Acupuncture is used by one in five fibromyalgia patients within 2 years of diagnosis and has a moderate effect on pain and stiffness compared with no treatment and standard therapy 1. The mechanism of acupuncture‐induced analgesia is likely based on the activation of different types of mechanical and nociceptive fibers that is induced by the insertion of needles into acupoints 2. The functional state of nociceptive pathways can be evaluated by multichannel laser‐evoked potentials (LEPs) 3. The aim of this study was to evaluate acute changes in multichannel LEPs induced by abdominal acupuncture in a cohort of acupuncture treatment‐naïve fibromyalgia patients.
Ten fibromyalgia patients were diagnosed according to the criteria of Wolfe et al. 4 and agreed to participate in this study, which was approved by local Ethic Committee of Bari Policlinico General Hospital. No patient had any peripheral or central nervous system disease. In the real acupuncture session, needles (40 × 0.2 mm) were inserted according to the abdominal meridian system and turtle system in CV 12, 10, 6, and 4, Ren 13 and 17, and ST 24 and 26 5. In the sham acupuncture session, the needles were inserted 3–5 mm away from the acupoints. In both sessions, the needles were inserted for 30 min. Each patient was submitted to real and sham acupuncture session, and the two sessions were separated by at least 15 days to avoid carryover effects. The order of the real and sham acupuncture sessions was randomized among patients.
Before needle insertion (baseline condition) and soon after needle removal, 30 CO2 laser stimuli (duration, 25 ms; interstimulus interval, 10 s; wavelength, 10.6 mm) were delivered to each of three sites: the right abdomen near the umbilicus, the dorsum of the right hand, and the skin over the chest tender point 6. The order of stimulation was randomized among patients. Stimulus intensity was determined on a subject‐specific basis and was above the pain threshold. Electroencephalography was recorded throughout the laser stimulation using 62 scalp electrodes positioned according to the enlarged 10–20 system. At the beginning and end of each session, the pain level at tender points was evaluated according to Okifuji et al. 6.
A preliminary EEG analysis was performed by an examiner blinded to the conditions (baseline, sham acupuncture, and real acupuncture) considering the CZ derivation, referred to nasion, and T3, referred to Fz, to evaluate the N2 and P2 vertex components and the N1 temporal component of each LEP 3. LEPs were analyzed using standardized low‐resolution brain electromagnetic tomography (s‐LORETA) software 7.
The N2‐P2 amplitude obtained by the abdomen stimulation was significantly increased after acupuncture session (repeated‐measures ANOVA with condition baseline, sham, acupuncture as factor F = 13.40, df = 2, P = 0.002), while it was reduced after acupuncture when the chest tender point was considered (F = 7.52; P = 0.012). (Table 1). No change was observed on LEPs obtained by the hand. (F = 2.78, n.s.). The subjective laser pain, measured using a visual analogical scale ranging from 0 to 100, did not change among the experimental conditions (F = 0.34, 1.24 and 0.98 for the hand, chest tender point and abdomen, respectively, df = 2, n.s.; Table 1).
Table 1.
Mean values and standard deviation of laser‐evoked potentials waves amplitudes recorded in the 10 FM patients by the stimulation of the right abdomen, hand dorsum, and chest tender point
| n2‐p2 amplitude (uv) | n1 amplitude (uv) | Vas | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Abdomen | Hand | Tender point | Abdomen | Hand | Tender point | Abdomen | Hand | Tender point | |
| Basal | 12.2 ± 8.9 | 12.4 ± 9.9 | 11.4 ± 10.9 | 2.53 ± 3.4 | 1.91 ± 3.2 | 2.2 ± 4.2 | 56.9 ± 24.5 | 50.4 ± 21.1 | 52 ± 22.6 |
| Sham | 12.5 ± 14.4 | 9.5 ± 11.4 | 12 ± 11.6 | 5.1 ± 6.5 | 2.27 ± 5.9 | 3.9 ± 5.6 | 65 ± 14.6 | 51 ± 12.7 | 51 ± 12.6 |
| Acupuncture | 16.3 ± 12.3* , ** | 8.5 ± 11.5 | 6.7 ± 6.3* , ** | 2.6 ± 3.5 | 4 ± 5.9 | 2.2 ± 3.8 | 63 ± 27.1 | 53.3 ± 26.7 | 53.3 ± 26.3 |
Results of Bonferroni test are shown: *acupuncture versus basal P < 0.05 **acupuncture versus sham P < 0.05.
The voxel‐by‐voxel log F ratio, employed to detect the dipolar source of LEPs 7, was computed for real acupuncture versus the baseline condition in the time interval of the N2 LEP component (180–250 ms) for abdomen stimulation and in the time window of the P2 LEP component (290–340 ms) for chest tender point stimulation. The N2 component of the potential evoked by abdomen stimulation was higher after real acupuncture than in the baseline condition in the cingulate gyrus (Brodmann area 24) and the inferior operculum (Brodmann areas 40 and 41; Figure 1A). The P2 component of the potential evoked by chest tender point stimulation was lower after real acupuncture than in the baseline condition in the bilateral middle and inferior frontal gyrus (Brodmann area 9; Figure 1B).
Figure 1.
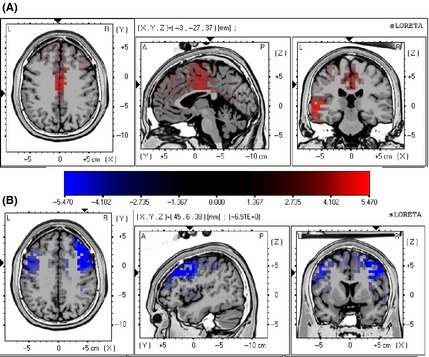
(A, B) s‐LORETA maps reporting statistical comparison of main dipolar sources of laser‐evoked potentials (LEPs) obtained by stimulation of the abdomen (A) and the right chest tender point (B) in acupuncture versus baseline conditions. The difference is significant at P = 0.01 for the two‐tailed test. In response to stimulation of the abdomen, activation of the cingulate gyrus (Brodmann area 24), insula, and inferior operculum (Brodmann areas 40 and 41) was higher after real acupuncture than in the baseline condition (A). In response to stimulation of the right chest tender point, activation of the bilateral middle and inferior frontal gyrus (Brodmann area 9) was lower after real acupuncture than in the baseline condition (B).
Pain at tender point was significantly lower after real acupuncture (78.5 ± 25.5) than after sham acupuncture (100.1 ± 45.5) and in the baseline condition (123.4 ± 34.5) (repeated‐measures ANOVA F = 7.52, P = 0.012; Bonferroni test: acupuncture versus basal and sham P < 0.05).
In our patients, needle insertion and manipulation at the abdomen increased cortical responses evoked by laser stimulation of skin zones close to the acupoints and reduced cortical responses evoked by laser stimulation of the chest tender point. This phenomenon supports the hypothesis that acupuncture‐induced analgesia involves integrative processes between afferent impulses from pain regions and afferent impulses from acupoints at different levels of the central nervous system 2. Increased activation of cortical areas devoted to processing nociceptive inputs from the abdomen may induce a complex inhibitory modulation of painful signals from other sites, particularly from sites that are prone to spontaneous or provoked pain. The results of our dipolar analysis suggest that a complex interaction may occur between cortical zones at the insula and the anterior cingulate, which process the cognitive and affective components of pain 8, and the prefrontal cortical zones that are involved in pain modulation 9. Such cognitive and emotional experience linked to acupuncture, induced an acute relief of pain at the tender point. Although the sample size was small, these results confirm the analgesic effect of acupuncture in fibromyalgia patients and support the presence of a real interaction between acupuncture and mechanisms of pain processing in this complex syndrome.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev 2013;(5):CD007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008;85:355–375. [DOI] [PubMed] [Google Scholar]
- 3. Treede RD, Lorenz J, Baumgärtner U. Clinical usefulness of laser‐evoked potentials. Neurophysiol Clin 2003;33:303–314. [DOI] [PubMed] [Google Scholar]
- 4. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- 5. Bo ZY, Niu QQ, Zhu WG, Xiang Y, Wang GK, Yuan SM. [Multicenter controlled study on abdominal acupuncture for treatment of nerve root type cervical spondylosis]. Zhongguo Zhen Jiu 2005;25:387–389. [PubMed] [Google Scholar]
- 6. Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey. I. development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol 1997;24:377–383. [PubMed] [Google Scholar]
- 7. Pascual‐Marqui RD. Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol 2002;24:5–12. [PubMed] [Google Scholar]
- 8. Stancak A, Fallon N. Emotional modulation of experimental pain: A source imaging study of laser evoked potentials. Front Hum Neurosci 2013;17:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126(Pt 5):1079–1091. [DOI] [PubMed] [Google Scholar]


