Abstract
This investigation measured the effects of a light pressure instrument assisted soft tissue mobilization (IASTM) technique on tactile discrimination and pain perception in individuals after strenuous exercise. Twenty-three subjects underwent three different testing sessions: baseline measures and exercise, 24-hours (post) treatment and measures, and 48-hours (post) treatment and measures. Outcomes included two-point discrimination (TPD) and pressure pain threshold (PPT). Statistical analysis included parametric tests. For TPD, a significant difference was observed between all time points (p <.001). Post-hoc testing revealed a significant difference from baseline to 24 hours post (p <.001) and 48 hours post (p <.001). For PPT, a significant difference was observed between all time points (p <.001). Post-hoc testing revealed a significant difference from baseline to 24 hours post (p =.005) and 48 hours post (p =.003). A significant difference was not observed between 24 to 48 hours post for TPD and PPT (p =1.00). The results suggest that a light IASTM technique may produce a neuromodulation effect on local tactile descrimination and pain perception in individuals with DOMS.
Keywords: mobilization, muscle soreness, perceived pain, recovery
Abstract
Cette étude a mesuré les effets d’une technique de mobilisation des tissus mous assistée par un instrument de pression légère (IASTM) sur la discrimination tactile et la perception de la douleur chez les individus après un exercice intense. Vingt-trois sujets se sont prêtés à trois séances d’évaluation différentes: mesures et exercices de base, 24 heures et 48 heures après le traitement et les mesures. Les résultats comprenaient la discrimination de deux points et le seuil de douleur à la pression. L’analyse statistique comprenait des tests paramétriques. Pour la discrimination de deux points, une importante différence entre tous les points dans le temps (p < 0,001) a été observée. L’examen post-hoc a révélé une importante différence entre le début de l’étude et les 24 heures (p < 0,001) et 48 heures (p < 0,001) qui ont suivi l’étude. Selon le seuil de douleur à la pression, une importante différence entre tous les points dans le temps (p < 0,001) a été observée. L’examen post-hoc a révélé une importante différence entre le début de l’étude et les 24 heures (p = 0,005) et 48 heures (p = 0,003) qui ont suivi l’étude. Aucune différence importante n’a été observée entre les 24 à 48 heures après la discrimination de deux points et le seuil de douleur à la pression (p = 1,00). Les résultats suggèrent qu’une technique de mobilisation des tissus mous assistée par un instrument de pression légère (IASTM) pourrait produire un effet de neuromodulation sur la discrimination tactile locale et la perception de la douleur chez les individus présentant des courbatures.
MOTS CLÉS: mobilisation, douleur musculaire, douleur perçue, récupération
Introduction
Instrument assisted soft-tissue mobilization (IASTM) has become a popular myofascial intervention utilized by allied health professionals across the world. The popularity has stimulated the creation of many different tools and treatment techniques. This popularity has stimulated a growth in research over the past 10 years. The body of IASTM research has produced variable outcomes with most of evidence coming from case reports or case studies. 1 The amount of high level evidence (e.g. randomized controlled trials) has grown but a disparity still exists.1 Current published research suggests that IASTM may improve joint ROM,2–5 modulate pain perception,3 and increase local circulation.6,7 However, little effects on muscle performance have been documented.8,9
Presently, there are several scientific theories regarding the effects of IASTM, most notable, mechanical and neurophysiological. The mechanical theory suggests that pressure and shearing from the instrument may release and breakdown scar tissue, adhesions, and fascial restrictions, and aid in tissue healing.7,10,11 The only studies supporting this theory have shown enhanced healing in rodent ligaments.7,11 No human studies have been conducted that support this theory. In contrast, the neurophysiological theory suggests that the compression from the instrument may stimulate local mechanoreceptors, nociceptors (e.g. C-tactile fibers),12–14 and ascending afferent pathways which may stimulate other physiological responses by the body.10 Portillo-Soto et al.6 reported local blood flow changes in the gastrocnemius after a 10 minute IASTM session. Ge et al.10 reported changes in mechanoreceptor activity after a 10 minute IASTM treatment to the anterior thigh. These researchers found a statistically significant post-treatment increase in 2-point discrimination (40.2 ± 9.4 mm to 44.9 ± 12.0 mm) but no significant changes in pressure pain threshold (PPT) with algometry (18.2 ± 6.6 lbs to 18.7 ± 6.8 lbs). The researchers concluded that the IASTM treatment may have a greater effects of mechanoreceptors than nociceptors.10
Despite the emerging research and popularity of IASTM, there is no consensus on the optimal treatment parameters such as: tool type, tool angle, stroke type (vector), rate, and amount of pressure being applied.1 Due to this gap in the literature, a clinician may choose their own preferred treatment parameters or follow the parameters taught by non-evidence based sources.1
Concomitantly, there are a limited number of controlled studies on the efficacy of IASTM with individuals in pain or with injury. The majority of evidence comes from case reports and case series.1 The existing evidence does suggest favorable outcomes after IASTM treatment for individuals with shoulder impingment,2 chronic ankle instability,15 carpal tunnel syndrome,16 and chronic low-back pain.3
Of interest are the therapeutic effects of IASTM as a posttreatment intervention after strenuous exercise. To the authors knowledge, no studies have measured these effects. The purpose of this investigation was to measure the therapeutic effects of a light pressure IASTM technique on tactile discrimination and pain perception in healthy individuals after strenuous exercise. The researchers hypothesized a light pressure IASTM technique may have a modulatory effect on local mechanoreceptors and ascending pain pathways.
Methods
Subjects
Twenty-three recreationally active adults (M=14, W=9) were recruited via convenience sampling (e.g. flyers) (Table 1). Recruited subjects reported participating in recreational fitness activities (e.g. walking) with no prior experience with IASTM treatment (naïve subjects). Exclusion criteria included the presence of any neurosensory impairments, musculoskeletal, systemic, or metabolic disease that would affect lower extremity tolerance to IASTM treatment, pressure pain threshold (PPT) testing, two-point discrimination (TPD), and the inability to avoid medications that may affect testing.10 This prestest, posttest clinical study was approved by the Institutional Review Board at California State University Dominguez Hills, Carson, CA, USA (#18-177).
Table 1.
Experimental procedures.
Instruments
For PPT, the Wagner (Midvale, UT) FDX algometer was used for this investigation. The manufacturer reports an accuracy error of < ± 0.3% for this technology.17 Algometry is a valid and reliable tool for measuring pressure pain thresholds.18–21 Algometry has also been used in prior IASTM research.10 For TPD, the Baseline (Fabrication Enterprises, Inc, White Plains, NY) two-point discrimination (12-1480) caliper was used to administer the testing. Calipers are a valid and reliable tool for measuring two-point discrimination22,23 and have been used in prior IASTM research.10
IASTM Treatment
For IASTM treatment, the Rock Blades® Mohawk tool (RockTape®, Campbell, CA) was used to administer the treatment. The Mohawk is a stainless steel shaped metal instrument with several beveled edges (Figure 1). The investigator administered a 90 second light pressure “feather stroke” using the weight of the tool (208 grams) intervention in line with the fibers of the rectus femoris using a rate of 120BPM with the Mohawk angled at 30°.24 The investigator used a metronome (The metronome by Soundbrenner App 2017) to ensure a consistent rate during treatment and calibrated the instrument angle with a digital goniometer prior to each subject’s treatment.
Figure 1.

Rock Blades® Mohawk tool.
Outcome Measures
Two outcome measures were used for the baseline, 24 hours, and 48-hours post exercise measures. For PPT, the subjects dominant (kicking leg) quadriceps group was tested with the subject lying supine on a mat table (2 measurements).25–27 The 1.0-cm2 probe of the algometer was placed in a predetermined area (see procedures) along the midline of the dominant quadriceps (rectus femoris). 28,29 The graded force was applied at a constant rate of 50–60 kilopascals per second (kPa/sec), perpendicular to the tissues, until the subject verbally reported the presence of pain.25–27 These outcome measures have been used in prior research.30–32 For TPD, subjects laid supine on the mat table. The examiner used the two-point discrimination caliper to the predetermined area using the 4-2-1 stepping algorithm for tactile sensory testing.33,34
Pilot Study
Prior to data collection, a two-session pilot training was conducted to establish intrarater reliability. The primary investigator took all measurements. The primary investigator is a licensed physical therapist with over 13 years of experience and board certified in orthopaedics. Ten independent subjects were recruited and tested for this portion of the study. The intrarater reliability was calculated using the Intraclass Correlation Coefficient (ICC model 3, k).35 There was good intrarater reliability for PPT with algometry (ICC= 0.95; 95% CI 0.83–0.99) and TPD (ICC= 0.94; 95% CI 0.61–0.97).
Procedures
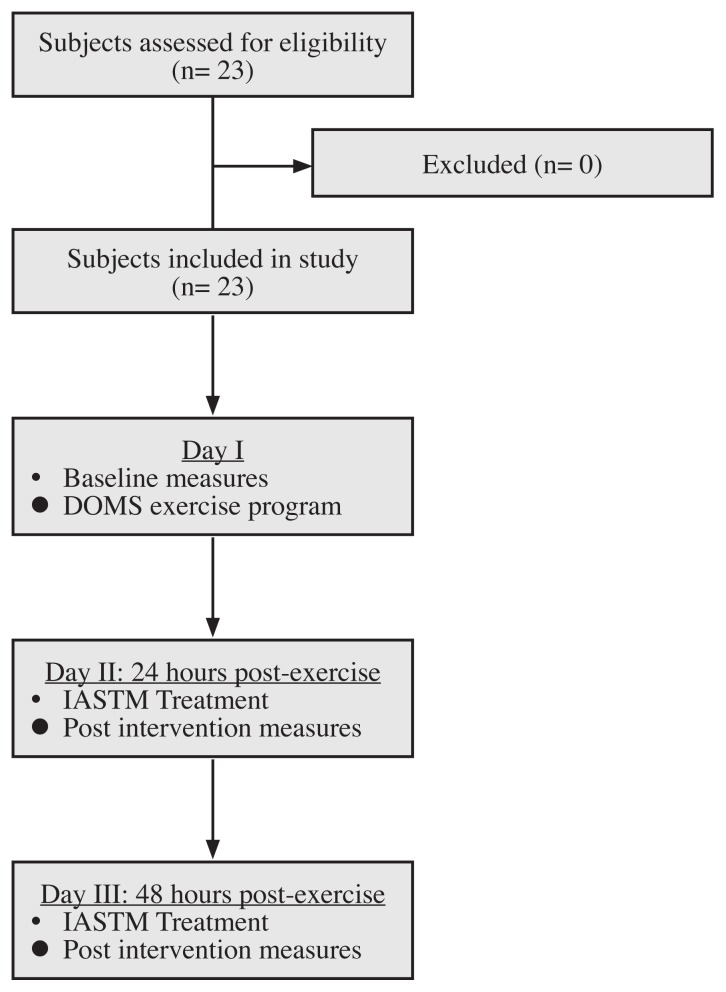
All eligible participants were given an IRB approved consent form to read and sign before testing. Participants then completed a questionnaire to provide demographic information. All participants were tested by the primary investigator and were blinded from the results and other participants enrolled in the study. A second investigator administered the IASTM treatment and was blinded to the results. The second investigator is a Certified Athletic Trainer with over 24 years of experience and certified in several IASTM intervention techniques. Testing was conducted between the hours of 11 A.M. and 3 P.M. and subjects were instructed to refrain from any strenuous activity five hours prior to testing and from taking any medications or supplements that would interfere with testing. All subjects underwent three days of testing which included: baseline measures and exercise, 24-hours (post) treatment and measures, and 48-hours (post) treatment and measures. The soreness that occurs after exercises arises 24 hours after exercise and peaks in intensity by 48 hours post-exercise.36
Day I: Baseline measures and exercise
The primary investigator first explained and demonstrated the testing process to each subject and answered any questions. The investigator then measured and marked two ends of a 6.4 cm (2.5 in) line approximately 7.62 cm (3.0 in) above the patella in the midline of the subjects dominant quadriceps. 37 Baseline measures were conducted on all subjects which included PPT and TPD within the predetermined area.28,29 Participants then underwent an induced muscle soreness exercise protocol which included a five-minute treadmill warm-up followed by 100 drop jumps (5 sets of 20 repetitions) from a 0.5 m box.38 The primary investigator monitored each subject and provided feedback to ensure adequate form and safety during the activity.
Day II: 24 hours post
The second investigator confirmed the predetermined marked area from Day I and administered the 90 second IASTM treatment to each subject. Upon completion, the second investigator left the area and the primary investigator retested each subject. The primary investigator confirmed the predetermined area and tested subjects within the marked area.
Day III: 48 hours post
The second investigator again confirmed the predetermined marked area from Day I and II and administered the 90 second IASTM treatment to each subject. Upon completion, the investigator left the area and the primary investigator then retested each subject within the predetermined marked area.
Statistical Analysis
Statistical analysis was performed using SPSS version 24.0 (IBM SPSS, Chicago, IL, USA). Subject descriptive data was calculated and reported as the mean and standard deviation (SD) for age, height, body mass, and body mass index (BMI). Baseline and post exercise differences were calculated using the repeated ANOVA statistic.39 Post hoc testing was calculated using the Bonferroni statistic. Effect size (ES) was calculated (d = M1 - M2 / spooled). Effect size of > 0.70 was considered strong, 0.41 to 0.70 was moderate, and < 0.40 was weak.40 Statistical assumptions were met for the ANOVA including normality and homgeneity of variance.35 Statistical significance was considered p< .05 using a conservative two-tailed test.
Results
Twenty-three subjects completed the study (M=14, W=9; mean age= 24.22 ± 3.07 years; height= 172.08 ± 8.53 cm; body mass=80.43 ± 16.18 kg; body mass index (BMI)= 26.60 ± 4.05 kg/m2). There were no adverse events or subject attrition during data collection.
For TPD, there was a significant difference between baseline, 24 hours, and 48 hours post [F (1, 21) =30.50, p <.001, partial η2 =.744]. Post-hoc testing revealed a significant difference from baseline to 24 hours post (p <.001, ES =1.12) and baseline to 48 hours post (p <.001, ES =1.10) (Table 2). There was no significant difference between 24 to 48 hours post (p =1.00, ES =.10). For PPT, there was a significant difference between baseline, 24 hours, and 48 hours post [F (1, 21) =9.56, p <.001, partial η2 =.477]. Post-hoc testing revealed a significant difference from baseline to 24 hours post (p =.005, ES =.33) and baseline to 48 hours post (p =.004, ES =.30). TA significant difference was not observed between 24 to 48 hours post for TPD and PPT (p =1.00, ES =.03) (Table 2).
Table 2.
Baseline and post-intervention descriptive results (N=23).
| Baseline | 24 hours | p-value | Baseline | 48 hours | p-value | |
|---|---|---|---|---|---|---|
| Two Point Discrimination (cm) | 4.33 ± 1.12 | 2.98 ± 1.28 | *<.001 | 4.33 ± 1.12 | 2.83 ± 1.57 | *<.001 |
| Pressure Pain Threshold (kPa) | 1132.08 ± 244.26 | 1214.91 ± 261.25 | *=.005 | 1132.08 ± 244.26 | 1207.22 ± 248.80 | *=.004 |
Discussion
This was the first investigation to measure the efficacy of a predetermined IASTM technique on local tactile discrimination and pain perception in healthy individuals 24 and 48 hours after strenuous exercise. DOMS is a common condition that arises 24 hours after strenuous exercise and peaks in intensity by 48 hours post-exercise.36 This soreness can affect an individual’s tactile sense and pain perception.12,38,41 The results of this study suggest that a light pressure longitudinal IASTM intervention using specific parameters may produce changes in local TPD and PPT for up to two days after strenuous exercise.
In related research, Ge et al.10 used similar methods to measure the immediate post-intervention effects of a 10 minute IASTM treatment to the anterior thigh using TPD and PPT in non-exercised healthy individuals. The researchers found a statistically meaningful change with TPD but not PPT. Additionally, the researchers reported using the Graston® concept for treatment, but only completed an IASTM intervention and did not follow the recommended steps in the paradigm.1 Furthermore, the researchers did not report any specific treatment parameters such as: tool angle, stroke rate, predetermined area, and pressure which created a methodological weakness and may have influenced the outcomes.10
In this investigation, the researchers attempted to use a strict treatment strategy to control for multiple variables. For TPD, the results revealed a post-intervention decrease in distance at 24 and 48 hours which suggests that subjects may have experienced improved local tactile sense through mechanoreceptor stimulation.10 For PPT, the results revealed a decrease in perceived pain (higher tolerance to pressure) at 24 and 48 hours after strenuous exercise which suggests that the light IASTM treatment modulated nociceptive activity (C-tactile fibers). C-tactile fibers are low threshold afferent mechanoreceptors that innervate the human skin and contribute to pain perception.24 These receptors respond to light tissue compressive forces (e.g. light brushing) and have been reported to modulate pain14 and mediate allodynia in DOMS.12 Thus, the light pressure (i.e. weight of tool) IASTM stroke at a 30° angle for 90 seconds at a rate of 120 BPM may have produced a short-term modulation of local C-tactile pain fibers at 24 and 48 hours post. Further research is needed to validate these findings.
Clinical Implications and Future Research
The results of this investigation suggests to clinicians that there may be merit in using a light IASTM stroke to stimulate local mechanoreceptors and nociceptors when treating patients in pain or following injury. The results of this study are preliminary evidence. Clinicians should consider this before integrating these treatment and assessment techniques into their clinical practice. Future research should focus on three gaps in the current literature. First, research should continue to study different treatment parameters such as pressure, dose time, stroke rate, and tool angle. Second, the therapeutic efficacy of the tool architecture needs to be studied such as type of metal, edge angle, tool surface, and weight. Third, the therapeutic effects of the treatment area need to be investigated. The optimal size of treatment area where changes occur may help clinicians become more efficient with their treatments.
Limitations
There are specific limitations to the investigation that need to be discussed. First, this investigation tested healthy subjects after a specific strenuous exercise protocol which limits the generalizability of the results to this population. Second, the IASTM technique used predetermined parameters on the dominant quadriceps muscle group. Other IASTM techniques may have produced different results. Third, a pre-determined small area on the quadriceps was investigated. A different or larger area on the body many have produced different results. Fourth, only the short-term effects (24 and 48 hours) of the IASTM intervention were measured. Future studies should measure the long-term effects of this intervention. Fifth, a randomized, larger sample size with a comparison control group may have produced other results. The researchers considered this investigation as exploratory and plan to use such methodology in future investigations.
Conclusion
IASTM is a popular intervention that is significantly understudied. This is the first investigation to study the effects of a light IASTM stroke and specific parameters on individuals with DOMS. Clinicians should consider these results as exploratory before integrating such strategies into their clinical practice.
Acknowledgements
We would like to thank RockTape for providing permission to use of the Rock Blades® Mohawk tool and accompanying image. We would like to thank Dr. Steven Capobianco and Steven Stahl for providing their insight and feedback with this manuscript.
Footnotes
The authors have no disclaimers, competing interests, or sources of support or funding to report in the preparation of this manuscript.
References
- 1.Cheatham SW, Lee M, Cain M, et al. The efficacy of instrument assisted soft tissue mobilization: a systematic review. J Can Chiropr Assoc. 2016;60(3):200–211. [PMC free article] [PubMed] [Google Scholar]
- 2.Coviello JP, Kakar RS, Reynolds TJ. Short-term effects of instruments-assisted soft tissue mobilization on pain free range of motion in a weightlifter with subacromial pain syndrome. Int J Sports Phys Ther. 2017;12(1):144–154. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Lee DK, Oh JS. The effect of Graston technique on the pain and range of motion in patients with chronic low back pain. J Phys Ther Sci. 2016;28(6):1852–1855. doi: 10.1589/jpts.28.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markovic G. Acute effects of instrument assisted soft tissue mobilization vs. foam rolling on knee and hip range of motion in soccer players. J Bodyw Mov Ther. 2015;19(4):690–696. doi: 10.1016/j.jbmt.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Laudner K, Compton BD, McLoda TA, et al. Acute effects of instrument assisted soft tissue mobilization for improving posterior shoulder range of motion in collegiate baseball players. Int J Sports Phys Ther. 2014;9(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Portillo-Soto A, Eberman LE, Demchak TJ, et al. Comparison of blood flow changes with soft tissue mobilization and massage therapy. J Altern Complement Med. 2014;20(12):932–936. doi: 10.1089/acm.2014.0160. [DOI] [PubMed] [Google Scholar]
- 7.Loghmani MT, Warden SJ. Instrument-assisted cross fiber massage increases tissue perfusion and alters microvascular morphology in the vicinity of healing knee ligaments. BMC Complement Altern Med. 2013;13:240. doi: 10.1186/1472-6882-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroiney DA, Mokris RL, Hanna GR, et al. Examination of self-myofascial release vs. instrument-assisted soft-tissue mobilization techniques on vertical and horizontal power in recreational athletes. J Strength Cond Res. 2018 doi: 10.1519/JSC.0000000000002628. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald N, Baker R, Cheatham SW. The effects of instrument assisted soft tissue moilization on lower extremity muscle performance: a randomized controlled trial. Int J Sports Phys Ther. 2016;11(7):1040–1047. [PMC free article] [PubMed] [Google Scholar]
- 10.Ge W, Roth E, Sansone A. A quasi-experimental study on the effects of instrument assisted soft tissue mobilization on mechanosensitive neurons. J Phys Ther Sci. 2017;29(4):654–657. doi: 10.1589/jpts.29.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loghmani MT, Warden SJ. Instrument-assisted crossfiber massage accelerates knee ligament healing. J Orthop Sports Phys Ther. 2009;39(7):506–514. doi: 10.2519/jospt.2009.2997. [DOI] [PubMed] [Google Scholar]
- 12.Nagi SS, Mahns DA. C-tactile fibers contribute to cutaneous allodynia after eccentric exercise. J Pain. 2013;14(5):538–548. doi: 10.1016/j.jpain.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Nagi SS, Rubin TK, Chelvanayagam DK, et al. Allodynia mediated by C-tactile afferents in human hairy skin. J Physiol. 2011;589(Pt 16):4065–4075. doi: 10.1113/jphysiol.2011.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaikh S, Nagi SS, McGlone F, et al. Psychophysical Investigations into the Role of Low-Threshold C Fibres in Non-Painful Affective Processing and Pain Modulation. PLoS One. 2015;10(9):e0138299. doi: 10.1371/journal.pone.0138299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer JL, Sandrey MA. Effects of a 4-week dynamic-balance-training program supplemented with Graston instrument-assisted soft-tissue mobilization for chronic ankle instability. J Sport Rehabil. 2012;21(4):313–326. doi: 10.1123/jsr.21.4.313. [DOI] [PubMed] [Google Scholar]
- 16.Burke J, Buchberger DJ, Carey-Loghmani MT, et al. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. J Manipulative Physiol Ther. 2007;30(1):50–61. doi: 10.1016/j.jmpt.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Wagner I. Wagner FDX Algometer Specification Page. [Accessed 5/12/18]. Available at. http://www.wagnerinstruments.com/products/force-gages/digital/fdx.
- 18.Chesterton LS, Sim J, Wright CC, et al. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23(9):760–766. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther. 1998;78(2):160–169. doi: 10.1093/ptj/78.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Walton DM, Macdermid JC, Nielson W, et al. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther. 2011;41(9):644–650. doi: 10.2519/jospt.2011.3666. [DOI] [PubMed] [Google Scholar]
- 21.Aboodarda SJ, Spence AJ, Button DC. Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskelet Disord. 2015;16:265. doi: 10.1186/s12891-015-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cashin AG, McAuley JH. Measuring two-point discrimination threshold with a caliper. J Physiother. 2017;63(3):186. doi: 10.1016/j.jphys.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Catley MJ, Tabor A, Wand BM, et al. Assessing tactile acuity in rheumatology and musculoskeletal medicine--how reliable are two-point discrimination tests at the neck, hand, back and foot? Rheumatology (Oxford) 2013;52(8):1454–1461. doi: 10.1093/rheumatology/ket140. [DOI] [PubMed] [Google Scholar]
- 24.Liljencrantz J, Olausson H. Tactile C fibers and their contributions to pleasant sensations and to tactile allodynia. Front Behav Neurosci. 2014;8:37. doi: 10.3389/fnbeh.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearcey GE, Bradbury-Squires DJ, Kawamoto JE, et al. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J Athl Train. 2015;50(1):5–13. doi: 10.4085/1062-6050-50.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheatham SW, Kolber MJ, MC Comparison of a video-guided, live instructed, and self-guided foam roll interventions on knee joint range of motion and pressure pain threshold: a randomized controlled trial. Int J Sports Phys Ther. 2017;12(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Cheatham SW, Stull KR, Kolber MJ. Comparison of a vibrating foam roller and a non-vibrating foam roller intervention on knee range of motion and pressure pain threshold: a randomized controlled trial. J Sport Rehabil. 2017:1–23. doi: 10.1123/jsr.2017-0164. [DOI] [PubMed] [Google Scholar]
- 28.Cheatham SW, Baker R. Differences in pressure pain threshold among men and women after foam rolling. J Bodyw Mov Ther. 2017;21(4):978–982. doi: 10.1016/j.jbmt.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Cheatham SW, Kolber MJ. Does self-myofascial release with a foam roll change pressure pain threshold of the ipsilateral lower extremity antagonist and contralateral muscle groups? An exploratory study. J Sport Rehabil. 2017:1–13. doi: 10.1123/jsr.2016-0196. [DOI] [PubMed] [Google Scholar]
- 30.Cheatham SW, Baker R. Differences in pressure pain threshold among men and women after foam rolling. J Bodywork Movement Ther. 2017;21(4):978–982. doi: 10.1016/j.jbmt.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Cheatham SW, Kolber MJ. Does self-myofascial release with a foam roll change pressure pain threshold of the ipsilateral lower extremity antagonist and contralateral muscle groups? An exploratory study. J Sport Rehabil. 2017:1–18. doi: 10.1123/jsr.2016-0196. [DOI] [PubMed] [Google Scholar]
- 32.Cheatham SW, Kolber MJ, Cain M. Comparison of video-guided, live instructed, and self-guided foam roll interventions on knee joint range of motion and pressure pain thresold: a randomized controlled trial. Int J Sports Phys Ther. 2017;12(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- 33.Wikstrom EA, Allen G. Reliability of two-point discrimination thresholds using a 4-2-1 stepping algorithm. Somatosens Mot Res. 2016;33(3–4):156–160. doi: 10.1080/08990220.2016.1227313. [DOI] [PubMed] [Google Scholar]
- 34.Snyder BA, Munter AD, Houston MN, et al. Interrater and intrarater reliability of the Semmes-Weinstein monofilament 4-2-1 stepping algorithm. Muscle Nerve. 2016;53(6):918–924. doi: 10.1002/mus.24944. [DOI] [PubMed] [Google Scholar]
- 35.Portney LG, Watkins MP. Foundations of Clinical Research: Applications To Practice. F.A. Davis Company/Publishers; 2015. [Google Scholar]
- 36.Torres R, Ribeiro F, Alberto Duarte J, et al. Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: systematic review and meta-analysis. Phys Ther Sport. 2012;13(2):101–114. doi: 10.1016/j.ptsp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Valagussa G, Meroni R, Cantarelli F, et al. Two point discrimination in lower limbs in healthy people: Average values and influence of gender, dominance, height and BMI. Manual Therapy. 2016;25:e95. [Google Scholar]
- 38.Romero-Moraleda B, La Touche R, Lerma-Lara S, et al. Neurodynamic mobilization and foam rolling improved delayed-onset muscle soreness in a healthy adult population: a randomized controlled clinical trial. PeerJ. 2017;5:e3908. doi: 10.7717/peerj.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dugard P, Todman J. Analysis of pre-test-post-test control group designs in educational research. Educational Psychology. 1995;15(2):181–198. [Google Scholar]
- 40.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 41.Frey Law LA, Evans S, Knudtson J, et al. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9(8):714–721. doi: 10.1016/j.jpain.2008.03.009. [DOI] [PubMed] [Google Scholar]