Abstract
Background
Variation exists in the initial treatment for the first episode of primary spontaneous pneumothorax (PSP), and no definitive consensus exists due to a lack of high-quality evidence. This study examined the outcomes of needle aspiration and closed thoracostomy in first episodes of PSP requiring intervention.
Methods
This study was a randomized, prospective, single-center trial conducted between December 2015 and August 2016. Patients of all ages with a documented first episode of PSP who were unilaterally affected, hemodynamically stable, and had a pneumothorax measuring over 25% in size were included. Patients with underlying lung disease, severe comorbidities, bilateral pneumothorax, tension pneumothorax, recurrent pneumothorax, traumatic pneumothorax, and pregnancy were excluded. Patients were randomly assigned to the needle aspiration or closed thoracostomy group using a random number table.
Results
Forty patients with a first episode of PSP were recruited, and 21 and 19 patients were included in the needle aspiration group and the closed thoracostomy group, respectively. The hospital stay of each group was 2.1±1.8 days and 5.4±3.6 days, respectively (p<0.01). However, no significant differences were found in the success rate of initial treatment or the 1-month and 1-year recurrence rates.
Conclusion
Needle aspiration is a favorable initial treatment in patients experiencing a first episode of PSP.
Keywords: Primary spontaneous pneumothorax, Chest aspiration, Thoracostomy
Introduction
Primary spontaneous pneumothorax (PSP) is defined as spontaneously occurring pneumothorax in a patient without underlying pulmonary disease [1,2]. The pathophysiology of PSP is heterogeneous, and involves the rupture of bullae or blebs, which are emphysema-like changes of the lung parenchyma caused by distal airway inflammation and obstruction [1,3–5]. Treatment options for PSP include observation with O2 inhalation, manual needle aspiration (NA), closed thoracostomy (CT), and surgical intervention [6]. In PSP patients with a small pneumothorax (size ≤25%), the general consensus is that observation with O2 inhalation is most appropriate [6,7]. However, there is no clear guideline for PSP patients with a large pneumothorax (size >25), and variations exist in international guidelines and clinical practice [8,9]. These discrepancies arise from a lack of high-quality evidence obtained from a variety of prospective studies [6,10]. Furthermore, in Korea, variation exists in the initial treatment of first episodes of PSP, and no definitive consensus has emerged. Herein, we report the results of a randomized prospective study conducted at our center for first episodes of large PSP requiring intervention.
Methods
1) Study design and population
This study was a randomized, prospective, and single-center trial conducted between December 2015 and August 2016. Patients of all ages with a documented first episode of PSP who were unilaterally affected, hemodynamically stable, and had a pneumothorax measuring over 25% in size were included. Patients with underlying lung disease (e.g., chronic obstructive pulmonary disease, interstitial lung disease, emphysematous lung, pulmonary tuberculosis, malignancy, and infectious lung disease), severe comorbidities, bilateral pneumothorax, tension pneumothorax, recurrent pneumothorax, traumatic pneumothorax, or pregnancy were excluded. The size of the pneumothorax was measured using the Collins method (Fig. 1) [11]. All patients provided informed consent. Patients were randomly assigned to the NA or CT group using a random number table. This study received the Institutional Review Board approval of Inje University Haeundae Paik Hospital, Inje University College of Medicine (IRB approval no., 129792-2015-116).
Fig. 1.
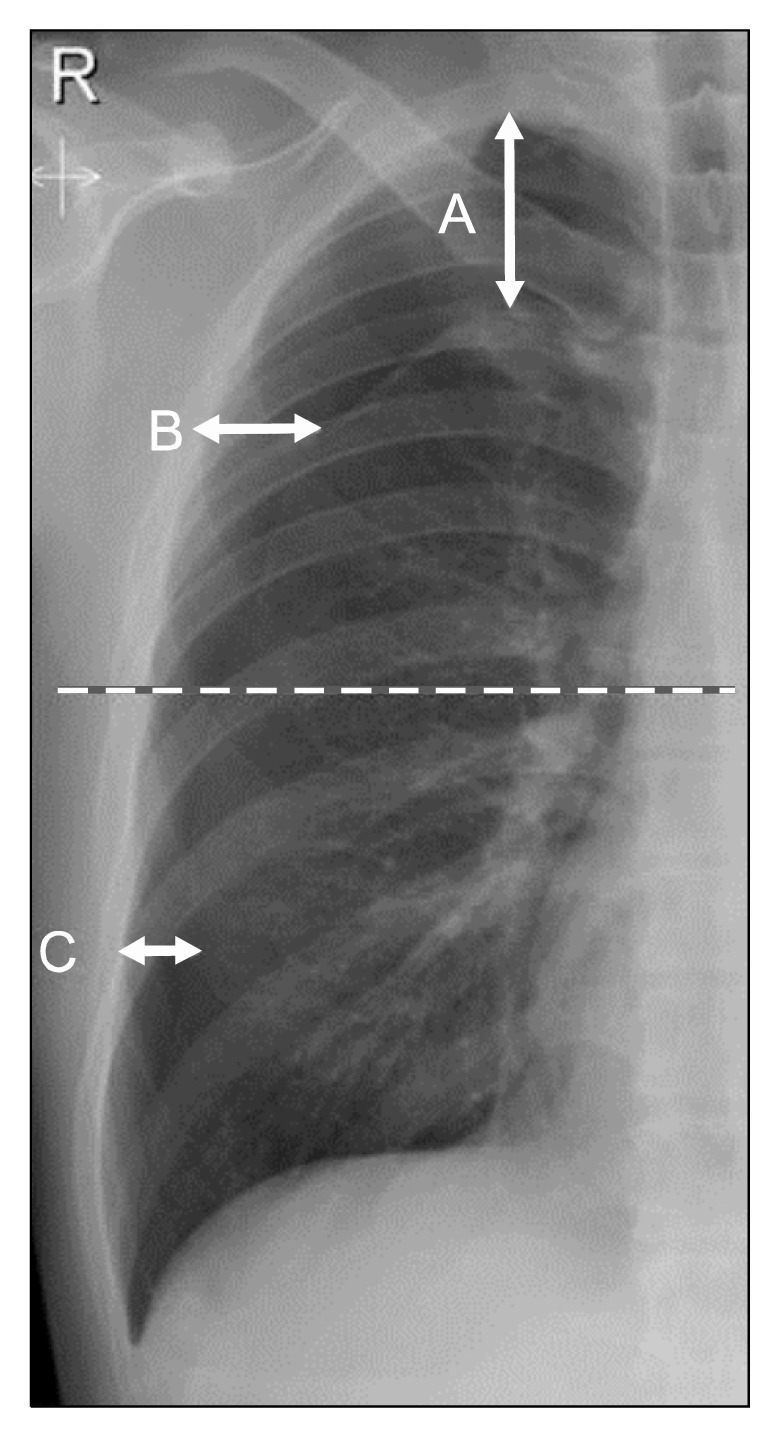
Quantification of pneumothorax size on chest radiograph using the Collins method. Size of pneumothorax is 4.2+{4.7×(A+ B+C)}.
2) Manual needle aspiration group
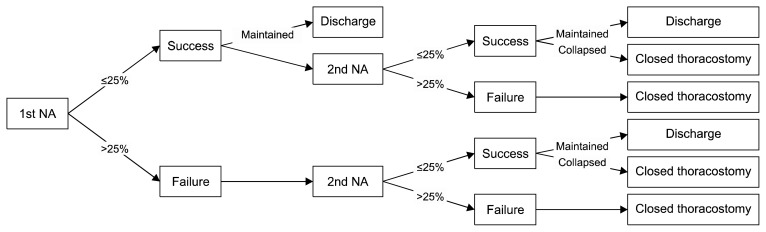
The treatment protocol for the NA group is shown in Fig. 2. Patients were placed in the semi-Fowler position and skin disinfection was performed. After local anesthesia using 2% lidocaine, a 16-gauge intravenous angio-catheter with a diameter of 1.7 mm was introduced through the second intercostal space, on the midclavicular line. When the catheter entered the pleural space, it was placed at the apex of the thoracic cavity and connected to a 3-way valve and syringe. After confirming the functionality of the catheter with a syringe, the air in the pleural cavity was suctioned using a mechanical suction system. After sufficient mechanical suction, manual aspiration was performed until resistance was felt. When no more air was evacuated, the catheter was removed and the procedure was terminated. Chest radiography was performed immediately after the procedure and 12 hours later to determine the results of NA.
Fig. 2.
Protocol of needle aspiration for a first episode of primary spontaneous pneumothorax. NA, needle aspiration; ≤25%, size of pneumothorax is same or less than 25% as estimated by Collins method on immediate follow-up chest radiography after NA; >25%, size of pneumothorax is greater than 25% on immediate follow-up chest radiography after NA; maintained, lung expansion is maintained on chest radiography 12 hours after needle aspiration; collapsed, worsening of lung expansion on chest radiography 12 hours after needle aspiration.
NA was considered successful when the pneumothorax measured ≤25% on chest radiography taken immediately after the procedure and when no signs of worsening were observed on follow-up chest radiography taken 12 hours later. We defined initial treatment success as achieving successful NA within 2 attempts. If NA was successful, the patient was discharged. If the first attempt to perform NA failed, a second attempt was made immediately. If the second attempt to performed NA failed, CT was performed.
3) Closed thoracostomy group
Under local anesthesia using 2% lidocaine, CT was performed using a 12-French (F) catheter (Argyle Suture Rib Trocar Catheter; Covidien, Mansfield, MA, USA) through the fourth intercostal space, on the anterior axillary line. The catheter was connected to an underwater seal drainage system with a negative pressure of −10 to −15 mm H2O. When complete lung expansion on chest radiography was confirmed and there was no air leakage, the chest tube was removed. If no signs of worsening were observed on chest radiography taken 12 hours after removal of the chest tube, the patient was discharged. If there was persistent air leakage (>5 days), a surgical intervention was performed. Successful CT was defined as removal of the chest tube within 5 days.
4) Statistical analysis
All statistical analyses were performed using PASW SPSS statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Baseline characteristics and information about the hospital stay were compared using the Student t-test, and the categorical variables were assessed with the chi-square test or the Fisher exact test. All p-values <0.05 were considered to indicate statistical significance.
Results
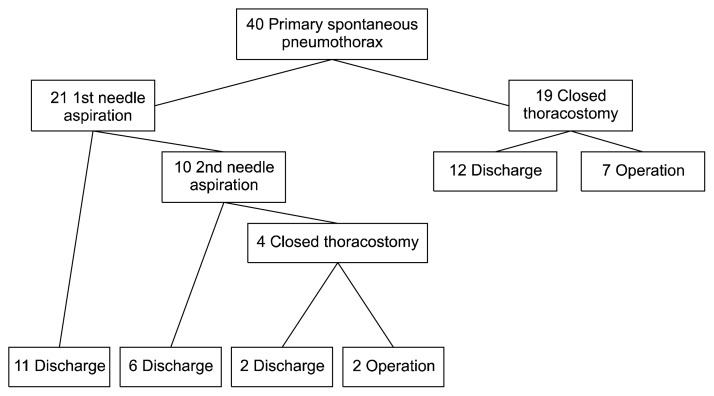
Forty patients with a first episode of PSP were recruited between December 2015 and August 2016. There were 21 patients in the NA group and 19 patients in the CT group. The characteristics of the patients in each group are shown in Table 1. There were no statistically significant differences in age, sex, body mass index, size of pneumothorax, the affected side, or smoking status. Fig. 3 shows the flow chart of study allocation and overall outcomes.
Table 1.
Patient characteristics
| Characteristic | Needle aspiration (N=21) | Closed thoracostomy (N=19) | p-value |
|---|---|---|---|
| Age (yr) | 24.0±10.9 | 24.8±11.5 | 0.83 |
| Sex (male:female) | 20:1 | 17:2 | 0.44 |
| Body mass index (kg/m2) | 20.2±2.8 | 20.7±3.3 | 0.55 |
| Size of pneumothorax (%) | 56.0±23.3 | 65.6±22.1 | 0.23 |
| Affected side (right:left) | 11:10 | 10:9 | 0.57 |
| Current smoker | 5 | 3 | 0.44 |
Values are presented as mean±standard deviation or number.
Fig. 3.
Flow chart of study allocation and outcomes.
Table 2 shows information about patients’ hospital stay, the success rate of initial treatment, the 1-month recurrence rate, the 1-year recurrence rate, and the size of pneumothorax in both groups. The hospital stay of the patients in the NA group was shorter than that of the patients in the CT group. This difference was statistically significant (p<0.01). The success rate of initial treatment in the NA group and CT group was 81.0% and 63.2%, respectively. However, this difference was not statistically significant. There was no significant difference in the 1-month or 1-year recurrence rate between the NA and CT groups.
Table 2.
Results comparing needle aspiration to closed thoracostomy
| Variable | Needle aspiration (N=21) | Closed thoracostomy (N=19) | p-value |
|---|---|---|---|
| Initial treatment success rate | 17/21 (81.0) | 12/19 (63.2) | 0.16 |
| Success, first attempt | 11/21 (52.4) | ||
| Success, second attempt | 6/10 (60.0) | ||
| 1-Month recurrence rate | 3/21 (14.3) | 1/19 (5.3) | 0.34 |
| Success | 3/17 (17.7) | 1/12 (8.3) | 0.44 |
| Failure | 0 | 0 | 0 |
| 1-Year recurrence rate | 4/21 (19.0) | 3/19 (15.7) | 0.79 |
| Success | 4/17 (23.5) | 3/12 (25.0) | 0.93 |
| Failure | 0 | 0 | 0 |
| Hospital stay | 2.1±1.8 | 5.4±3.6 | <0.01 |
| Success | 1.4±0.5 | 3.3±1.4 | <0.01 |
| Failure | 5.5±1.3 | 8.9±3.4 | 0.05 |
| Size of pneumothorax | |||
| Success, first attempt | 48.5±22.1 | 58.4±19.6 | |
| Success, first attempt | 55.9±19.9 | ||
| Failure | 77.2±22.7 | 77.0±22.3 | |
Values are presented as number (%) or mean±standard deviation. The category of ‘success’ refers to patients in whom the intervention succeeded, while ‘failure’ refers to the patients in which the intervention failed.
We also conducted a subgroup analysis according to whether treatment succeeded or failed. In the NA group, the success rate of the first attempt was 52.4% (11 of 21) and that of the second attempt was 60.0% (6 of 10). There was no difference in the recurrence rate between these 2 subgroups, and the hospital stay of each of these subgroups was 1.4±0.5 days and 3.3±1.4 days, respectively (p<0.01). In the NA group, a statistically significant difference was found in the size of the pneumothorax among subgroups in which the first round of treatment succeeded, the second round of treatment succeeded, or treatment failed (p=0.04).
Table 3 shows the success rate according to multiple clinicopathologic factors in the NA group. Pneumothorax size was identified as an independent variable that affected the initial treatment success rate. The success rate was significantly higher in patients in whom the size of the pneumothorax was less than 75%.
Table 3.
Results of subgroup analysis of the patients who received needle aspiration
| Variable | Mean success rate (%) | p-value |
|---|---|---|
| Age (yr) | 0.41 | |
| ≤20 | 75.0 | |
| >20 | 88.9 | |
| Sex | 0.81 | |
| Male | 80.0 | |
| Female | 100.0 | |
| Body mass index (kg/m2) | 0.40 | |
| ≤22 | 85.7 | |
| >22 | 71.4 | |
| Size of pneumothorax (%) | 0.04 | |
| ≤75 | 93.3 | |
| >75 | 50.0 | |
| Affected side | 0.67 | |
| Right | 81.2 | |
| Left | 80.0 | |
| Current smoker | 0.70 | |
| Yes | 81.3 | |
| No | 80.0 |
Discussion
There is an extensive spectrum of treatment for PSP throughout the world. The variation in PSP treatment is caused by differences in treatment goals and a lack of high-quality evidence from a variety of prospective studies. Schramel et al. [12] suggested that all symptomatic patients with PSP should undergo video-assisted thoracoscopic surgery (VATS) because of its cost-effectiveness and lower morbidity. However, they also reported that the recurrence rate after a first episode of PSP was approximately 30%, and that 73% of the VATS procedures were unnecessary operations. Additionally, some studies have suggested that procedures to prevent recurrence should be reserved for cases of recurrent pneumothorax [6]. Currently, patients with a first episode of PSP are usually treated with simple air removal without taking specific steps to prevent recurrence, and we agree with this trend.
Although a general consensus exists regarding the appropriateness of conservative care in patients with a first episode of a small PSP [6,7], no definite guideline exists for management of large PSPs, and many options exist. The American College of Chest Physicians recommends CT with a small-bore catheter (≤14F) or a 16F to 22F chest tube connected to a Heimlich valve or to a water-seal device [7]. In contrast, the British Thoracic Society guidelines and some other studies recommend manual aspiration [13]. Although NA is known to be simpler, less invasive, and less painful [14], CT is a more popular procedure to remove air. In many patients with PSP, there is a large amount of air leakage after CT and the air leakage stops immediately. In our opinion, this is because the ruptured bullae or blebs heal spontaneously before the patient arrives at the hospital. Therefore, we hypothesized that simple air removal through NA would be effective in many patients with a first episode of PSP, and we designed this randomized and prospective study to evaluate the effectiveness of NA. In our study, no significant differences were found in the success rate of initial treatment or the recurrence rate between the NA and CT groups. However, the duration of hospital stay was significantly shorter in the NA group.
Some authors have reported that a second attempt to perform NA did not contribute to the success rate [6,15]. However, Harvey and Prescott [16] reported that a second attempt could increase the success rate. In order to shed light on these discrepancies, we performed a second attempt when the first attempt had failed. Six cases (6 of 10, 60.0%) were successful on the second attempt. Therefore, according to the results of our study, a second attempt would be helpful to increase the success rate.
Complications including hemothorax, subcutaneous emphysema, vasovagal reaction, and retained catheter tip have been reported to occur about 1% of cases [6], but no specific complications occurred in our study. Furthermore, there were no urgent readmissions after discharge in our study, in accordance with the observations of other studies, leading to the conclusion that neither procedure poses particular safety concerns.
In other studies, the hospital stay of patients who received NA was shorter than in our study [17,18]; in fact, in those studies, patients were discharged immediately if the procedure was successful. In our study, however, all patients in the NA group remained hospitalized to monitor their overall condition and to check the results of chest radiography taken 12 hours after the procedure. Therefore, the hospital stay was slightly longer than that of other studies.
Pre-procedural chest CT can reveal the existence of bullae and identify characteristics of the bullae (size, multiplicity, and location) that affect the success of initial treatment. However, in our study, only simple chest radiography was used as a diagnostic tool. Therefore, the relationship between the success of initial treatment and chest CT findings could not be assessed in our study. Other limitations of this study include a small sample size and short study period. We will continue the study and report higher-quality results in future publications.
In conclusion, NA may be a safe, less invasive, and effective initial treatment option in well-selected patients with a first episode of PSP. Further prospective studies are necessary to evaluate the effectiveness of NA as an initial treatment for first episodes of PSP.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-120).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Light RW. Pneumothorax. In: Light RW, editor. Pleural diseases. 3rd ed. Baltimore (MD): Williams and Wilkins; 1995. pp. 242–77. [Google Scholar]
- 2.Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul) 2014;76:99–104. doi: 10.4046/trd.2014.76.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987;92:1009–12. doi: 10.1378/chest.92.6.1009. [DOI] [PubMed] [Google Scholar]
- 4.Bense L, Eklund G, Wiman LG. Bilateral bronchial anomaly: a pathogenetic factor in spontaneous pneumothorax. Am Rev Respir Dis. 1992;146:513–6. doi: 10.1164/ajrccm/146.2.513. [DOI] [PubMed] [Google Scholar]
- 5.Ohata M, Suzuki H. Pathogenesis of spontaneous pneumothorax: with special reference to the ultrastructure of emphysematous bullae. Chest. 1980;77:771–6. doi: 10.1378/chest.77.6.771. [DOI] [PubMed] [Google Scholar]
- 6.Noppen M, Alexander P, Driesen P, Slabbynck H, Verstraeten A. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med. 2002;165:1240–4. doi: 10.1164/rccm.200111-078OC. [DOI] [PubMed] [Google Scholar]
- 7.Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 8.Subotic D, Van Schil P. Spontaneous pneumothorax: remaining controversies. Minerva Chir. 2011;66:347–60. [PubMed] [Google Scholar]
- 9.Wang C, Lyu M, Zhou J, Liu Y, Ji Y. Chest tube drainage versus needle aspiration for primary spontaneous pneumothorax: which is better? J Thorac Dis. 2017;9:4027–38. doi: 10.21037/jtd.2017.08.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bintcliffe OJ, Hallifax RJ, Edey A, et al. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med. 2015;3:578–88. doi: 10.1016/S2213-2600(15)00220-9. [DOI] [PubMed] [Google Scholar]
- 11.Collins CD, Lopez A, Mathie A, Wood V, Jackson JE, Roddie ME. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol. 1995;165:1127–30. doi: 10.2214/ajr.165.5.7572489. [DOI] [PubMed] [Google Scholar]
- 12.Schramel FM, Sutedja TG, Braber JC, van Mourik JC, Postmus PE. Cost-effectiveness of video-assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. Eur Respir J. 1996;9:1821–5. doi: 10.1183/09031936.96.09091821. [DOI] [PubMed] [Google Scholar]
- 13.MacDuff A, Arnold A, Harvey J BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 14.Chan SS, Lam PK. Simple aspiration as initial treatment for primary spontaneous pneumothorax: results of 91 consecutive cases. J Emerg Med. 2005;28:133–8. doi: 10.1016/j.jemermed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Parlak M, Uil SM, van den Berg JW. A prospective, randomised trial of pneumothorax therapy: manual aspiration versus conventional chest tube drainage. Respir Med. 2012;106:1600–5. doi: 10.1016/j.rmed.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Harvey J, Prescott RJ. Simple aspiration versus intercostal tube drainage for spontaneous pneumothorax in patients with normal lungs. British Thoracic Society Research Committee. BMJ. 1994;309:1338–9. doi: 10.1136/bmj.309.6965.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J. 2006;27:477–82. doi: 10.1183/09031936.06.00091505. [DOI] [PubMed] [Google Scholar]
- 18.Thelle A, Gjerdevik M, SueChu M, Hagen OM, Bakke P. Randomised comparison of needle aspiration and chest tube drainage in spontaneous pneumothorax. Eur Respir J. 2017;49 doi: 10.1183/13993003.01296-2016. 1601296. [DOI] [PubMed] [Google Scholar]