The blood–brain barrier (BB) is a dynamic interference which regulates the flow of substances into and out of the brain. It consists of endothelial cells of microvessels, astrocytes, and pericytes, and is surrounded by neurons. In normal conditions, BBB represents a major barrier for most substances to enter the brain from the circulation. Stroke compromises the barrier function of BBB 1. Many substances that could augment tissue damage, including excitotoxic glutamate, the activated form of proteases, transmembrane fluxes of calcium, and inflammation mediators, could easily enter brain parenchyma through the BBB and aggravating the damaged brain tissues 2.
Stroke is a leading cause of the mortality, and it requires new drugs to improve the treatment of stroke. In developing new drugs to treat ischemic stroke, a historical major concern is the ability of the candidate drugs to pass BBB. We propose that BBB is not a significant barrier in such efforts, based on the following arguments.
The medical treatment of ischemic stroke mainly includes three classes of drugs. The first class consists of intravenous fibrinolytics as is exemplified by the now widely used tissue‐type plasminogen activator (rtPA) 3, 4, anticoagulants, and antiplatelets agents. Many candidate fibrinolytic agents (e.g., tenecteplase, reteplase, desmoteplase, urokinase, and ancrod) are being developed and are currently under testing in clinical trials 5. Aspirin is the prototype antiplatelet and is recommended for the treatment of the stroke within 24–48 h after disease onset 6. Novel antiplatelet agents (e.g., dabigatran) need more evidences for clinical use 7.
The second class of drugs acts on cerebral vessels. The penumbra remains viable for hours after stroke onset due to compensatory arteriolar dilation of partially occluded vessels and collateral circulation. Increasing blood flow to the penumbra with vasodilating drugs, such as methylxanthine derivatives, specially pentoxifylline, propentofylline, and pentifylline, has been shown to reduce blood viscosity, increase erythrocyte flexibility, and inhibit platelet aggregation 5. More studies are needed to verify the efficacy and safety in stroke patients.
The two classes of drugs mentioned above produce action at intravascular sites and do not involve the BBB.
The third class of drugs acts by protecting cerebral parenchyma in the penumbra. Rather than reversing the necrotic brain tissue, the aim of the neuroprotective agents is to rescue or delay the infarction of the penumbra. The ability to pass through the BBB is clearly important for these agents. An important consideration here is increased permeability of the BBB upon stroke. However, peripheral actions, such as antiinflammatory effects, could also produce therapeutic effects. Our group found that neostigmine combined with anisodamine had neuroprotective effect by reducing the infarct size from 34.2 ± 9.86% to 22.2 ± 7.59% in an animal stroke model 8. A mechanistic investigation showed that the protective effects of the neostigmine/anisodamine combination could be attributed to decrease of the pro‐inflammatory cytokines levels and increase of the expression of antiapoptotic proteins, playing a critical role in the outcome after stroke. Both neostigmine and anisodamine do not cross the BBB under physiological condition; whether these could cross the BBB during stroke was not examined in our experiment.
In our opinion, ability to cross the BBB has been over‐emphasized in the R&D efforts to develop novel agents to treat ischemic stroke for two primary reasons (Figure 1). First, the permeability of BBB is increased upon stroke, thus allowing many “impermeable” drugs to enter the brain. Second, many novel protective agents could produce beneficial effects via peripheral or systemic actions.
Figure 1.
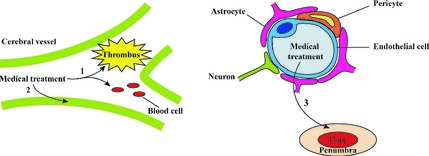
A schematic diagram of medical treatments of ischemic stroke.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Zhang GS, Tian Y, Huang JY, et al. The gamma‐secretase blocker, DAPT, reduces the permeability of the blood‐brain barrier by decreasing the ubiquitination and degradation of occludin during permanent brain ischemia. CNS Neurosci Ther 2013;19:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu WY, Wang ZB, Zhang LC, Wei X, Li L. Tight junction in blood‐brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 2012;18:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang YJ, Zhang SM, Zhang L, et al. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther 2012;18:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jauch EC, Saver JL, Adams HP, Jr , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 6. European Stroke Organisation Executive C, Committee ESOW . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 7. Brouns R, Van Hooff RJ, De Smedt A, et al. Acute stroke management in patients taking dabigatran. CNS Neurosci Ther 2012;18:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu AJ, Zang P, Guo JM, et al. Involvement of acetylcholine‐alpha7nAChR in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther 2012;18:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]


