The efficacy of L‐3,4‐dihydroxyphenylalanine (L‐DOPA) in the treatment of Parkinson's disease (PD) is impaired by anxiety or depression in some patients and iatrogenic side effects such as dyskinesia 1. Classical pharmacological tools such as selective serotonin reuptake inhibitors could be interesting to limit motor or nonmotor undesirable effects, but they directly target the activity of serotonergic neurons. Indeed, serotonergic (5‐HT) neurons are responsible for L‐DOPA‐induced dopamine (DA) release and some of the inherent behavioral effects at the expense of 5‐HT itself 2. Therefore, the difficulty emerging with the therapies associated with L‐DOPA is to enhance central DA tone, without further alteration of 5‐HT transmission and excessive striatal DA tone correlated to the possible appearance of dyskinesia 2, 3.
Our study makes reference to a recent work published by Mendez‐David et al. 4 confirming that 5‐HT4 ligands display interesting and fast onset of anxiolytic and antidepressant action 5. Part of these effects would be related to their indirect modulatory action of 5‐HT neuronal activity where 5‐HT4 receptor stimulation and blockade could enhance and reduce dorsal raphe (DRN) 5‐HT neuron activity and release at terminals, respectively 5. Due to the expression of 5‐HT4 receptors being preserved in the brain of Parkinsonian humans 6, the stimulation and blockade of 5‐HT4 receptors could enhance and reduce the ability of L‐DOPA to stimulate the depolarization‐dependent outflow of DA from 5‐HT neurons, respectively.
We used multisite intracerebral microdialysis in the unilateral 6‐hydroxydopamine (6‐OHDA) rat model of PD to study the effect of the 5‐HT4 agonist prucalopride or the 5‐HT4 antagonist GR 125487 on the release of DA induced by intraperitoneal (i.p.) 12 mg/kg L‐DOPA. Microdialysis probes were simultaneously implanted in the striatum, hippocampus (HIPP), substantia nigra pars reticulata (SNr), and the prefrontal cortex (PFC) 7. We have chosen a dose for each 5‐HT4 compound that guaranteed selective and efficient action toward 5‐HT4 receptors 8. The entire experimental procedures were similar to those recently reported 7 and agreed with French National Committee (décret 87/848, Ministère de l'Agriculture et de la Forêt) and European Economic Community (86‐6091 EEC) guidelines for the care and use of laboratory animals.
As previously reported 3, 7, L‐DOPA (12 mg/kg, i.p.) increased DA outflow in the striatum, SNr, PFC, and the HIPP (Figure 1). The 5‐HT4 antagonist GR 125487 (0.1 mg/kg, i.p.) did not modify the effect of L‐DOPA suggesting that 5‐HT4 receptors do not contribute to the regulation of the basal activity of 5‐HT neurons in the presence of L‐DOPA. This is consistent with the fact that L‐DOPA tends to decrease 5‐HT extracellular levels in the brain 2. Conversely, 5‐HT4 receptor agonist prucalopride (5 mg/kg, i.p.) selectively enhanced L‐DOPA‐stimulated DA release in the SNr and the PFC (Figure 1). The use of multisite intracerebral microdialysis allows us to discard peripheral or central pharmacokinetic interaction because the prucalopride‐dependent enhancement of L‐DOPA‐stimulated DA release was not observed in the striatum or the HIPP of the same rats. Consistently, the DA metabolite 3,4‐dihydroxyphenylacetic acid (DOPAC) extracellular levels, reflecting circulating L‐DOPA, were homogeneously enhanced in all brain regions. The lack of effect of prucalopride on DOPAC extracellular levels further rules out regional changes of L‐DOPA availability (data not shown).
Figure 1.
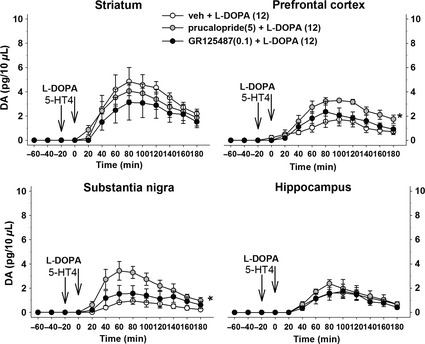
Effect of the 5‐HT4 ligands prucalopride and GR 125487 on L‐DOPA‐induced DA release in the striatum, the prefrontal cortex, the substantia nigra pars reticulata, and the hippocampus of 6‐OHDA rats. Data represent the mean ± standard error of the mean (SEM) of DA extracellular levels in each sample (n = 4–5 animals/group) and are expressed in raw data (pg/10 μL of sample). Three to four weeks after the unilateral injection of 6‐OHDA in the median forebrain bundle, L‐DOPA or its vehicle (0) was administered intraperitoneally (i.p.) at 12 mg/kg (time indicated by the vertical arrows) and was preceded 20 min before by the i.p. administration of benserazide (15 mg/kg, i.p.) 7. The 5‐HT4 compounds, prucalopride and GR 125487, were administered intraperitoneally 20 min before L‐DOPA (as benserazide) at 5 and 0.1 mg/kg i.p., respectively 8. All drugs were freshly prepared and dissolved as the free base (excepted benserazide) in saline. The effect of 5‐HT4 compounds was studied on the overall effect of L‐DOPA using one‐way ANOVA using group as the main factor. It reached significance in the SNr [F(2,12) = 4.7, P < 0.05] and the PFC [F(2,13) = 5.4, P < 0.05] but not in the striatum [F(2,13) = 0.6, ns] or the HIPP [F(2,13) = 0.5, ns]. Asterisks refer to the overall statistical effect * P < 0.05 versus vehicle + L‐DOPA group (Fisher's PLSD test).
The pharmacodynamic interaction might involve the indirect stimulation of 5‐HT neurons located in the raphe nuclei and increase release at terminals, thereby enhancing a depolarization‐dependent release of L‐DOPA‐derived DA at terminals of 5‐HT neurons 3. The selective L‐DOPA‐stimulated DA release in the SNr and the PFC is likely related in part to the heterogeneous function of 5‐HT terminals in the brain 2. In particular, such depolarization‐induced L‐DOPA‐derived DA outflow might be bypassed in the HIPP. The release of DA induced by L‐DOPA may involve an impulse‐independent mechanism in the HIPP which might be less present in other brain regions 3. The role of 5‐HT4 receptors in the striatum is unclear. No data have been reported regarding its ability to control the activity of 5‐HT terminals yet, and previous data indicate that 5‐HT4 receptors do not interfere with DA‐mediated behaviors attributed to striatal functions 9. Regional changes in 5‐HT4 receptor densities or coupling could account for the heterogeneous and regional responses to prucalopride, in particular in the SNr with respect to other brain regions investigated 10.
The main interest here is to report that a 5‐HT agonist changes the pattern of L‐DOPA‐induced DA release. The pattern of L‐DOPA‐stimulated DA release would be more important than striatal DA release per se in the neurochemical and behavioral outcomes of L‐DOPA 3. A heterogeneous response is, for example, difficult to obtain with blockers of noradrenaline or serotonin transporters which display a widespread expression 3, 7. The increase in DA release in the PFC and the SNr but not in the striatum might correspond to a favorable neurochemical profile. Indeed, the efficacy of L‐DOPA cannot be simply related to striatal DA release, while L‐DOPA‐induced dyskinesia could be consequent to the dramatic loss of DA responses in the SNr and/or PFC compared to the striatum after chronic L‐DOPA 3. Additional studies are warranted to study the effect of 5‐HT4 ligands in rats chronically treated with L‐DOPA.
5‐HT4 agonists, including prucalopride, can already be given in the treatment of constipation to patients with PD 11. The nonselective 5‐HT4 agonist cisapride has been reported to aggravate tremor in PD, although this effect is probably related to other components of its pharmacological profile 9. The enterokinetic properties of 5‐HT4 receptor agonists suggest their potential use against L‐DOPA‐induced fluctuations in patients with PD 11. Moreover, 5‐HT4 receptor agonists might act as potential antidepressant or anti‐Parkinsonian agents in the treatment of PD, although no evaluation has been reported yet in humans. This assumption is based on the work of Mendez‐David et al. 4 which recalls that 5‐HT4 agonists display anxiolytic/antidepressant properties in the mouse corticosterone model. The 5‐HT4 agonist mechanism of action differs from classical 5‐HT antidepressant drugs as they indirectly act on the function of 5‐HT terminals 5. In addition, our findings showing favorable neurochemical pattern of DA release obtained in the presence of L‐DOPA, in terms of efficacy without motor side effects 3, would uphold 5‐HT4 agonists as an alternative approach to the treatment of anxiety and/or depression in L‐DOPA‐treated patients with PD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The project was funded by the Fondation de France and the Centre National de la recherche scientifique. SN received the price of the “Société Française de Physiologie et de Pharmacologie” for this project. PDD and GDG acknowledge the support given by the Cooperation for Science and Technology (COST) action CM1103. The authors thank Dr Jan A. Schuurkes (Movetis, Belgium) for the generous gift of prucalopride.
References
- 1. Djaldetti R1, Melamed E. New drugs in the future treatment of Parkinson's disease. J Neurol 2002;249(Suppl 2):II30–II35. [DOI] [PubMed] [Google Scholar]
- 2. Navailles S, De Deurwaerdere P. Contribution of serotonergic transmission to the motor and cognitive effects of high‐frequency stimulation of the subthalamic nucleus or levodopa in Parkinson's disease. Mol Neurobiol 2012;45:173–185. [DOI] [PubMed] [Google Scholar]
- 3. Navailles S, De Deurwaerdere P. Imbalanced dopaminergic transmission mediated by serotonergic neurons in L‐DOPA‐induced dyskinesia. Parkinsons Dis 2012;2012:323686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendez‐David I, David DJ, Darcet F, et al. Rapid anxiolytic effects of a 5‐HT₄ receptor agonist are mediated by a neurogenesis‐independent mechanism. Neuropsychopharmacology 2014;39:1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lucas G. Serotonin receptors, type 4: a new hope? Curr Drug Targets 2009;10:1085–1095. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds GP, Mason SL, Meldrum A, et al. 5‐Hydroxytryptamine (5‐HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol 1995;114:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navailles S, Milan L, Khalki H, Di Giovanni G, Lagière M, De Deurwaerdère P. Noradrenergic Terminals Regulate l‐DOPA‐derived Dopamine Extracellular Levels in a Region‐dependent Manner in Parkinsonian Rats. CNS Neurosci Ther 2014;20:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porras G, Di Matteo V, De Deurwaerdère P, Esposito E, Spampinato U. Central serotonin 4 receptors selectively regulate the impulse‐dependent exocytosis of dopamine in the rat striatum: in vivo studies with morphine, amphetamine and cocaine. Neuropharmacology 2002;43:1099–1109. [DOI] [PubMed] [Google Scholar]
- 9. De Deurwaerdère P, Cervo L, Stinus L, Spampinato U. Central 5‐HT(4) receptors and dopamine‐dependent motor behaviors: searching for a functional role. Pharmacol Biochem Behav 2002;71:627–633. [DOI] [PubMed] [Google Scholar]
- 10. Bonaventure P, Hall H, Gommeren W, et al. Mapping of serotonin 5‐HT(4) receptor mRNA and ligand binding sites in the post‐mortem human brain. Synapse 2000;36:35–46. [DOI] [PubMed] [Google Scholar]
- 11. Asai H, Udaka F, Hirano M, et al. Increased gastric motility during 5‐HT4 agonist therapy reduces response fluctuations in Parkinson's disease. Parkinsonism Relat Disord 2005;11:499–502. [DOI] [PubMed] [Google Scholar]


