal regeneration. Front Biosci 2008;1:6999–7007. [DOI] [PubMed] [Google Scholar]
- 65. Takasawa K, Kitagawa K, Yagita Y, et al. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2002;22:299–307. [DOI] [PubMed] [Google Scholar]
- 66. Huang ZY, Wu CJ, Zhu XF, Dong SX, Wei CJ. Survival and migration of transplanted neural stem cells: Can it elevate the efficiency of transplantation by cerebellar fastigial nucleus stimulation? J Clin Rehabil Tissue Eng Res 2010;14:985–991. [Google Scholar]
- 67. Xu X. Application of cerebellar fustigial nucleus electric stimulation on rehabilitation of cerebrovascular diseases. Lab Med Clin 2010;7:1318–1321. [Google Scholar]
- 68. Shen HQ. Cerebral circulation function therapeutic apparatus of the role of the mechanism and clinical effect. Prog Biomed Eng 2009;30:245–246. [Google Scholar]
- 69. He WY, Li BP, Huang XZ. Clinical observation of treatment of acute cerebral infarction by electrical stimulation of the fastigial nucleus. J Apoplexy Nerv Dis 2003;20:470. [Google Scholar]
- 70. Dow RS. Some novel concepts of cerebellar physiology. Mt Sinai J Med 1974;41:103–119. [PubMed] [Google Scholar]
- 71. Schmahman JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 1998;121:561–579. [DOI] [PubMed] [Google Scholar]
- 72. Frick RB. The ego and the vestibulocerebellar system: Some theoretical perspectives. Psychoanal Q 1982;51:93–112. [PubMed] [Google Scholar]
- 73. Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, Frackowiak RS. Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry 1992;55:768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tan XL, Dong WW, Yang QD. The prophylactic and curative effects of electrical stimulation of cerebellar fastigial nucleus on vascular dementia in rats. Stroke Nerv Dis 2004;11:349–352. [Google Scholar]
- 75. Sui R, Zhang L, Min L, Yuan J, Li X. Cerebellar dysfunction may play an important role in post‐stroke depression. Med Hypotheses 2009;72:643–646. [DOI] [PubMed] [Google Scholar]
- 76. Baldaçara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Rev Bras Psiquiatr 2008;30:281–289. [DOI] [PubMed] [Google Scholar]
- 77. Lakusic N, Mahovic D, Babic T. Gradual recovery of impaired cardiac autonomic balance within first six months after ischemic cerebral stroke. Acta Neurol Belg 2005;105:39–42. [PubMed] [Google Scholar]
- 78. Chen PL, Kuo TB, Yang CC. Parasympathetic activity correlates with early outcome in patients with large artery atherosclerotic stroke. J Neurol Sci 2012;314:57–61. [DOI] [PubMed] [Google Scholar]
- 79. Wang YM, Liu XD, Dong WW. Electrical stimulation of cerebellums fastigial nucleus protects neurogenic autonomic function in rats after ischemic stroke. Chin J Phys Med Rehabil 2006;28:221–224. [Google Scholar]
- 80. Wang YM, Liu XD, Dong WW, Yang ZC. The investigation on the effect of treatment in cerebrogenic cardiac autonomic nerve function disturbances. J Clin Intern Med 2004;21:267–269. [Google Scholar]
- 81. Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar projections to the limbic system. J Neurophysiol 1959;22:451–458. [DOI] [PubMed] [Google Scholar]
- 82. Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: Effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry 1978;13:501–529. [PubMed] [Google Scholar]
- 83. Dietrichs E. Divergent axon collaterals to cerebellum and amygdala from neurons in the parabrachial nucleus locus coeruleus and some adjacent nuclei: A fluorescent double labeling study using rhodamine labeled latex microspheres and fast blue as retrograde tracers. Anat Embryol (Berl) 1985;172:375–382. [DOI] [PubMed] [Google Scholar]
- 84. Deitrichs E, Haines DE. Observations on the cerebellohypothalamic projection, with comments on non‐somatic cerebellar circuits. Arch Ital Biol 1985;123:133–139. [PubMed] [Google Scholar]
- 85. Li L, Li X, Zhang RF. Effect of fastigial nucleus electro‐stimulation on neuropeptide Y levels in myocardial infarction heart in rats. Chin J Mod Med 2007;17:1717–1722. [Google Scholar]


