Summary
Aims
Different trophic factors are known to promote retinal ganglion cell survival and regeneration, but each had their own limitations. We report that hepatocyte growth factor (HGF) confers distinct advantages in supporting ganglion cell survival and axonal regeneration, when compared to two well‐established trophic factors ciliary neurotrophic factor (CNTF) and brain‐derived neurotrophic factor (BDNF).
Methods
Ganglion cells in adult hamster were injured by cutting the optic nerve. HGF, CNTF, or BDNF was injected at different dosages intravitreally after injury. Ganglion cell survival was quantified at 7, 14, or 28 days postinjury. Peripheral nerve (PN) grafting to the cut optic nerve of the growth factor–injected eye was performed either immediately after injury or delayed until 7 days post‐injury. Expression of heat‐shock protein 27 and changes in microglia numbers were quantified in different growth factor groups. The cellular distribution of c‐Met in the retina was examined by anti‐c‐Met immunostaining.
Results
Hepatocyte Growth Factor (HGF) was equally potent as BDNF in promoting short‐term survival (up to 14 days post‐injury) and also supported survival at 28 days post‐injury when ganglion cells treated by CNTF or BDNF failed to be sustained. When grafting was performed without delay, HGF stimulated twice the number of axons to regenerate compared with control but was less potent than CNTF. However, in PN grafting delayed for 7 days after optic nerve injury, HGF maintained a better propensity of ganglion cells to regenerate than CNTF. Unlike CNTF, HGF application did not increase HSP27 expression in ganglion cells. Microglia proliferation was prolonged in HGF‐treated retinas compared with CNTF or BDNF. C‐Met was localized to both ganglion cells and Muller cells, suggesting HGF could be neuroprotective via interacting with both neurons and glia.
Conclusion
Compared with CNTF or BDNF, HGF is advantageous in sustaining long‐term ganglion cell survival and their propensity to respond to favorable stimuli.
Keywords: Heat‐shock protein, Microglia, Neuroprotection, Optic nerve, Retinal ganglion cell, Trophic factor
Introduction
It is well known that most neurons in the adult central nervous system (CNS) undergo cell death after axonal injury, and those that survive do not regenerate, leading to significant functional deficits. Different CNS models have been used to explore the effects of trophic factors on neuronal survival and regeneration, one of which is retinal ganglion cells and their axons projecting to the brain via the optic nerve. Of the multitude of growth factors that have been examined in the ganglion cell model, only brain‐derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) have been demonstrated to exhibit significant survival‐promoting and axonal growth–promoting effects, respectively 1, 2, 3. However, there are limitations with respect to the actions of these two factors, for example, BDNF only promotes short term survival (for the first 2 weeks post‐injury) even after either multiple intravitreal injections, delivery by osmotic minipump or adenovirus infection 1, 4, 5, but it does not support or even inhibit axonal regeneration 6, 7. On the other hand, although CNTF stimulates more ganglion cells to regenerate axons into a peripheral nerve graft compared with other trophic factors 2, it only slightly enhances survival 8. Thus, it becomes imperative to search for other trophic factors that could provide additional beneficial effects, for example, a longer survival‐promoting period as well as stimulating regeneration from ganglion cells that are not responsive to CNTF.
Hepatocyte Growth Factor (HGF) is a pleiotropic cytokine with multiple actions on a wide range of target cells. HGF was first isolated from serum and blood platelets of animals with liver damage 9, 10. It is a 90kD heparin‐binding polypeptide comprised of two subunits and having a 4‐kringle domain as well as an inactive serine protease domain, thus sharing similarities with other protein molecules involved in blood coagulation like plasminogen 11. Originally characterized as a potent mitogen for hepatocytes, HGF is now known to be produced by many types of cells, including fibroblasts, endothelial cells, macrophages, and some types of epithelial and tumor cells, and it exerts multiple actions like migration, proliferation, and differentiation on many different cells and tissues through binding with its receptor c‐Met (mesenchymal–epithelial transition) which is a proto‐oncogene product with tyrosine kinase activity 12. It comes to no surprise that HGF also has a wide range of activity on the nervous system. It promotes the survival and differentiation of motor, sensory, and sympathetic neurons in culture 13, 14, as well as the survival of spinal, hypoglossal, and facial motor neurons after peripheral nerve damage in vivo 15, 16, 17. In the developing CNS, HGF stimulates oligodendrocyte precursor cells and neural precursors to proliferate, and migrate 18, 19, and promotes hippocampal neuron dendritic maturation 20. On the other hand, its effect on the in vivo CNS after injury is just starting to be explored. In models of cerebral ischemia, Alzheimer's disease and amyotrophic lateral sclerosis, HGF delivery to the affected brain regions delays disease progression or promotes functional recovery 21, 22, 23. HGF also reduces secondary degeneration of the contused rat spinal cord and promotes functional recovery when introduced to the damaged site via a viral vector carrying the gene 24.
With respect to the retina and visual pathway, intravitreal HGF injection reduces retinal degeneration after ischemia 25 and photoreceptor cell death in Royal College of Surgeons (RCS) rats 26. Due to its pleiotropic activities on a wide range of cells including neurons, we hypothesize that HGF may be beneficial to ganglion cells after axotomy. In pilot studies, we first reported that similar to BDNF and CNTF, HGF promoted the survival of ganglion cells after optic nerve injury in the hamster and stimulated ganglion cell axons to regenerate into a peripheral nerve (PN) graft 27. In the rat, it has also been shown that HGF stimulates ganglion cell survival and axonal regeneration in the optic nerve 28. However, it is not known whether HGF would provide more beneficial outcomes with respect to the limitations in neuroprotection by BDNF and CNTF. Here, we report that by comparing different dosages on the spatiotemporal course of ganglion cell survival among treatment by the three trophic factors, HGF is superior to BDNF and CNTF in promoting long‐term ganglion cell survival as well as axonal regeneration into a PN graft.
Materials and Methods
Experiments were performed in adult Syrian golden hamsters (Mesocricetus auratus) 8–12 weeks old. All surgical manipulations were performed after induction of general anesthesia by i.p. injection of ketamine/xylazine (200 mg/7.5 mg per kg body weight). The experimental protocols have been approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
Transection of the Optic Nerve to Damage Ganglion Cell Axons
The right optic nerve was exposed in the orbit and cut with microsurgical scissors at 2 mm behind the globe, care being taken not to injure the ophthalmic artery running along the inferior aspect of the dura.
Intravitreal Injection of HGF after Optic Nerve Transection
A single dose of either 1 μg or 4 μg of HGF (dissolved in 2 μl phosphate‐buffered saline [PBS]) was injected intravitreally after ON transection. Injection was performed by a fine‐tipped glass micropipette attached to an electrically controlled microinjector (World Precision Instruments, Sarasota, FL, USA). A small hole was made in the superior limbal region of the eye, and the micropipette (loaded with growth factor) tip was gently inserted through the hole into the vitreous under microscopic guidance via a micromanipulator. The growth factor was injected into the vitreous in a series of pulses within 1 min, after which the pipette tip was maintained in situ for another minute before being withdrawn from the eye. To act as a control for nonspecific effects of HGF injection, either PBS, or bovine serum albumin (BSA, 4 μg dissolved in PBS), was injected in the same volume. BSA (an inert protein with no trophic action) served as a control to exclude the possibility of any nonspecific stimuli being elicited due to injection of a foreign protein. The results indicated that BSA injection did not differ from the vehicle PBS in terms of influencing ganglion cell survival.
Comparison of Trophic Activity of HGF, BDNF, or CNTF
The three trophic factors (recombinant proteins from Invitrogen) were studied in separate paradigms of intravitreal injection to compare their effects on ganglion cell survival after optic nerve injury:
A single dose of 1 μg of trophic factor was injected;
A single dose of 4 μg of trophic factor was injected;
Multiple injection of 1 μg of trophic factor each day for four consecutive days (1 μg × 4).
Quantification of Ganglion Cell Survival
The number of ganglion cells in the normal retina as well as their surviving numbers at 7, 14, or 28 days post‐optic nerve injury were determined by immuostaining with anti‐βIII‐tubulin (clone TuJ1, Covance, Princeton, NJ, USA) which is a well‐established ganglion cell marker 29. The animal was sacrificed by an overdose i.p. injection of chloral hydrate and perfused transcardially with phosphate‐buffered saline. The retina was removed and fixed as a wholemount in 4% paraformaldehyde for 1 h. The retina was incubated in TuJ1 antibody (mouse monoclonal, 1:1000) overnight at 4°C followed by anti‐mouse IgG coupled to Cy3 (Jackson ImmunoResearch, West Grove, PA, USA, 1:400) overnight at 4°C. After staining was finished, the retina was mounted in glycerol and observed under the microscope with epifluorescent illumination.
The number of ganglion cells in the wholemount retina was quantified using the morphometric analysis system NeuroLucida (MicroBrightfield, Williston, VT, USA), using a sampling technique. Briefly, sites (delimited by a square grid 200 × 200 μm) spaced at 500 μm along two lines extending from the optic disk to the retinal margin in each retinal quadrant were examined, and the number of labeled cells within the grid boundary were recorded; all sites in the retina were then averaged to deduce the mean cell density, and the number of surviving ganglion cells in the whole retina was calculated by relating to the retinal surface area. The number of surviving cells in each experimental retina was then divided by the mean number of ganglion cells in the normal retina (80618 ± 671 cells per retina, estimated from a total of five retinas) and expressed as a percentage of ganglion cell survival of that retina.
The ganglion cell densities at central and peripheral regions of each retina at 28 days post‐injury were also calculated. This was performed by dividing the whole retina into four quadrants (superior temporal, superior nasal, inferior temporal, inferior nasal), and each quadrant was further delineated into a central and peripheral region (each comprising one half of the retinal radius). Ganglion cell counts of the sampling grid sites in either the central or the peripheral region were averaged and divided by the area of the grid to obtain the ganglion cell density. Ganglion cell densities of the central and peripheral regions of the four quadrants were separately tabulated and compared among the growth factors.
Soma sizes of the sampled ganglion cells were deduced by tracing the outlines of the somata in the NeuroLucida program from which the soma area could be derived. The data of all sampled cells from retinas of each time point were collected and plotted as a histogram of soma size frequency distribution (with a bin size of 50 μm2) for each growth factor.
Regeneration of Ganglion Cell Axons into a Peripheral Nerve (PN) Graft
To assess whether ganglion cell axonal regeneration would be enhanced by HGF treatment and to compare its efficacy against the stimulus of CNTF or BDNF, the optic nerve was cut and HGF or other growth factors was injected intravitreally at a single 4 μg dose. Grafting of a PN to the optic nerve of the growth factor‐injected eye was performed in two paradigms. In the first experiment, immediately following optic nerve cut and growth factor injection, a 1.5 cm long PN (common peroneal branch of sciatic nerve from the same animal) was opposed to the cut optic nerve without delay, as described previously 30. In another group of experiment, the optic nerve was cut and growth factor was injected, but PN grafting was delayed until the seventh day postoptic nerve cut. The rationale for performing the delayed grafting was to see whether HGF or other growth factor treatment could sustain the ability of ganglion cell axons to respond to a favorable support like the PN graft if it was provided not immediately after injury but some time after—a circumstance that is a better mimic of clinically encountered CNS injury.
The number of ganglion cell axons that had regenerated into the PN graft was quantified at 28 days post‐grafting by retrograde labeling of the cell bodies of regenerating ganglion cells with a fluorescent marker Granular Blue (GB) applied to the PN 30. All GB‐labeled cells (representing cells that have regenerated axons into the PN) in the retina were counted with NeuroLucida.
Expression of the Heat‐Shock Protein HSP27 after Optic Nerve Injury and Growth Factor Treatment
The expression of HSP27 by ganglion cells after optic nerve cut and injection of growth factors was examined at 7 and 14 days post‐injury in the wholemount retina by immunofluorescence with anti‐HSP27 (rabbit polyclonal, StressGene; 1:1000) and visualized by anti‐mouse‐Cy3 (Jackson; 1:500). All HSP27‐stained ganglion cells in the whole retina were systematically counted by the NeuroLucida software.
Changes in Microglia Number after Optic Nerve Injury and growth Factor Treatment
Microglial number and activity is well known to be increased after neuronal injury 31, and such increases have been thought to be detrimental to neuronal survival. For instance, inhibition of microglial activation after optic nerve injury or in glaucoma promotes ganglion cell survival and retards optic axon degeneration 32, 33. However, there have also been recent studies suggesting enhancement of neuronal survival by activated microglia 34. To test whether the influence of ganglion cell survival by HGF or other growth factors correlates with changes in microglial activity, their numbers were quantified at 7 and 14 days post‐optic nerve cut. Wholemount retinas were incubated in anti‐Iba‐1 (rabbit polyclonal, Wako Chuo‐Ku, Osaka, Japan) which is a pan‐microglia marker and visualized with anti‐rabbit‐biotin plus Streptavidin‐Cy2. The number of microglia in distinct sublaminae of the retina, namely nerve fiber–ganglion cell layer, inner plexiform layer, and outer plexiform layer, was separately quantified by sampling as described for TuJ1 above.
C‐Met Localization in the Retina
To gain further insights into the cellular targets that respond to exogenous HGF treatment, the distribution of c‐Met in normal and optic nerve‐injured retinas was studied. Ten micrometer‐thick cryostat sections of normal and 7 days post‐optic nerve‐cut retinas were prepared and double‐immunostained with goat anti‐c‐Met (1:200; Sigma, St. Louis, MO, USA), together with either TuJ1, glutamine synthetase (1:1000 Chemicon, Temecula, CA, USA) ‐ a marker for Muller cells, or Iba‐1. Anti‐c‐Met was visualized with anti‐goat‐Cy3 and the other primary antibody with biotin‐conjugated secondary antibody plus Streptavidin‐Cy2, followed by counterstaining with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI) nuclear stain.
Number of Animals used and Statistical Analysis
For quantification of the number of surviving ganglion cells and ganglion cell densities distribution, each experimental group consisted of five retinas. Soma size frequency distribution histograms were compiled from four retinas in each group.
In the study of the influence of growth factor on ganglion cell axonal regeneration, four to five animals were employed in each growth factor grouping.
For the study of HSP27 expression and microglia change dynamics, four to five retinas were included in each growth factor group.
For c‐Met localization in retinal sections, four retinas were taken from normal animals as well as animals with optic nerve cut for 7 days.
All quantitative data were presented as the mean ± SEM. For statistical comparison of the outcomes from the experimental groups, one‐way ANOVA followed by post hoc pairwise comparison was employed, with the level of statistical significance set at P < 0.05.
Results
A Single Intravitreal HGF Injection Promoted Ganglion Cell Survival after Optic Nerve Cut
Retinal ganglion cells were labeled by TuJ1 (Figure 1B) and their numbers quantified in normal retinas as well as in retinas after optic nerve injury. The mean number of ganglion cells in normal animals was 80618 ± 671 (n = 5), and surviving cells in each experimental retina was quantified and expressed as a percentage survival of this number.
Figure 1.
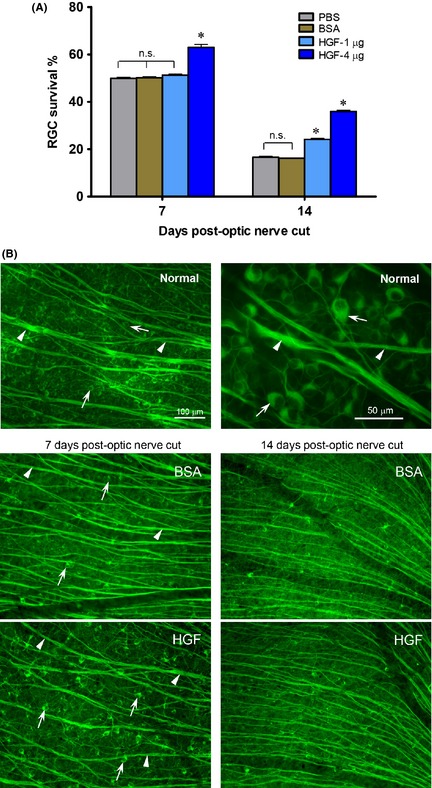
A single intravitreal HGF injection after optic nerve injury promotes ganglion cell survival. (A) Graph showing the effect of either 1 or 4 μg HGF on ganglion cell survival at 7 or 14 days post‐optic nerve cut. *P < 0.01 significantly higher than the vehicle control PBS or BSA. n.s. not significantly different from control PBS or BSA. (B) Photomicrographs illustrating the normal retina (upper panel), and part of the mid‐superior retina at 7 or 14 days post‐optic nerve cut and HGF or BSA control injection (middle and lower panels), immunostained by TuJ1. Arrows point to ganglion cell bodies and arrowheads to axon bundles. The 100 μm scale bar in the normal retina panel is also applicable to the HGF and BSA panels below. Note that although optic nerve cut led to ganglion cell death in both HGF and BSA control groups, more ganglion cells survived after HGF treatment compared with BSA control at 7 or 14 days post‐injury.
A single dose of either 1 or 4 μg HGF was injected intravitreally after cutting the optic nerve. At 7 days post‐injury, 4 μg HGF treatment resulted in enhanced ganglion cell survival (63 ± 1.3%) compared with PBS vehicle control (49.9 ± 0.5%, P < 0.01, one‐way ANOVA followed by Tukey post hoc analysis) or BSA (50.1 ± 0.4%, P < 0.01), while 1 μg HGF did not promote survival (P > 0.1, Figure 1A). The beneficial effect of HGF was still evident at 14 days post‐injury, when both 1 and 4 μg HGF sustained better ganglion cell survival compared with PBS or BSA (P < 0.01, Figure 1A). Examples of the influence of 4 μg HGF on axotomized ganglion cells were shown in Figure 1B. It could be seen that in the normal retina, many ganglion cells and their axon bundles were labeled by TuJ1. Optic nerve injury in either BSA control or HGF group led to a decrease in ganglion cell numbers as well as thinner axon bundles. However, more ganglion cell somata were preserved, and cell shrinkage was less, after HGF injection compared to BSA control. As BSA treatment did not alter ganglion cell survival as compared to PBS (Figure 1A), this suggested that injection of an inert protein did not elicit any nonspecific stimulus on ganglion cells. In subsequent experiments, BSA was used as the control.
Different Dosages of HGF Promoted Long‐Term Ganglion Cell Survival Compared with BDNF or CNTF
Single 1 or 4 μg Intravitreal Injection
The potency of HGF to stimulate ganglion cell survival, especially at longer survival times post‐injury, was compared to BDNF or CNTF, two well‐known trophic factors for ganglion cell survival. When a single dose of 1 μg of the respective growth factors was administered intravitreally after optic nerve cut, only BDNF promoted ganglion cell survival at 7 days post‐injury (P < 0.05 compared with BSA control), whereas at 14 days post‐injury, HGF also exhibited survival‐promoting effect (P < 0.05 compared with BSA control), the magnitude of which was not different from that of BDNF (Figure 2A). More importantly, only HGF continued to protect ganglion cells at 28 days post‐injury (P < 0.05 compared with BSA control), while both BDNF and CNTF failed to do so (P > 0.1 compared with BSA control). A similar picture was observed when 4 μg growth factor was delivered (Figure 2B): at 7 days post‐injury, all three growth factors promoted survival above that of the BSA control (P < 0.05 compared with BSA control), with BDNF being the most potent; however, by 14 days post‐injury, HGF treatment resulted in a similar extent of protection as BDNF, while the beneficial effect of CNTF was dwindling. As in the case of 1 μg treatment, only 4 μg HGF still afford significant protection at 28 days, with ganglion cell survival (16.4 ± 0.4%) being double that of BDNF (8.4 ± 0.2%), CNTF (7.5 ± 0.2%), or BSA control (7.6 ± 0.1%) (P < 0.05 compared with either BDNF, CNTF, or BSA, Figure 2B).
Figure 2.
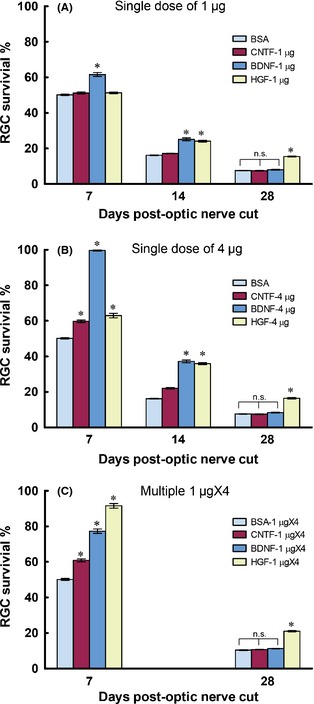
Graphs comparing the ganglion cell survival–promoting effects of three trophic factors CNTF, BDNF, and HGF delivered intravitreally at different dosages. BSA was used as the control. (A) A single dose of 1 μg of trophic factor was injected immediately after optic nerve cut. (B) A single dose of 4 μg trophic factor was injected. (C) A dose of 1 μg trophic factor was injected per day for a consecutive period of 4 days (multiple 1 μg × 4), starting from the day of injury. For the single dose experiments, ganglion cell survival was quantified at 7, 14, and 28 days postinjury, while in the multiple dose group, only 7 and 28 days were examined. *P < 0.05 significantly higher than the BSA control at the corresponding time period. n.s. not significantly different compared with BSA control.
1 μg × 4 Intravitreal Injection
We also asked whether multiple injections of growth factors could result in better neuroprotection than single dose treatment. Each growth factor was injected intravitreally at 1 μg per day for a consecutive 4 days (1 μg × 4) after optic nerve cut, and ganglion cell survival was assessed at 7 and 28 days post‐injury. Interestingly, 1 μg × 4 HGF resulted in a dramatic increase in ganglion cell survival (91.6 ± 1.3%) at 7 days post‐injury which was much better than either 1 μg × 4 BDNF (77.3 ± 1.2%) or CNTF (60.9 ± 0.8%) (P < 0.05 compared with either BDNF or CNTF, Figure 2C). In fact, this value was not much different to that achieved by a single 4 μg BDNF at 7 days post‐injury (99.6 ± 0.4%, Figure 2B), showing that 1 μg × 4 HGF could almost obliterate ganglion cell death at this time point. We did not assess ganglion cell survival at 14 days post‐injury for this dosage, but when examined at 28 days postinjury, 1 μgX4 HGF treatment resulted in ganglion cell survival that was doubled that of 1 μg × 4 BDNF, CNTF, or BSA control ((P < 0.05 compared with either BDNF, CNTF, or BSA, Figure 2C). The overall conclusion from these dosage studies was that although all three growth factors promoted ganglion cell survival at short post‐injury times (14 days or less), only HGF was able to slow down the degeneration of ganglion cells at longer post‐injury times like 28 days.
HGF Protection Extended Across the Entire Retina
The extent of protection of ganglion cells and their axons by 4 μg HGF in different parts of the retina at 28 days post‐injury was illustrated in Figure 3A. HGF treatment led to an obvious preservation of ganglion cell axon bundles that converge onto the optic disk, as well as significant increases in the densities of ganglion cell somata and axons located in both the central and peripheral retinas, whereas both BDNF and CNTF treatment resembled that of the BSA control (Figure 3A). These observations were confirmed by topographic quantification of the densities of surviving ganglion cells in Figure 3B. In the normal retina, mean ganglion cell densities varied from about 1000 cells to 1500 cells per mm2, with the density at the central region higher than that of the peripheral. Twenty‐eight days after optic nerve injury, ganglion cell densities decreased to about 150 cells per mm2 in all groups except the HGF one. Across different quadrants of the retina, only the HGF‐treated retina exhibited higher ganglion cell densities in both central and peripheral retinas, their values being at least twice of that in retinas treated with BDNF, CNTF, or BSA control (P < 0.05 compared with either BDNF, CNTF, or BSA, Figure 3B). This indicated that the beneficial effect of HGF was global and not topographically restricted to subsets of ganglion cells.
Figure 3.
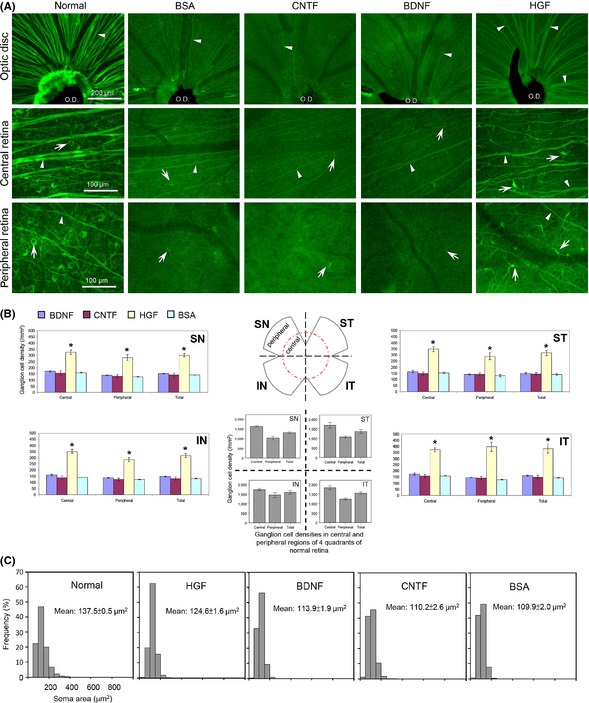
(A) Only HGF treatment resulted in higher ganglion cell survival at 28 days post‐injury. Photomicrographs of wholemount retina at different regions (optic disk, central and peripheral regions) showing surviving ganglion cells and axons at 28 days post‐injury in different trophic factor groups. The appearance of the normal retina is shown on the left column. Note that a lot of ganglion cells and their axons have degenerated by this time in the BSA control group, as well as in the CNTF and BDNF groups (which appeared similar to the BSA group). In contrast, HGF treatment resulted in markedly better preservation of axon bundles (arrowheads) converging at the optic disk (O.D.) region, as well as more ganglion cell bodies (arrows) in both central and peripheral retinal regions. Cells in the HGF group also exhibited less shrinkage compared to the other three groups. The dark strand at the optic disk region is the remnant of the hyaloid vessels. (B) Topographic quantification of surviving ganglion cell densities at 28 days post‐injury in different trophic factor groups. The distribution of ganglion cell densities at the four quadrants of the normal retina was shown in the middle for comparison. Note that only HGF treatment resulted in enhanced ganglion cell survival at all retinal regions sampled. *P < 0.05 significantly higher density than BSA, CNTF, or BDNF. (C) Frequency distribution of soma sizes (represented by soma areas) of surviving ganglion cells at 28 days post‐injury in the trophic factor‐treated groups. The soma size distribution in normal retinas on the left was used for comparison. Note that in the HGF group the distribution pattern of ganglion cell sizes most resembled that of the normal retina, with the mean cell size closest to that of the control, reflecting lesser atrophy of the surviving cells.
HGF Alleviated the Cellular Atrophy Associated with Axotomy of Ganglion Cells
Besides protecting ganglion cells from degeneration, HGF treatment also reduced cell shrinkage seen after axotomy at 28 days post‐injury. This could be seen from Figure 3A where ganglion cells in the HGF‐treated retina had larger somata than those of BDNF, CNTF, or BSA. Quantification of the soma size of the surviving ganglion cells substantiated this view (Figure 3C): the histogram of soma size distribution in the HGF‐treated retina resembled most closely that of the normal retina, whereas those of the BDNF or CNTF group were similar to the BSA control in that there were higher proportions of shrunken (smaller) somata among the surviving cells. This was also reflected by the mean cell size of the surviving population: although the mean cell size in all treated groups was less than that of cells in the normal retina, HGF treatment led to a significantly larger mean soma size than that of BDNF, CNTF, or BSA (Figure 3C). Similar preservation of soma sizes by HGF treatment was also seen at 7 and 14 days post‐injury (quantitative data not shown, but see photomicrograph in Figure 1), suggesting that HGF maintained the structural integrity of surviving cells until at least 28 days post‐injury.
HGF Stimulated Ganglion Cells to Regenerate into a PN Graft and also Maintain their Propensity to Regenerate in a Delayed Grafting Paradigm
The number of ganglion cells that regenerated axons into a PN grafted to the optic nerve immediately after injury was almost doubled when a single 4 μg HGF intravitreal injection was given (2245 ± 131, P < 0.01 compared with BSA) as compared to BSA control (1214 ± 80), although this magnitude was less than that of CNTF (4966 ± 720) which was the most robust trophic factor for ganglion cell regeneration (Figure 4A,B). BDNF injection, on the other hand, led to a decrease in axonal regeneration (645 ± 26, P < 0.01 compared with BSA), in agreement with previous studies 6, 7. However, the potency of CNTF was rapidly lost if PN grafting was delayed for 7 days post‐injury: the number of ganglion cells that could still regenerate dropped to 1237 ± 73, this being only 25% of the number stimulated by immediate grafting (Figure 4A). A similar lost of response to the delayed PN occurred in both BSA group (231 ± 24 cells, 19% of the immediate grafting number) and BDNF group (162 ± 13 cells, 25% of the immediate grafting number). In contrast, HGF treatment followed by delayed grafting resulted in 966 ± 52 regenerating ganglion cells which was 45% of the number induced by immediate grafting—a much better response than CNTF, BSA, or BDNF. In fact, when PN grafting was delayed, the number of ganglion cells that could still respond to and regenerate into the PN became comparable in the CNTF and HGF groups (P > 0.1, Figure 4A). Thus, only HGF was able to sustain the responsiveness of injured ganglion cells to a delay‐presented regenerating stimulus.
Figure 4.
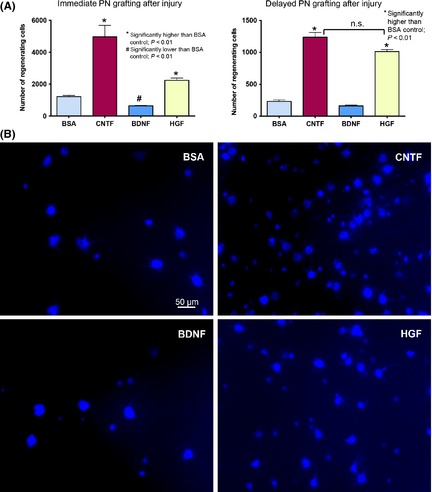
(A) Graphs comparing the number of ganglion cells that have regenerated axons into a PN graft under the influence of trophic factors. Left panel shows the result from immediate PN grafting after optic nerve cut and injection of growth factors, and right panel illustrates the results obtained when PN grafting was delayed for 7 days after optic nerve cut. n.s. not significantly different between the CNTF and HGF groups when PN grafting was delayed. (B) Photomicrographs depicting ganglion cells in the wholemount retina that have regenerated axons into a PN grafted to the cut optic nerve immediately after injury and injection of trophic factors. The regenerating cells have been retrogradely labeled by the fluorescent dye GB applied to the PN graft at 25 days after grafting and the retina examined 3 days later (at 28 days post‐grafting). Note that ganglion cells of different sizes have regenerated into the graft in each group, and that HGF and CNTF stimulated more ganglion cells to regenerate (~ 2 and 4 times, respectively, of BSA control), while BDNF reduced regeneration.
HGF‐Enhanced Survival did not Correlate with Changes in HSP27 Expression
We sought to determine whether the beneficial support of HGF on ganglion cell survival depended on enhanced HSP27 expression after injury, as have been shown in a number of neuronal systems 35. In the normal retina, no ganglion cells expressed HSP27, and only blood vessels exhibited prominent anti‐HSP27 staining (Figure 5B). After optic nerve injury and control BSA injection, a population of ganglion cells (~700) expressed HSP27 at 7 days post‐injury (Figure 5A,B), but by 14 days post‐injury, this transient expression had vanished. Treatment by CNTF dramatically elevated ganglion cell HSP27 expression by five fold at 7 days (P < 0.001 compared with BSA, Figure 5A,B) but again most cells had disappeared by 14 days post‐injury, while BDNF injection actually led to a reduction in HSP27 expression compared with BSA control (P < 0.01 compared with BSA). However, HGF delivery to ganglion cells did not alter the number of HSP27‐positive ganglion cells as compared to the control (P > 0.1 compared with BSA, Figure 5A), regardless after a single 4 μg or multiple 1 μg × 4 injections (despite the latter treatment stimulating 90% survival at 7 days post‐injury). Thus, HGF promoted ganglion cell survival via mechanisms distinct from HSP27 induction and protection.
Figure 5.
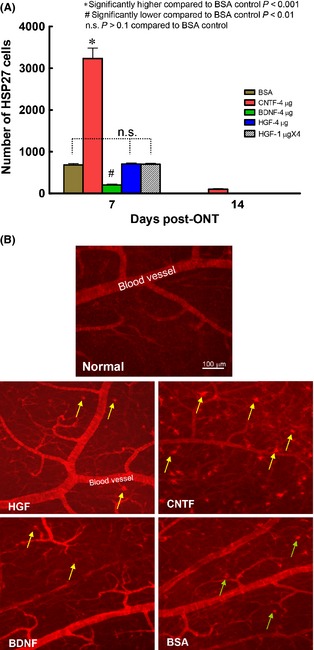
HSP27 expression induced by optic nerve cut and trophic factor injection. (A) Graph comparing the expression of HSP27 in ganglion cells after axotomy and trophic factor treatment. Note that at 7 days postinjury, both single dose and multiple injections of HGF failed to up‐regulate HSP27 expression in ganglion cells, while CNTF treatment led to a drastic increase in ganglion cells expressing HSP27 compared with BSA control. At 14 days postinjury, most groups (except CNTF) had almost no HSP27 cell expression, thus the bars seemed to be missing. (B) Photomicrographs of part of the superior temporal retina depicting HSP27‐positive staining in the normal retina or 7 days optic nerve‐injured retinas in different trophic factor‐treated groups. Note ganglion cells (pointed by arrows) present in all the experimental groups except the normal retina. Blood vessels were also prominently stained.
HGF Stimulated more Microglial Proliferation than CNTF or BDNF
Retinal microglia resided in distinct retinal sublaminae which could be well visualized in wholemount preparations (Figure 6B). Microglia in different layers proliferated after optic nerve injury, especially in the nerve fiber/ganglion cell layer where they tripled in number at 7 days post‐injury (Figure 6A). Injection of CNTF or HGF further increased their numbers in the nerve fiber‐ganglion cell layer and outer plexiform layer over that of the BSA control (P < 0.05 compared with BSA), while BDNF treatment led to numbers similar to that of BSA control (P > 0.1 compared to BSA). At 14 days post‐injury, microglial numbers of all injury groups were still above that of the normal animal. However, while microglial numbers in CNTF and BDNF groups had subsided to the same level as that of the BSA control, microglial proliferation in the HGF group was still elevated in all three sublaminae (P < 0.05 compared with either BDNF, CNTF, or BSA, Figure 6A,B). Figure 6B illustrated the appearance of microglia in the nerve fiber layer at 14 days post‐injury: after HGF treatment microglia density was higher and their cell bodies also tend to be larger with more robust processes.
Figure 6.
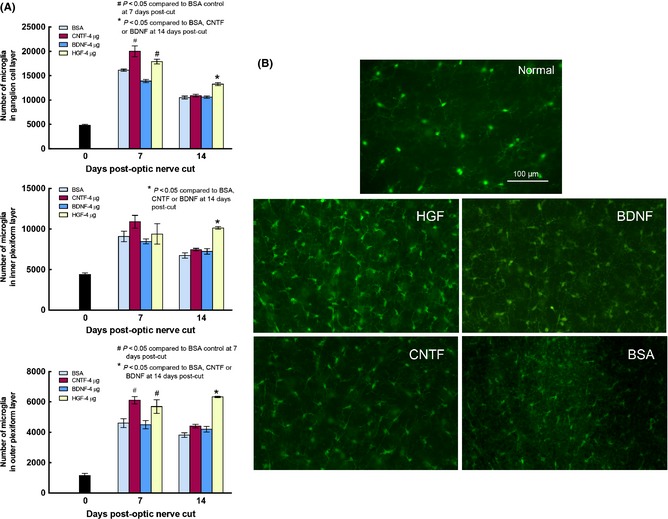
(A) Graphs depicting changes in microglia numbers in the three sublaminae of normal retina (Day 0), and retinas at 7 and 14 days post‐optic nerve cut, in different trophic factor groups. Note that microglia numbers were higher than normal in all three sublaminae at 7 and 14 days after injury, but there was a trend of decrease from 7 to 14 days post‐injury in most groups. HGF treatment was notable in that, at 14 days post‐injury, microglia numbers in the three sublaminae were still higher than in other trophic factors or BSA control. (B) Photomicrographs depicting Iba‐1 stained microglia in wholemount retinas of normal animal, or the different trophic factor‐treated groups at 14 days post‐optic nerve cut. Note that in the HGF‐treated retina microglial density was higher and cell bodies appeared larger and more robustly stained than the other treated groups.
Increased c‐Met Expression after Optic Nerve Injury
In the normal retina, c‐Met was expressed mainly in cells in the ganglion cell and inner nuclear layer (Figure 7A). Double labeling of anti‐c‐Met and TuJ1 showed that many ganglion cells expressed c‐Met (Figure 7G‐I), while double labeling of anti‐c‐Met and anti‐glutamine synthetase revealed that Muller cells in the inner nuclear layer were also positive for c‐Met (Figure 7A‐C). When the retina was examined at 7 days post‐optic nerve cut, an increase in anti‐c‐Met staining was seen, especially in the ganglion cell layer and inner plexiform layer (Figure 7D). Many radial processes of Muller cells positive for anti‐c‐Met could now be seen coursing through the inner plexiform layer, as confirmed by double staining with anti‐glutamine synthetase (Figure 7D,E). In fact, the laminated appearance of the inner plexiform layer (formed by branches coming off from the radial processes of Muller cells) exhibited in the anti‐glutamine synthetase staining was also well visualized in the anti‐c‐Met staining, showing that Muller cell processes in the inner plexiform layer possessed c‐Met (Figure 7D). Thus, the increased c‐Met staining seen in the inner retinal layers after optic nerve injury was most likely contributed by the increased localization of c‐Met on Muller cell processes that ramified extensively in the inner retina. Interestingly, the Muller cell radial processes and their branches that ramified in the outer retina (including the outer nuclear layer and their terminations at the outer limiting membrane) did not exhibit any obvious increase in c‐Met staining after optic nerve injury.
Figure 7.
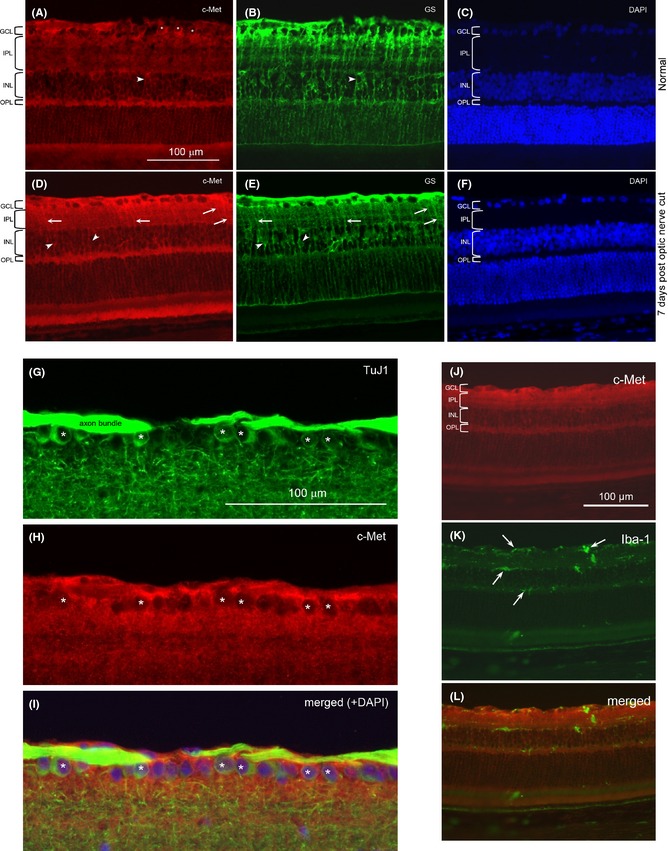
C‐Met localization in the retina. A.‐F. Double staining of retinal sections with anti‐c‐Met (red) and anti‐glutamine synthetase (GS, green), and counterstained with DAPI nuclear stain: from a normal retina (A‐C) or retina at 7 days post‐optic nerve cut (D‐F). The various retinal layers have been marked: GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer. Asterisks (*) indicate c‐Met‐labeled cell bodies in the GCL that are confirmed to be ganglion cells by double‐labeling with TuJ1 (see G‐I below). Arrowheads point to cell bodies in the INL that belong to Muller cells, while the arrows point to stained processes in the IPL that belong to radial processes of Muller cells. Note the increase in anti‐c‐Met staining after optic nerve injury, especially in GCL and IPL. The c‐Met‐labeled radial processes of Muller cells in the IPL are also more prominent after optic nerve cut. G.‐I. Double staining of TuJ1 and anti‐c‐Met from a normal retina. The micrographs showed the GCL and part of the IPL to illustrate that many ganglion cells labeled by TuJ1 (marked by *) in G are also positive for anti‐c‐Met in H. The merged view of the 2 labeling together with DAPI counterstain is shown in I. The apparent “emptiness” of the c‐Met‐labeled cells in H is due to the fact that c‐Met is localized on the cell membrane so that the red label surrounds the unstained cytoplasm. J.‐L. Double staining of a retinal section at 7 days post‐optic nerve cut with anti‐c‐Met and anti‐Iba‐1. Note that the Iba‐1‐stained cells (arrows) do not have any correspondence with the anti‐ c‐Met staining, showing that microglia do not express c‐Met.
When anti‐c‐Met was double stained with anti‐Iba‐1, either in the normal retina or in the retina with optic nerve injury (Figure 7J‐L), no colocalization was seen, suggesting that microglia (normal resting or injury‐activated) did not express c‐Met.
Discussion
By comparing different dosages and ganglion cell survival times among the three trophic factors in this study, we have shown that HGF stimulates ganglion cell survival much better than that of BDNF and CNTF. Although BDNF at its optimal dosage could protect almost all ganglion cells from degeneration at 7 days post‐optic nerve cut 1, it ceases to sustain ganglion cell survival at longer post‐injury times (28 days). Similarly, CNTF only affords mild protection at 7 days post‐injury, but no longer rescues ganglion cells at 14 and 28 days post‐injury. In contrast, HGF could rescue over 90% ganglion cells at 7 days post‐injury after 1 μg × 4 treatment and is the only trophic factor that continues to increase ganglion cell survival above that of the control and other growth factors at 28 days postinjury. It is also notable that HGF is able to protect different populations of ganglion cells across the entire retina as opposed to other growth factors that only promote survival at some but not all retinal eccentricities 36. Unlike BDNF and CNTF, HGF could alleviate somatic atrophy of surviving ganglion cells, one of the hallmarks of the injury response of CNS neurons 37. The ability of HGF to sustain the survival of diverse ganglion cell populations coupled with maintenance of their structural integrity makes it particularly attractive as a potential treatment of chronic neurodegenerative diseases like glaucoma.
Hepatocyte Growth Factor (HGF) is also unique among many trophic factors in its ability to promote both substantial survival and regeneration. A previous study has reported that of a multitude of growth factors tested, only CNTF could promote significant ganglion cell axonal regeneration into a PN 2. Here, we showed that HGF could stimulate twice as many ganglion cell axons to regenerate into a PN graft compared with the BSA control, although CNTF is still more potent. In the rat, it was found that HGF could stimulate more axons to regrow in the damaged optic nerve compared to vehicle‐treated animals 28. More importantly, however, is the ability of HGF to maintain injured ganglion cells in a relatively healthy state to respond to a delayed growth stimulus, as reflected by our result that ganglion cells stimulated by HGF maintain their propensity to regenerate even when the PN is grafted at 7 days post‐injury. It is well known that CNS axons rapidly lose their regenerative propensity if the stimulus is delayed for some time post‐injury 38. Although rapid neuronal degeneration after injury could account to a certain extent the lost of responsiveness to a growth stimulus, this factor could not explain the observed difference in regenerative propensity to the delayed grafting between HGF and CNTF, since the extent of ganglion cell survival at 7 days post‐injury was similar between them (HGF = 63 ± 1.3% vs. CNTF = 59.7 ± 0.7%, Figure 2). Rather, cellular mechanisms induced by HGF that help to sustain long‐term survival and structural integrity may be involved in maintaining a better regenerative propensity of ganglion cells, for example, via better preservation of cytoskeletal organization.
The molecular targets of exogenous HGF application that account for its benefits remain to be determined. There was a dramatic difference among the three trophic factors with respect to influencing HSP27 expression after optic nerve injury. HSP27 was not up‐regulated above that of the vehicle control by HGF treatment, suggesting that the chaperone protective mechanisms of this small molecular weight heat shock protein that account for enhanced neuronal survival are not associated with neuroprotection by HGF 39, 40. In stark contrast, CNTF stimulated a drastic albeit transient increase in ganglion cells expressing HSP27 concomitant with only modest promotion of survival. Because CNTF strongly promotes ganglion cell regeneration 2, 3, it is tempting to associate the enhanced HSP27 expression by CNTF with mechanisms involved in regeneration 41. The observation that BDNF down‐regulates HSP27 in ganglion cells 42 and also induces fewer ganglion cells to regenerate compared with the control supports the notion that, at least in axotomized ganglion cells, HSP27 is more involved in supporting regeneration than survival. However, our finding that HGF doubles the number of ganglion cells to regenerate but without increasing HSP27 expression compared with the control suggests that other molecular targets are involved in HGF's axon‐growth benefits.
The potent induction of HSP27 by CNTF as compared to HGF and BDNF could be due to differences in the major downstream intracellular signaling cascades elicited by the binding of growth factors to their receptors. These cascades include the Janus kinase/signal transducers and activators of transcriptase 3 (JAK‐STAT3), phosphatidylinositol 3‐kinase (PI3‐K/Akt), and mitogen‐activated protein kinase/extracellular signal–regulated kinase (MAPK/ERK) pathways, which are differentially employed by growth factors to promote cell survival. CNTF and various stress stimuli stimulate the expression of STAT3 in ganglion cells, Muller cells and astrocytes 43, while BDNF promotes ganglion cell survival via both the MAPK/ERK and PI3/Akt pathways 44. Similarly, it has been observed that binding of HGF to ganglion cells activate the MAPK/ERK and PI3/Akt pathways 28. It has been shown that in tumor cells STAT3 is a potent stimulator of HSP27 synthesis as well as its phosphorylation 45. Thus, it is likely that the up‐regulation of STAT3 by CNTF in ganglion cells leads to the enhanced expression of HSP27 and its phosphorylation which will play a role in promoting regeneration, whereas HGF will likely utilize downstream targets of the PI3‐K/Akt pathway like mammalian target of rapamycin and glycogen synthase kinase 3 46 as mechanisms to enhance ganglion cell survival and regeneration.
Previous studies in rodent retina suggest that c‐Met is present in ganglion cells and cells in the inner nuclear layer 47. In the present study with hamster retina, both ganglion cells and Muller cells are found to express c‐Met. Moreover, c‐Met expression is increased in the inner retina after optic nerve injury, as a result of its increased localization on the radial processes and branches of Muller cells associated with the inner retina. It has also been reported that c‐Met is present in glial cells of epiretinal membranes in proliferative vitreoretinopathy 48 and is increased in the inner retina after ischemia 25. Thus, a common reaction of Muller cells to retinal and optic nerve injury may be by up‐regulating c‐Met expression. Moreover, we found that such up‐regulation seems to be differentially distributed with respect to the radially elongated Muller cell that spans almost the entire retinal thickness, and in that it is associated with their branches in the inner retina where ganglion cells (and their processes) are undergoing degeneration after optic nerve injury. Such polarized distribution of molecules on the surface of Muller cells is well known, for example, the enrichment of aquaporin‐4 and other channel molecules on vitreal end‐feet of Muller cells 49. The functional significance of increased localization of c‐Met on inner retinal Muller cell processes is not known but could be related to autocrine/paracrine interactions with endogenous HGF that is known to be released from the retina in various diseases 50.
The enhanced expression of c‐Met by Muller cells and its preferential localization in the inner retina after optic nerve injury may be one of the bases for the beneficial effects of exogenous application of HGF, as HGF could activate Muller cells in addition to its direct action on ganglion cells. Muller cells are now well established to be involved in virtually all disease processes of the retina and contribute to both neuroprotection, neuroinflammation and toxicity mechanisms, via cell/cell interactions and secretions of a wide variety of neurotrophic factors, antioxidants as well as cytokines 51. It could be hypothesized that exogenous HGF delivered after optic nerve injury activates Muller cells directly by binding to c‐Met on their cell surfaces to result in the production of supportive factors for ganglion cell survival and regeneration. On the other hand, the receptors for CNTF and BDNF (CNTF receptor alpha and TrkB, respectively) have not been conclusively demonstrated to be expressed by Muller cells in vivo 52, 53. Although both CNTF and BDNF activate Muller cells to express GFAP 54, such “gliosis” might not be supportive to ganglion cell survival as compared to HGF activation of Muller cells. Future studies aimed at elucidating whether HGF could differentially stimulate Muller cells to engage in a neuroprotection status may help to resolve this issue.
We have observed that of the three growth factors examined, only HGF led to a persistent increase in microglia numbers in all three sublaminae where they are located. This raises the possibility that HGF has a direct action on microglial proliferation. However, double staining of c‐Met and anti‐Iba‐1 failed to show that microglia expressed c‐Met. This accords with other brain injury models where only macrophages but not microglia express c‐Met 55. Presumably, the stimulus of HGF on microglia is indirect, of which one potential candidate are Muller cells that have become activated by HGF. It is known that Muller cells and retinal microglia engage in a complex bi‐directional communication in both the normal and diseased retina. For instance, it has been found that activated retinal microglia stimulate Muller cells to secrete trophic factors and pro‐inflammatory cytokines that in turn further boost microglial activation 56. It could be envisioned that Muller cells activated by HGF secrete various bioactive molecules that maintain a heightened microglial proliferation above that of other trophic factors.
Although we have observed both a parallel increase in microglial proliferation and enhanced ganglion cell survival only after exogenous HGF treatment, it is not known for sure whether microglia play a supportive role in this case. Microglial proliferation and activation after neuronal injury are well‐established phenomena, but their significances to survival and regeneration are still debatable 57. Previous studies have shown that an influx of vitreal macrophages is a potent stimulus for ganglion cell survival and regeneration 58, 59, but the influence of endemic retinal microglia on ganglion cell survival and regeneration is not well defined 60. Inhibition of activated microglia has been shown to promote ganglion cell regeneration 32, but the contribution of the total microglial population (activated and resting) residing in different retinal laminae has not been addressed. On the other hand, it has been found that microglial proliferation in the ganglion cell layer stimulated by pituitary adenylate cyclase‐activating polypeptide correlated with improved survival of neurons in the ganglion cell layer after NMDA injury 61. In contrast, such increase in microglia numbers was not observed in our study of the neuroprotective effect of a remote ischemic postconditioning lesion on ganglion cells 62. The present viewpoint is that microglia possess both neuroprotective and neurotoxic properties that require fine tuning to achieve optimal repair of the CNS after injury 63. If microglia are involved in the protection of ganglion cells by HGF, they may act by secreting trophic factors that stimulate ganglion cell survival and/or modulation of phagocytic activity to achieve optimal removal of degenerating neuronal debris 63.
In conclusion, glial cells like Muller cells and microglia in the retina may become activated directly or indirectly by exogenous HGF and in turn provide trophic support to ganglion cells, in addition to the direct stimulus of HGF acting on ganglion cells. Elucidation of the complex interactions of neurons and glia in the retina in response to HGF will help in understanding its role in the treatment of different retinal diseases.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the General Research Fund CUHK463309 of the Research Grants Council of Hong Kong (E.Y.P. Cho), and a postgraduate studentship of the Chinese University of Hong Kong (W.K. Wong).
References
- 1. Mansour‐Robaey S, Clarke DB, Wang YC, et al. Effects of ocular injury and administration of brain‐derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA 1994;91:1632–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui Q, Lu Q, So KF, et al. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci 1999;40:760–766. [PubMed] [Google Scholar]
- 3. Jo SA, Wang E, Benowitz LI. Ciliary neurotrophic factor is and axogenesis factor for retinal ganglion cells. Neuroscience 1999;89:579–591. [DOI] [PubMed] [Google Scholar]
- 4. Clarke DB, Bray GM, Aguayo AJ. Prolonged administration of NT‐4/5 fails to rescue most axotomized retinal ganglion cells in adult rats. Vision Res 1998;38:1517–1524. [DOI] [PubMed] [Google Scholar]
- 5. Di Polo A, Aigner LJ, Dunn RJ, et al. Prolonged delivery of brain‐derived neurotrophic factor by adenovirus‐infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA 1998;95:3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sawai H, Clarke DB, Kittlerova P, et al. Brain‐derived neurotrophic factor and neurotrophin‐4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci 1996;16:3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pernet V, Di Polo A. Synergistic action of brain‐derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 2006;129:1014–1026. [DOI] [PubMed] [Google Scholar]
- 8. Cho KS, Chan PM, So KF, et al. Ciliary neurotrophic factor promotes the regrowth capacity but not the survival of intraorbitally axotomized retinal ganglion cells in adult hamsters. Neuroscience 1999;94:623–628. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 1984;122:1450–1459. [DOI] [PubMed] [Google Scholar]
- 10. Michalopoulos G, Houck KA, Dolan ML, et al. Control of hepatocyte replication by two serum factors. Cancer Res 1984;44:4414–4419. [PubMed] [Google Scholar]
- 11. Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989;342:440–443. [DOI] [PubMed] [Google Scholar]
- 12. Boros P, Miller CM. Hepatocyte growth factor: A multifunctional cytokine. Lancet 1995;345:293–295. [DOI] [PubMed] [Google Scholar]
- 13. Maina F, Klein R. Hepatocyte growth factor, a versatile signal for developing neurons. Nat Neurosci 1999;2:213–217. [DOI] [PubMed] [Google Scholar]
- 14. Thompson J, Dolcet X, Hilton M, et al. HGF promotes survival and growth of maturing sympathetic neurons by PI‐3 kinase‐ and MAP kinase‐dependent mechanisms. Mol Cell Neurosci 2004;27:441–452. [DOI] [PubMed] [Google Scholar]
- 15. Okura Y, Arimoto H, Tanuma N, et al. Analysis of neurotrophic effects of hepatocyte growth factor in the adult hypoglossal nerve axotomy model. Eur J Neurosci 1999;11:4139–4144. [DOI] [PubMed] [Google Scholar]
- 16. Koyama J, Yokouchi K, Fukushima N, et al. Neurotrophic effect of hepatocyte growth factor on neonatal facial motor neurons. Neurol Res 2003;25:701–707. [DOI] [PubMed] [Google Scholar]
- 17. Hayashi Y, Kawazoe Y, Sakamoto T, et al. Adenoviral gene transfer of hepatocyte growth factor prevents death of injured adult motoneurons after peripheral nerve avulsion. Brain Res 2006;1111:187–195. [DOI] [PubMed] [Google Scholar]
- 18. Yan H, Rivkees SA. Hepatocyte growth factor stimulates the proliferation and migration of oligodendrocyte precursor cells. J Neurosci Res 2002;69:597–606. [DOI] [PubMed] [Google Scholar]
- 19. Garzotto D, Giacobini P, Crepaldi T, et al. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met‐Grb2 coupling. J Neurosci 2008;28:5901–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim CS, Walikonis RS. Hepatocyte growth factor and c‐Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell Signal 2008;20:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimamura M, Sato N, Waguri S, et al. Gene transfer of hepatocyte growth factor gene improves learning and memory in the chronic stage of cerebral infarction. Hypertension 2006;47:742–751. [DOI] [PubMed] [Google Scholar]
- 22. Ishigaki A, Aoki M, Nagai M, et al. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J Neuropathol Exp Neurol 2007;66:1037–1044. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi D, Sato N, Shimamura M, et al. Alleviation of Abeta‐induced cognitive impairment by ultrasound‐mediated gene transfer of HGF in a mouse model. Gene Ther 2008;15:561–571. [DOI] [PubMed] [Google Scholar]
- 24. Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007;85:2332–2342. [DOI] [PubMed] [Google Scholar]
- 25. Shibuki H, Katai N, Kuroiwa S, et al. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia‐reperfusion injury. Invest Ophthalmol Vis Sci 2002;43:528–536. [PubMed] [Google Scholar]
- 26. Machida S, Tanaka M, Ishii T, et al. Neuroprotective effect of hepatocyte growth factor against photoreceptor degeneration in rats. Invest Ophthalmol Vis Sci 2004;45:4174–4182. [DOI] [PubMed] [Google Scholar]
- 27. Cho EYP, Wong WK. Hepatocyte growth factor sustains retinal ganglion cell survival after axotomy for longer periods than brain‐derived neurotrophic factor and ciliary neurotrophic factor, and also promotes their regeneration. 38th Annual Meeting of the Society for Neuroscience 2008;725.22.
- 28. Tonges L, Ostendorf T, Lamballe F, et al. Hepatocyte growth factor protects retinal ganglion cells by increasing neuronal survival and axonal regeneration in vitro and in vivo. J Neurochem 2011;117:892–903. [DOI] [PubMed] [Google Scholar]
- 29. Cui Q, Yip HK, Zhao RC, et al. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor‐induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 2003;22:49–61. [DOI] [PubMed] [Google Scholar]
- 30. Cho EYP, So KF. Characterization of the sprouting response of axon‐like processes from retinal ganglion cells after axotomy in adult hamsters: A model using intravitreal implantation of a peripheral nerve. J Neurocytol 1992;21:589–603. [DOI] [PubMed] [Google Scholar]
- 31. Cao T, Thomas TC, Ziebell JM, et al. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 2012;225:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia‐suppressing factors retards axotomy‐induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci 1993;13:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosco A, Inman DM, Steele MR, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 2008;49:1437–1446. [DOI] [PubMed] [Google Scholar]
- 34. Walter L, Neumann H. Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol 2009;31:513–525. [DOI] [PubMed] [Google Scholar]
- 35. Latchman DS. HSP27 and cell survival in neurones. Int J Hyperthermia 2005;21:393–402. [DOI] [PubMed] [Google Scholar]
- 36. Yan Q, Wang J, Matheson CR, et al. Glial cell line‐derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: Comparison to and combination with brain‐derived neurotrophic factor (BDNF). J Neurobiol 1999;38:382–390. [DOI] [PubMed] [Google Scholar]
- 37. Janssen KT, Mac Nair CE, Dietz JA, et al. Nuclear atrophy of retinal ganglion cells precedes the bax‐dependent stage of apoptosis. Invest Ophthalmol Vis Sci 2013;54:1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richardson PM, Miao T, Wu D, et al. Responses of the nerve cell body to axotomy. Neurosurgery 2009;65:A74–A79. [DOI] [PubMed] [Google Scholar]
- 39. Benn SC, Perrelet D, Kato AC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 2002;36:45–56. [DOI] [PubMed] [Google Scholar]
- 40. Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci 2007;1113:147–158. [DOI] [PubMed] [Google Scholar]
- 41. Williams KL, Rahimtula M, Mearow KM. Hsp27 and axonal growth in adult sensory neurons in vitro. BMC Neurosci 2005;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krueger‐Naug AM, Emsley JG, Myers TL, et al. Administration of brain‐derived neurotrophic factor suppresses the expression of heat shock protein 27 in rat retinal ganglion cells following axotomy. Neuroscience 2003;116:49–58. [DOI] [PubMed] [Google Scholar]
- 43. Peterson WM, Wang Q, Tzekova R, et al. Ciliary neurotrophic factor and stress stimuli activate the Jak‐STAT pathway in retinal neurons and glia. J Neurosci 2000;20:4081–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakazawa T, Tamai M, Mori N. Brain‐derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci 2002;43:3319–3326. [PubMed] [Google Scholar]
- 45. Song H, Ethier SP, Dziubinski ML, et al. Stat3 modulates heat shock 27 kDa protein expression in breast epithelial cells. Biochem Biophys Res Commun 2004;314:143–150. [DOI] [PubMed] [Google Scholar]
- 46. Lu Y, Belin S, He Z. Signaling regulations of neuronal regenerative ability. Curr Opin Neurobiol 2014;27C:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun W, Funakoshi H, Nakamura T. Differential expression of hepatocyte growth factor and its receptor, c‐Met in the rat retina during development. Brain Res 1999;851:46–53. [DOI] [PubMed] [Google Scholar]
- 48. Hollborn M, Krausse C, Iandiev I, et al. Glial cell expression of hepatocyte growth factor in vitreoretinal proliferative disease. Lab Invest 2004;84:963–972. [DOI] [PubMed] [Google Scholar]
- 49. Enger R, Gundersen GA, Haj‐Yasein NN, et al. Molecular scaffolds underpinning macroglial polarization: An analysis of retinal Muller cells and brain astrocytes in mouse. Glia 2012;60:2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salom D, az‐Llopis M, Quijada A, et al. Aqueous humor levels of hepatocyte growth factor in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2010;51:3157–3161. [DOI] [PubMed] [Google Scholar]
- 51. Bringmann A, Wiedemann P. Muller glial cells in retinal disease. Ophthalmologica 2012;227:1–19. [DOI] [PubMed] [Google Scholar]
- 52. Grishanin RN, Yang H, Liu X, et al. Retinal TrkB receptors regulate neural development in the inner, but not outer, retina. Mol Cell Neurosci 2008;38:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sarup V, Patil K, Sharma SC. Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Res 2004;1013:152–158. [DOI] [PubMed] [Google Scholar]
- 54. Bringmann A, Iandiev I, Pannicke T, et al. Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Prog Retin Eye Res 2009;28:423–451. [DOI] [PubMed] [Google Scholar]
- 55. Moransard M, Sawitzky M, Fontana A, et al. Expression of the HGF receptor c‐met by macrophages in experimental autoimmune encephalomyelitis. Glia 2010;58:559–571. [DOI] [PubMed] [Google Scholar]
- 56. Wang M, Wong WT. Microglia‐muller cell interactions in the retina. Adv Exp Med Biol 2014;801:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, saboteur, or something else? Science 2013;339:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yin Y, Cui Q, Gilbert HY, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A 2009;106:19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. London A, Itskovich E, Benhar I, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte‐derived macrophages. J Exp Med 2011;208:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Raibon E, Sauve Y, Carter DA, et al. Microglial changes accompanying the promotion of retinal ganglion cell axonal regeneration into peripheral nerve grafts. J Neurocytol 2002;31:57–71. [DOI] [PubMed] [Google Scholar]
- 61. Wada Y, Nakamachi T, Endo K, et al. PACAP attenuates NMDA‐induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neurosci 2013;51:493–502. [DOI] [PubMed] [Google Scholar]
- 62. Liu X, Sha O, Cho EYP. Remote ischemic postconditioning promotes the survival of retinal ganglion cells after optic nerve injury. J Mol Neurosci 2013;51:639–646. [DOI] [PubMed] [Google Scholar]
- 63. Sierra A, Abiega O, Shahraz A, et al. Janus‐faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 2013;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]


