Summary
Aims
Muscarinic acetylcholine receptor agonist pilocarpine reduces intraocular pressure (IOP) of glaucoma mainly by stimulating ciliary muscle contraction and then increasing aqueous outflow. It is of our great interest to know whether pilocarpine has the additional properties of retinal neuroprotection independent of IOP lowering in vitro and in vivo models.
Methods
In rat primary retinal cultures, cell viability was measured using an MTT assay and the trypan blue exclusion method, respectively. Retinal ganglion cells (RGCs) were identified by immunofluorescence and quantified by flow cytometry. For the in vivo study, the retinal damage after retinal ischemia/reperfusion injury in rats was evaluated by histopathological study using hematoxylin and eosin staining, transmission electron microscopy, and immunohistochemical study on cleaved caspase‐3, caspase‐3, and ChAT.
Results
Pretreatment of pilocarpine attenuated glutamate‐induced neurotoxicity of primary retinal neurons in a dose‐dependent manner. Protection of pilocarpine in both retinal neurons and RGCs was largely abolished by the nonselective muscarinic receptor antagonist atropine and the M1‐selective muscarinic receptor antagonist pirenzepine. After ischemia/reperfusion injury in retina, the inner retinal degeneration occurred including ganglion cell layer thinning and neuron lost, and the optic nerve underwent vacuolar changes. These degenerative changes were significantly lessened by topical application of 2% pilocarpine. In addition, the protective effect of pilocarpine on the ischemic rat retina was favorably reflected by downregulating the expression of activated apoptosis marker cleaved caspase‐3 and caspase‐3 and upregulating the expression of cholinergic cell marker ChAT.
Conclusions
Taken together, this highlights pilocarpine through the activation of muscarinic receptors appear to afford significant protection against retinal neurons damage and optic nerve degeneration at clinically relevant concentrations. These data also further support muscarinic receptors as potential therapeutic neuroprotective targets in glaucoma.
Keywords: Glaucoma, Glutamate, Ischemia/reperfusion, Neuroprotection, Pilocarpine
Introduction
Glaucoma is now recognized as a progressive neurodegeneration as the result of neuronal death in glaucoma, rather than merely a condition relating to intraocular pressure (IOP) alone 1, 2. Thus, it is becoming increasingly accepted that it is necessary for ideal antiglaucoma drugs to have additional properties of neuroprotection besides IOP lowering 3, 4. Pilocarpine, as a muscarinic receptor agonist, remains among the major classes of drugs clinically used for the treatment of glaucoma. Pilocarpine topically applying can reduce IOP mainly by stimulating ciliary muscle contraction and afterward increasing aqueous outflow. It is of our great interest to know whether pilocarpine has the additional neuroprotective property both in vitro and in vivo glaucoma‐relevant models.
Mounting evidence supports the idea that the progressive loss of retinal ganglion cells (RGCs) is a critical mechanism of glaucomatous neuropathy 5, 6, 7. It has been suggested that vascular insufficiency at the optic nerve head leading to ischemic‐like insults may be an important part of the death process of RGCs in all forms of glaucoma 8, 9. Although the precise mechanisms of ischemia‐induced neuronal cell death are unknown, excitotoxicity triggered by the overactivation of glutamate receptors is considered to be a central component of postischemic damage in the retina 10. Glutamate is the major neurotransmitter of the retina in which excitotoxic lesions were described in glaucoma 11. Toxic levels of glutamate might contribute to damage or death of retinal neurons, especially RGC loss seen in glaucoma 12. The previous study has demonstrated that excessive activation of glutamate receptors triggers a calcium‐induced intracellular cascade, ultimately leading to cell death through an apoptotic mechanism 13. Some studies have confirmed the importance of both NMDA and non‐NMDA receptors in the mechanism underlying RGC death with the neuroprotective action of specific GluR antagonists 14. Other pharmacological agents including β‐adrenoceptor antagonist, α2 adrenergic agonist, and neurotrophic factors 15, which do not directly affect GluRs, were also found to have neuroprotective effects. Activation of muscarinic receptors with relatively low concentrations of carbamylcholine was found to significantly increase the survival of retinal ganglion cells via M1 muscarinic acetylcholine receptors (M1 mAChR) under normal physiological conditions 16. Systemic administration of galantamine, an acetylcholinesterase inhibitor, promoted protection of RGCs occurred by activation of M1 mAChR 17. In our previous study, we found that muscarinic receptor agonist pilocarpine could markedly prevent the apoptotic cell death in primary retinal culture and RGC‐5 cells 18, 19. Thus, the neuroprotective effect of muscarinic receptor agonist at clinically relevant concentrations or doses against glaucoma is worthy to be further investigated in glaucoma‐relevant models.
The retinal ischemia/reperfusion (I/R) model has been used as an acute model of glaucoma in many laboratories 20, 21. Pathological findings of the rat retina after ischemia/reperfusion are similar in the retina of a glaucoma patient 22. The most susceptible retinal neurons to ischemia, in terms of the earliest and fastest degeneration, are RGCs 23. After ischemia/reperfusion, RGCs loss and thinning of the inner retinal layers are observed 24. These types of damage can be prevented and protected by various agents, such as free‐radical scavengers, Ca2+‐channel blockers, growth factors. But there are no reports regarding the neuroprotective effect of the antiglaucoma agent pilocarpine in the ischemia/reperfusion model. In addition, experimental data suggest that excitotoxicity occurs during retinal ischemia and that this process plays a role in the pathogenesis of ischemia‐induced retinal damage 14. Hence, in the present study, we further used glutamate excitotoxicity model in vitro and ischemia/reperfusion injury model in vivo to explore a novel mechanism of pilocarpine at clinically relevant concentrations in glaucoma therapy as a neuroprotectant through activation of muscarinic receptors.
Materials and Methods
Retinal Neuron Cultures
Primary cultures were obtained from the retinas of Sprague‐Dawley (SD) rats at postnatal day 1, following a described procedure 25. Briefly, the retinas were placed in phosphate‐buffered saline (PBS) and dissected free from scleral tissue and pigmented epithelium. The tissues were incubated for ˜7 min at 37°C in PBS containing 0.125% trypsin, 32 U per ml papain, 0.01% L‐cysteine, and 0.02% EDTA. The enzyme was inactivated by washing the tissue with DMEM containing 10% heat‐inactivated fetal calf serum. The tissue was triturated gently through three polished glass pipettes of decreasing tip size to produce a suspension of dissociated cells, and the cells were seeded in cell culture plates previously coated with poly‐L‐lysine (0.01%) (Sigma Chemical Co., St. Louis, MO, USA). After incubation at 37°C, 5% CO2 for 24 h, the culture media were replaced by Neurobasal media supplemented with B‐27 (Gibco, Gaithersburg, MD, USA), which produced cultures with >99% neuronal composition. We continued to incubate the cells for 6 days prior to experimentation. Glial and fibroblast proliferation was prevented by the presence of cytosine arabinoside (10 μM) in Neurobasal media.
Identification of Neurons
Retinal cultures were used for experiment after 8 days in culture. The neurons of retinal cells were studied using the immunofluorescence method. Briefly, cells were fixed with 4% paraformaldehyde solution for 15 min at room temperature and treated with 1% hydrogen peroxide for 10 min and then incubated for 1 h with blocking solution (PBS containing BSA, Triton X‐100 and appropriate serum). After that, the cells were incubated with antineurofilament 200 antibody (1:200; Sigma Chemical Co.) overnight at 4°C. After washing in buffer, cells were incubated for 1 h at room temperature with the secondary antibodies coupled to FITC. After sequential washes, cells were mounted on slides and examined under a fluorescence microscope.
Viability and Cytotoxicity Assays
Retinal cell monolayer cultures were maintained for 6 days in culture. Retinal neurons were treated with glutamate at different concentration (0.1–3 mM). Based on the pilot dose–effect study, subsequent experiments were carried out by pretreatment with pilocarpine (Sigma Chemical Co.) at different concentrations (0.1–10 μM) following by 1 mM glutamate for 24 h. Cell viability was evaluated using a rapid colorimetric assay 26. In brief, the retinal neurons were seeded at 105 cells per well on a 96‐well microplate. After 6 days, cells were treated with the glutamate in the presence of pilocarpine. Cultures were supplied with 20 μL tetrazolium salt, that is, 3‐[4,5‐dimethylthiazol‐2yl]‐2,5‐diphenyltetrazolium bromide (MTT), which is converted to an insoluble blue formazan product by living cells, but not by dying cells or their lytic debris. After solubilization in DMSO, the color density was measured using a multiwell scanning spectrophotometer, at a wavelength of 490 nm. The optic density of the result in MTT assay was directly proportional to the number of viable retinal neurons. We performed at least three experiments.
Cell viability was also assayed by trypan blue exclusion method 27. Cultured cells were stained with 1.5% trypan blue solution (Worthington Biochemical Co., Freehold, NJ, USA) at a room temperature for 10 min and then fixed in isotonic formalin (pH 7.0, 2–4°C). The fixed cultures were rinsed with physiological saline and examined using microscopy at 200× magnification. More than 500 cells chosen at random were counted to determine their viability in cell culture. The cell viability rate was calculated as the percentage of viable cells among the total number of cells examined. When required, atropine (nonselective antagonist) (Sigma Chemical Co.) or pirenzepine (M1‐selective muscarinic receptor antagonist) (Sigma Chemical Co.) was added to the culture medium.
Identification and Quantitative Assay of RGCs
The RGCs were identified using immunofluorescence method. Briefly, cells were fixed with 4% paraformaldehyde solution for 15 min at room temperature and treated with 1% hydrogen peroxide for 10 min and then incubated for 1 h with blocking solution (PBS containing BSA and appropriate serum). After that, the cells were incubated with mouse anti‐rat THY 1.1 antibody labeled by FITC (Serotec, Oxford, UK) overnight at 4°C. After sequential washes, cells were mounted on coated slides and examined under a fluorescence microscope.
To evaluate the neuroprotective effects of pilocarpine, the number of RGCs was measured by flow cytometry. Retinal cells were digested by 0.125% trypsin in PBS. After removal, cells were incubated with mouse anti‐rat THY‐1.1 antibody labeled by FITC at 37°C for 45 min and then diluted in PBS. Next, cells were rinsed three times in PBS. Finally, cells were fixed in 1% paraformaldehyde solution and quantified by flow cytometry.
Animals
Adult male Sprague‐Dawley rats (weighing 200–300 g) were used in this study. Rats were maintained at room temperature on a 12 h light/dark schedule, with ad lib access to food and water. All experimental animals were handled in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications, No. 80‐82) and according to the tenets of the ARVO statement for the use of Animals in Ophthalmic and Vision Research.
Retinal Ischemia/Reperfusion Model
Retinal ischemia/reperfusion model was produced according to the method reported previously 6. Briefly, rats were anesthetized with an intraperitoneal injection of 3% of sodium pentobarbital. The pupil was fully dilated with 0.5% tropicamide and 0.5% phenylephrine hydrochloride. The anterior chamber was cannulated with a 30‐gauge needle connected to a container of saline. Retina ischemia was induced by elevating IOP to generate a hydrostatic pressure of 110 mmHg for 50 min (by raising the container). After 50‐min ischemia, the needle was withdrawn from the anterior chamber, and reperfusion of the retinal vasculature was confirmed by examination of the fundus. Pilocarpine (2%) was administered topically four times per day 6 days (3 days before and 3 days after the ischemia). For the pharmacological studies, three groups (n = 5 animals per group) of rats were studied including control (sham‐operated) group, ischemic/reperfused group, and pilocarpine‐treated group.
Histopathological Study
The experimental eyeballs were enucleated after 3 days of the ischemia/reperfusion insult. They were fixed in 4% paraformaldehyde in PBS and rinsed with PBS. Anterior segments and lenses of the eyes were dissected, and posterior segments (eye cups) were dehydrated and embedded in paraffin. Horizontal section of eye cups through the optic disk at 4 μm thickness was prepared and stained with hematoxylin and eosin (H&E). The retinal layers in each section at a distance of ~1.5 mm of the center of the optic nerve head (ONH) were recorded onto disk as image data. For this, we used a digital camera connected to a light microscope. The cell number in the ganglion cell layer (GCL) and the thickness of each layer was measured to evaluate retinal damage. The numbers of nuclear cells in the GCL were counted per 200‐μm length, and the mean cell count was then used to determine a representative cell number of the GCL.
Expression of Cleaved Caspase‐3, Caspase‐3, and ChAT in Retina
For immunohistochemistry analysis, cryosections from control, ischemia‐treated, and pilocarpine‐treated retinas were processed, using the avidin–biotin–perosidase complex (ABC) technique with a cleaved caspase‐3 antibody (1:50; Cell Signaling Technology, Danvers MA, USA), a caspase‐3 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and a polyclonal goat ChAT antibody (1:100; Boster Company, Wuhan, China), followed by incubation in affinity‐purified biotinylated rabbit anti‐goat IgG and ABC complex. All antisera were diluted in 0.1 M PBS containing 0.5% Triton X‐100. Incubation with primary antibody was performed at 4°C overnight, while the second antibody was incubated at room temperature for 1 h. To quantify the extent of labeled retinal immunoreactive positive cells, the number of immunoreactive positive cells in the GCL was counted on five adjacent retinal sections.
Electron Microscopy
To detect the optical nerve by electron microscopy, retina samples were fixed by immersion in 2.5% glutaraldehyde in phosphate buffer and postfixed in 1% osmium tetroxide in the same buffer. After dehydrating in ethanol gradients at room temperature, the tissue samples were embedded in araldite. Thin sections were cut, stained with uranyl acetate and lead citrate, and examined under transmission electron microscopy and photographed.
Statistical Analysis
All data are expressed as mean±SD. These results were analyzed using one‐way analysis of variance (ANOVA) followed by the Dunnett's post hoc test, in which P < 0.05 was considered to be statistically significant. All measurements were taken using an image analysis program (IPP, Olympus, Japan).
Results
Muscarinic Receptor Agonist Attenuated Glutamate‐induced Neurotoxicity in Rat Retinal Neurons
Neurons could be firstly identified in a mixed retinal culture on the basis of their morphology and special marker for neurons. In previous study, we used β‐III‐tubulin to identify the retinal neurons 18. Here, Neurofilament 200, as a highly specific marker, is also identified for neurons. Our studies indicate that neurofilament 200 positive neurons occupy predominantly in retinal cultures (Figure 1A).
Figure 1.
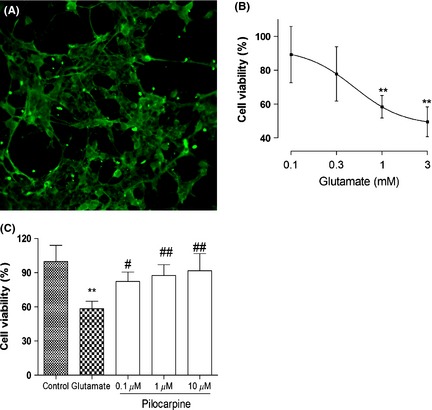
Dose‐dependent effect of glutamate and neuroprotection of pilocarpine on retinal neuronal survival. (A) Immunofluorescent illumination of retinal neurons stained with Neurofilament 200 monoclonal antibody in rat primary retinal cultures (200×). (B) Retinal neurons were incubated with different concentration of glutamate for 24 h. (C) Retinal neurons were incubated with different concentration of pilocarpine prior to exposure to 1 mM glutamate for 24 h in the continued presence of pilocarpine. Data are reported as the percentage of retinal cells survival compared with control culture (100%) and represent the mean ± SD (n = 4 per group, triplicate data per independent experiment). **P<0.01 versus control group, # P < 0.05, ## P < 0.01 versus glutamate‐treated group.
To determine the impact of glutamate on cytotoxicity, rat primary retinal neurons with elaborate neurite networks were treated with different concentration of glutamate for 24 h. As depicted in Figure 1B, increasing concentration of glutamate resulted in a dose‐dependent decrease in cell viability measured by MTT assays. Generally as the amount of glutamate increased, cell death increased progressively. Based on these preliminary data, all subsequent experiments were carried out for 24 h at 1 mM glutamate.
To determine the effect of pilocarpine on glutamate‐induced neurotoxicity, rat primary retinal neurons were pretreated with pilocarpine prior to exposure to 1 mM glutamate for 24 h in the continued presence of pilocarpine. Increasing concentration of pilocarpine resulted in a dose‐dependent increase in neuron viability measured by MTT method. Obvious protective effects were observed at pilocarpine concentrations of 0.1 μM (P < 0.05), 1 μM (P < 0.01), and 10 μM (P < 0.01) (Figure 1C). Based on these preliminary data, all subsequent experiments were carried out in the pretreatment with 1 μM pilocarpine following by 1 mM glutamate.
Trypan blue staining study was carried out to elucidate the mechanism of protection by pilocarpine against glutamate‐induced neurotoxicity. Rat primary retinal neurons were pretreated with pilocarpine in the presence and absence of antagonists prior to exposure to 1 mM glutamate. All photographs were taken immediately after trypan blue staining. As depicted in Figure 2a, here, dead cells but not living ones were stained by trypan blue. Most untreated (normal) cells were not stained, and natural cell death occurred in around 11% of cells in our study. However, cells exposed to 1 mM glutamate showed marked staining. The addition of pilocarpine clearly reduced the number of stained cells. Co‐application of atropine (the nonselective muscarinic receptors antagonist) or pirenzepine (the selective M1 muscarinic receptor antagonist) along with pilocarpine both increased the number of dead cells. A quantitative assessment of these effects was shown (Figure 2b). Cell viability decreased by 57% in glutamate‐treated cells compared with normal control cells. The addition of pilocarpine (1 μM) reduced glutamate‐induced neurotoxicity and cell viability returned to 85% of normal control cells. When treated with 1 μM atropine or 1 μM pirenzepine, the retinal neuronal viability significantly decreased. These results suggest that pilocarpine has protective effects against glutamate‐induced neurotoxicity, and the neuroprotection involves activation of M1 muscarinic receptors.
Figure 2.
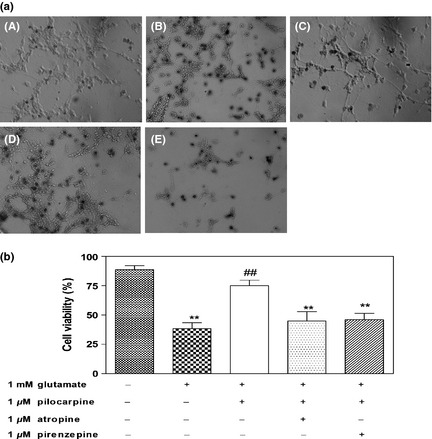
Protective effects of pilocarpine against glutamate‐induced neurotoxicity in rat primary retinal neurons by trypan blue staining. (a) Trypan blue staining. Photomicrographs showed the effect of pilocarpine on glutamate‐induced neuronal death (200 × ). A: Control (untreated) cells (without glutamate being applied). B: Cells were treated with glutamate for 24 h. C: Cells pretreated with pilocarpine were incubated with glutamate for 24 h. D, E: Cells pretreated with pilocarpine and atropine (D) or pirenzepine (E) were incubated with glutamate for 24 h. (b) Quantification of cell viability after exposure to glutamate in the presence of pilocarpine. Pilocarpine prevented cell death of retinal neurons induced by glutamate. Application of atropine or pirenzepine inhibited the protective effect of pilocarpine. Values are expressed as the mean ± SD (n = 4–6 per group). **P<0.01 versus control group, ## P<0.01 versus glutamate‐treated group.
Muscarinic Receptor Agonist Attenuated Glutamate‐induced Cytotoxicity in RGCs
Changes in RGCs in a mixed culture deserved to be closely investigated because the dysfunction and degeneration of RGCs are associated with glaucoma. RGCs were firstly identified immunofluorescence using an antiserum against THY 1.1, CD90, a specific ganglion cell surface marker. Ringlike staining suggests that only cell membranes were stained (Figure 3a).
Figure 3.

Effect of pilocarpine on glutamate‐induced neurotoxicity of retinal ganglion cells (RGCs). (a) Immunofluorescent illumination of retinal neurons stained with THY1.1 monoclonal antibody in rat primary retinal cultures (200×). (b) Percentage of RGCs positively labeled by mouse anti‐rat THY1.1 antibody in retinal neurons using flow cytometry. (c) Graphs of flow cytometry in RGCs. A: Control (untreated) cells (without glutamate being applied). B: Cells were treated with glutamate for 24 h. C: Cells pretreated with pilocarpine were incubated with glutamate for 24 h. D, E: Cells pretreated with pilocarpine and atropine (D) or pirenzepine (E) were incubated with glutamate for 24 h. Data are reported as the percentage of RGCs in retinal cells (100%) and represent the mean ± SD (n = 3 per group). **P<0.01 versus control group, ## P < 0.01 versus glutamate‐treated group.
Using flow cytometry, RGCs can be quantitatively identified in the mixed retinal neurons depending on THY 1.1 antibody targeting RGCs. The protective effect of pilocarpine on RGCs was quantitatively confirmed by flow cytometry (Figure 3b). The representative graph of flow cytometry was shown in Figure 3c. RGCs accounted for about 30% of total cells in a mixed retinal culture. When treated with glutamate, the proportion of RGCs was about 14% of total cells in a mixed retinal culture. The number of RGCs decreased by approximately 54% in glutamate‐treated retinal cultures compared with normal control cells. When cells were pretreated with pilocarpine, RGCs accounted for 26% of total cells in a mixed retinal culture, and the number of RGCs was reduced by 13% compared with normal control cells, which suggested that pilocarpine reverses glutamate‐induced decreases in RGCs. Similarly, the effect of cholinergic activity on RGCs was also evaluated when cells were treated with atropine or pirenzepine. When RGCs were treated with atropine and pirenzepine, their number was reduced by about 52% and 40% compared with normal control cells, respectively, which indicates that muscarinic antagonists can suppress the effect of pilocarpine and that M1 muscarinic receptors mediate this effect (Figure 3b).
Muscarinic Receptor Agonist Prevented Ischemia/Reperfusion Insults in the Retina
Hematoxylin–eosin staining of retinal sections showed degeneration after ischemia/reperfusion injury. In ischemic/reperfused animals, degenerative changes were observed in the inner plexiform layer (IPL) and the inner nuclear layer (INL), and GCL of the ischemic eye, a characteristic of ischemic atrophy (Figure 4). Both the number of cells in GCL and thickness of the IPL and INL were significantly reduced by the ischemia/reperfusion injury.
Figure 4.
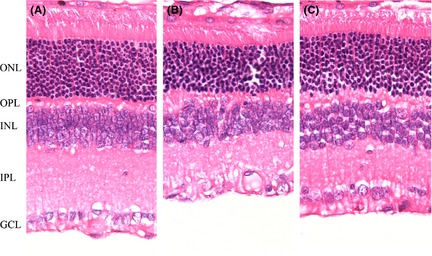
Histological features of hematoxylin‐/eosin‐stained rat retina slices under different experimental conditions (400×). (A) Sham‐operated retina (Control). Five well‐organized retinal layers include ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (B) Ischemic/reperfused retinas. (C) Pilocarpine‐treated retinas. The overall retinal thickness was decreased with marked thinning of the inner retina in the ischemic/reperfused retina. Note that administration of pilocarpine prevents the reduction of retinal thickness due to ischemia/reperfusion.
A quantitative assessment of the protective effect of pilocarpine is given in Table 1. The thickness of IPL decreased to 48% of controls in the ischemic/reperfused group and the corresponding figure was 81% of controls in the pilocarpine‐treated group. The thickness of INL decreased to 56% of controls in the ischemic/reperfused group, and the corresponding figure was 86% of controls in the pilocarpine‐treated group. The density of cells in GCL decreased to 67% of controls in the ischemic/reperfused group, and the corresponding figure was 87% of controls in the pilocarpine‐treated group. When 2% pilocarpine topically applied, the density of cells in GCL, the thickness of IPL and INL all showed the significant thicker than the ischemic/reperfused group. These results demonstrated that pilocarpine has a protective effect on inner retinal neurons after ischemia/reperfusion injury.
Table 1.
Effect of pilocarpine on ischemia‐/reperfusion‐induced depth of inner plexiform layer (IPL) and inner nuclear layer (INL), number of cells in ganglion cell layer (GCL)
| IPL thickness (μm) | INL thickness (μm) | Number of cells in GCL (per mm) | |
|---|---|---|---|
| Control (sham‐operated) | 49.72 ± 8.79 | 36.39 ± 7.18 | 54.67 ± 6.06 |
| Ischemia/reperfusion (I/R) | 24.10 ± 5.45** | 20.30 ± 3.13** | 36.67 ± 7.45** |
| I/R + pilocarpine | 40.02 ± 3.64## | 31.12 ± 4.58# | 47.33 ± 2.79# |
At 3 days after ischemia, eyes were enucleated and cross‐sections were prepared. Retinal ischemia/reperfusion induced a significant decrease in INL and IPL thickness and GCL cell number. The alterations in INL and IPL thickness and in GCL number were partially prevented by pilocarpine. Values are expressed as the mean ± SD (n = 5 rats per group). **P < 0.01 versus Control group; #P < 0.05, ##P < 0.01 versus Ischemic/reperfused group.
Protective effect of pilocarpine was confirmed by cleaved caspase‐3, caspase‐3, and ChAT immunoreactivity in the retinas (Figure 5a–c). Caspase‐3, especially cleaved caspase‐3, is the critical executioner of apoptosis, showing cytoplasmic localization in apoptotic cells. ChAT‐immunoreactive cells are associated with two types of certain amacrine cells located in both the INL and the GCL of the control retinas. To quantify the extent of labeled cleaved caspase‐3, caspase‐3, and ChAT immunoreactivity cells in the GCL, cleaved caspase‐3, caspase‐3‐positive, and ChAT‐positive cells in the GCL were analyzed (Figure 5d–f). The ischemic retina after reperfusion showed augmentation of cleaved caspase‐3 and caspase‐3 immunoreactivity and a decrease in ChAT immunoreactivity. However, topical application of 2% pilocarpine showed clear protection against ischemia‐/reperfusion‐induced increase in cleaved caspase‐3 and caspase‐3 immunoreactivity and loss of ChAT immunoreactivity. These results suggested that pilocarpine suppressed the activation of apoptosis and prevented ischemia/reperfusion injury.
Figure 5.

Cleaved caspase‐3, caspase‐3, and ChAT immunoreactivity in the sham‐operated retina (Control, A), in the ischemic/reperfused retina (B), and in the pilocarpine‐treated retina (C) (400 × ). Cleaved caspase‐3, caspase‐3, and ChAT immunoreactivity were observed in the INL and GCL (a–c). Cleaved caspase‐3, caspase‐3‐positive, and ChAT‐positive cells in the GCL were quantitatively analyzed (d–f, n = 5 per group). The ischemic/reperfused retina showed a decrease in ChAT‐positive cells and an increase of cleaved caspase‐3 and caspase‐3‐positive cells. Pilocarpine treatment restored ChAT‐positive cells and prevented caspase‐3‐positive cells after injury. *P<0.05, **P<0.01 versus Control group, # P < 0.05, ## P < 0.01 versus Ischemic/reperfused group.
Electron microscopy of optic nerve sections revealed that the myelin sheets were well organized and optic nerve fibers were intact in normal group (Figure 6A). In ischemia/reperfused group, the myelin sheets got thinner. They were unorganized and contained vacuolar structures in between them. Moreover, the optic nerve fibers contained granular accumulations Figure 6B). When 2% pilocarpine topically applied, the myelin sheets were thicker and vacuolar degeneration became to lessen (Figure 6C). It indicated that pilocarpine prevented degenerative changes in optical nerve.
Figure 6.

Transmission electron microscopic graphs of the optic nerves collected from rat retinas. (A) Sham‐operated optic nerve (Control, 7400×). (B) Ischemic/reperfused optic nerve (7400×). (C) Pilocarpine‐treated optic nerve (7400×). The myelin sheets observed in ischemic/reperfused group were unorganized and contained vacuolar structures in between them. The tissue in between the glial cells and optic nerve fibers lost its intact appearance, and the optic nerve fibers contain granular accumulations. When 2% pilocarpine topically applied, the myelin sheets were thicker and vacuolar degeneration become to lessen.
Discussion
Excitotoxicity is defined as neuronal cell death caused by excessive excitatory neurotransmitter and has been linked to various diseases of the eye including ischemia and glaucoma 28 and has become one of particularly attractive targets for neuroprotection. Glutamate may play an excitotoxic role in RGCs loss because increased levels of extracellular glutamate might exist in the RGC microenvironment rather than in the vitreous humor 29. Therefore, except for the amacrine cells, studies of ganglion cells are also crucially needed. Using flow cytometry, identification and quantification of RGCs were performed in rat primary retinal cultures. Our results indicate that pilocarpine exerted a neuroprotective effect on glutamate‐induced retinal neuron death as well as RGCs death apparently independent of IOP lowering.
Cholinergic activity modulates events during neuronal development, and lesions in cholinergic pathways are characteristics of neuro‐degenerative diseases 30, 31. Our results showed that the protective effect of pilocarpine against glutamate‐induced neurotoxicity in retinal neurons including RGCs was abolished by nonselective muscarinic receptor agonist atropine or M1‐selective muscarinic receptor agonist pirenzepine, suggesting its involvement of M1 muscarinic receptor activation. It was well documented that β‐adrenoceptor antagonist, also remaining among the major classes of drugs clinically used for the treatment of glaucoma, has been shown not only to lower IOP but also to exert neuroprotective effects against neuronal damage in the retina. However, neuroprotection of β‐adrenoceptor antagonist can be attributable to the calcium and sodium channel blocking activity, rather than to any direct interaction with β‐receptors 32. In contrast, both neuroprotection and IOP lowering of pilocarpine maybe share the same mechanism through activation of muscarinic receptor.
Ischemia/reperfusion injury is characterized by retinal degeneration, including extensive loss of neurons in the GCL and in the INL 22. Following periods of transient retinal ischemia between 45 and 50 min, retinal degeneration subsequently occurs over several weeks 33. The degree of ischemia followed by reperfusion causes damage to the whole retina with the inner part, particularly RGCs, being drastically affected 34, 35. Ischemic/reperfused insult in the inner retina is reflected in changes including depth of the inner plexiform layer and the inner nuclear layer, a number of inner retinal neurons (RGCs and amacrine cells). Our histological results showed that retinal damage after ischemia/reperfusion injury was most obvious in the inner retina. Optic nerve hypoplasia due to ischemia/reperfusion was observed. Alteration in myelinization of optic nerve fibers was at least partially implicated in the mechanism of this observation. Topical administration of a clinical dose (2%) of pilocarpine significantly reduced the inner retinal damage and optical nerve hypoplasia caused by ischemia/reperfusion.
Caspase‐3, a marker of activated apoptosis, was found to be involved in experimental glaucoma 36. A number of studies have shown that retinal neuronal death following ischemia/reperfusion is mediated by apoptosis and mainly executed by caspase‐3 in the inner retina 7. This is consistent with the case in the present study where it was shown that active caspase‐3 and caspase‐3 were upregulated in the GCL following ischemia/reperfusion. The fact that pilocarpine significantly attenuated the upregulation of caspase‐3 induced by ischemia/reperfusion therefore provided evidence that pilocarpine could prevent neurons from apoptosis.
In addition, the protective effect of pilocarpine on the ischemia rat retina was also reflected by ChAT immunoreactivity changes, as ChAT is a reliable marker for cholinergic amacrine cells. This study showed that retinal cells were negatively affected in the rat retina by ischemia/reperfusion and pilocarpine partially blunted these effects. Pilocarpine counteracted the expression of ChAT‐positive cells caused by ischemia/reperfusion injury, which can be also interpreted as an index of pilocarpine neuroprotective action.
Although there was the report by Vorwerk and coworkers in 1999 that pilocarpine is toxic to retinal ganglion cells in a dose‐dependent fashion from 2 to 40 mM, it was confirmed that both 0.4 and 0.8 mM pilocarpine did not affect ganglion cell survival 37. In our study, 0.1, 1, and 10 μM pilocarpine are much <0.4 mM pilocarpine and do not affect the retinal neuron survival. Conversely, they exert protection on the retinal neurons. It has been demonstrated that 10−1 M pilocarpine administered topically in the rabbit eye for 60 min results in concentrations of ~0.26 μg per gram in vitreous humor 38. It was also confirmed that the pharmacokinetics of pilocarpine distribution are similar between rabbit and human eyes 39. Based on these studies, 0.4 mM pilocarpine is roughly 1000‐fold higher than the vitreal concentration and 20‐fold higher than the scleral concentration obtainable with topical administration of 2% pilocarpine 37. And 0.1 and 1 μM pilocarpine are roughly less than the vitreal concentration obtainable with topical administration of 2% pilocarpine. There is a probability that pilocarpine at clinical concentration can be play a neuroprotective role.
Conclusion
Thus, the present in vitro and in vivo study demonstrates that pilocarpine at clinically relevant concentrations appears to afford significant protection against retinal neurons damage and optic nerve degeneration and provides potential therapeutic avenues for the use of muscarinic receptor agonists in glaucoma therapy.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
We thank all participating colleagues for contributing to this study. This study was supported by National Key Basic Research Program (2010CB529800), the International Cooperation Project of China (No. 2011DFA33180), the National Natural Science Foundation of China (No. 30472012, 30700280 and 81273519), the Shanghai Municipal Science and Technology Commission (No. 11ZR1419300), and the Shanghai Education Commission (No. 12YZ037, 12YZ200).
The first two authors contributed equally to this work.
References
- 1. Nilforushan N. Neuroprotection in glaucoma. J Ophthalmic Vis Res 2012;7:91–93. [PMC free article] [PubMed] [Google Scholar]
- 2. Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012;119:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tătaru CP, Purcărea VL. Antiglaucoma pharmacotherapy. J Med Life 2012;5:247–251. [PMC free article] [PubMed] [Google Scholar]
- 4. Nucci C, Strouthidis NG, Khaw PT. Neuroprotection and other novel therapies for glaucoma. Curr Opin Pharmacol 2013;13:1–4. [DOI] [PubMed] [Google Scholar]
- 5. Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma 2009;18:93–100. [DOI] [PubMed] [Google Scholar]
- 6. Goto W, Ota T, Morikawa N, et al. Protective effects of timolol against the neuronal damage induced by glutamate and ischemia in the rat retina. Brain Res 2002;958:10–19. [DOI] [PubMed] [Google Scholar]
- 7. Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol 2003;48:S21–S24. [DOI] [PubMed] [Google Scholar]
- 8. Mozaffarieh M, Grieshaber MC, Flammer J. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis 2008;14:224–233. [PMC free article] [PubMed] [Google Scholar]
- 9. Desai PV, Caprioli J. The treatment of normal‐tension glaucoma. Prog Brain Res 2008;173:195–210. [DOI] [PubMed] [Google Scholar]
- 10. Ju WK, Kim KY. Measuring glutamate receptor activation‐induced apoptotic cell death in ischemic rat retina using the TUNEL assay. Methods Mol Biol 2011;740:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vallazza‐Deschamps G, Fuchs C, Cia D, et al. Diltiazem‐induced neuroprotection in glutamate excitotoxicity and ischemic insult of retinal neurons. Doc Ophthalmol 2005;110:25–35. [DOI] [PubMed] [Google Scholar]
- 12. Dkhissi O, Chanut E, Wasowicz M, et al. Retinal TUNEL‐positive cells and high glutamate levels in vitreous humor of mutant quail with a glaucoma‐like disorder. Invest Ophthalmol Vis Sci 1999;40:990–995. [PubMed] [Google Scholar]
- 13. Aarts MM, Tymainski M. Molecular mechanisms underlying specificity of excitotoxic signaling in neurons. Curr Mol Med 2004;4:137–147. [DOI] [PubMed] [Google Scholar]
- 14. Nucci C, Tartaglione R, Rombolà L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)‐induced retinal ganglion cell death in rat. Neurotoxicology 2005;26:935–941. [DOI] [PubMed] [Google Scholar]
- 15. Shih GC, Calkins DJ. Secondary neuroprotective effects of hypotensive drugs and potential mechanisms of action. Expert Rev Ophthalmol 2012;7:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pereira SP, Medina SV, Araujo EG. Cholinergic activity modulates the survival of retinal ganglion cells in culture: the role of M1 muscarinic receptors. Int J Dev Neurosci 2001;19:559–567. [DOI] [PubMed] [Google Scholar]
- 17. Almasieh M, Zhou Y, Kelly ME, Casanova C, Di Polo A. Structural and functional neuroprotection in glaucoma: role of galantamine‐mediated activation of muscarinic acetylcholine receptors. Cell Death Dis 2010;1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou W, Zhu X, Zhu L, et al. Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate‐induced apoptosis in retinal neurons. Cell Mol Neurobiol 2008;28:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X, Zhou W, Cui Y, et al. Pilocarpine protects cobalt chloride‐induced apoptosis of RGC‐5 cells: involvement of muscarinic receptors and HIF‐1 alpha pathway. Cell Mol Neurobiol 2010;30:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Invest Ophthalmol Vis Sci 2002;42:471–478. [DOI] [PubMed] [Google Scholar]
- 21. Piras A, Gianetto D, Conte D, Bosone A, Vercelli A. Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PLoS ONE 2011;6:e22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osborne NN, Ugarte M, Chao M, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol 1999;42:S102–S128. [DOI] [PubMed] [Google Scholar]
- 23. Neufeld AH, Kawai S, Das S, Gachie E, Connor JR, Manning PT. Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase. Exp Eye Res 2002;75:521–528. [DOI] [PubMed] [Google Scholar]
- 24. Hangai M, Miyamoto K, Hiroi K, et al. Roles of constitutive nitric oxide synthase in postischemic rat retina. Invest Ophthalmol Vis Sci 1999;40:450–458. [PubMed] [Google Scholar]
- 25. Vorwerk CK, Naskar R, Schuettauf F, et al. Depression of retinal glutamate transporter function leads to elevated intravitreal glutamate levels and ganglion cell death. Invest Ophthalmol Vis Sci 2000;41:3615–3621. [PubMed] [Google Scholar]
- 26. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;16:55–63. [DOI] [PubMed] [Google Scholar]
- 27. Yamauchi T, Kashii S, Yasuyshi H, Zhang S, Honda Y, Akaike A. Mitochondrial ATP‐sensitive potassium channel: a novel site for neuroprotection. Invest Ophthalmol Vis Sci 2003;44:2750–2756. [DOI] [PubMed] [Google Scholar]
- 28. Ray K, Mookherjee S. Molecular complexity of primary open angle glaucoma: current concepts. J Genet 2009;88:451–467. [DOI] [PubMed] [Google Scholar]
- 29. Chintala SK. The emerging role of proteases in retinal ganglion cells. Exp Eye Res 2006;82:5–12. [DOI] [PubMed] [Google Scholar]
- 30. Tobin AB, Budd DC. The anti‐apoptotic response of the Gq/11‐coupled muscarinic receptor family. Biochem Soc Trans 2003;31:1182–1185. [DOI] [PubMed] [Google Scholar]
- 31. Pereira SP, Medina SV, Araujo SG. Chronic depolarization induced by veratridine increase the survival of retinal ganglion cells in vitro . Int J Dev Neurosci 2000;18:773–780. [PubMed] [Google Scholar]
- 32. Arai K, Wood JP, Osborne NN. Beta‐adrenergic receptor agonists and antagonists counteract LPS‐induced neuronal death in retinal cultures by different mechanisms. Brain Res 2003;984:176–186. [DOI] [PubMed] [Google Scholar]
- 33. Selles‐Navarro L, Villegas Perez MP, Salvador Silva M, Ruiz Comez JM, Vidal Sanz M. Retinal ganglion cell death after different transient periods of pressure‐induced ischemia and survival intervals, a quantitative in vivo study. Invest Ophthalmol Vis Sci 1996;37:2002–2014. [PubMed] [Google Scholar]
- 34. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004;23:91–147. [DOI] [PubMed] [Google Scholar]
- 35. Zhang B, Safa R, Rusciano D, Osborne NN. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res 2007;1159:40–53. [DOI] [PubMed] [Google Scholar]
- 36. Ji J, Chang P, Pennesi ME, et al. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vision Res 2005;45:169–179. [DOI] [PubMed] [Google Scholar]
- 37. Vorwerk CK, Simon P, Gorla M, et al. Pilocarpine toxicity in retinal ganglion cells. Invest Ophthalmol Vis Sci 1999;40:813–816. [PubMed] [Google Scholar]
- 38. Makoid MC, Robinson JR. Pharmacokinetic of topically applied pilocarpine in the albino rabbit eye. J Pharm Sci 1979;68:435–453. [DOI] [PubMed] [Google Scholar]
- 39. Krohn DL, Breitfeller JM. Transcorneal flux of topically pilocarpine to the human aqueous. Am J Ophthalmol 1979;187:50–56. [DOI] [PubMed] [Google Scholar]


