ABSTRACT
Stroke is the second leading cause of mortality worldwide and one of the main causes of adult disability. Many studies have suggested that combination therapies provide better outcomes in patients with stroke than monotherapies. The combination of botulinum-A toxin (BTX) injection with rehabilitation methods, such as modified constraint-induced movement therapy (BTX-mCIMT), has emerged as a highly promising intervention for promoting motor recovery after stroke. Thus, the present study compared the effectiveness of the combination of BTX with high-dose conventional therapy (BTX-ICT) and BTX-mCIMT for improving motor recovery and reducing spasticity of the upper limb in patients with stroke. This study recruited 64 patients with stroke. The patients were randomly allocated to two groups, namely, BTX-ICT and BTX-mCIMT. Modified Ashworth scale (MAS), Fugl–Meyer assessment (FMA), and Barthel index (BI) assessment scores were determined for the patients in both the groups before and at 4 weeks after the BTX injection. After four weeks of treatment, the MAS, FMA, and BI assessment scores of the patients in both groups were significantly higher than the scores before the treatments (P < 0.05). At the end of 4 weeks, the patients in the BTX-mCIMT group showed significantly higher mean FMA and BI assessment scores than the patients in the BTX-ICT group (P < 0.05). However, no significant statistical difference was observed in the MAS score of the patients in the two groups (P > 0.05). Our results indicated that while both BTX-mCIMT and BTX-ICT promoted motor function recovery in patients with stroke, BTX-mCIMT exerted higher therapeutic effects than BTX-ICT on motor function recovery and in the activities of daily living of patients with stroke.
KEYWORDS: Rehabilitation, botulinum toxin, CIMT, constraint-induced movement therapy, spasticity, stroke
1. Introduction
Stroke is one of the most serious diseases affecting thousands of people worldwide. Approximately 15–30% stroke survivors experience long-term upper extremity (UE) paralysis, which significantly decreases their quality of life. Several studies have attempted to overcome these complications in stroke survivors, resulting in the development of various treatments [1,2].
Hydrotherapy, electrotherapy, and therapeutic exercise are the most common conventional treatment approaches for the rehabilitation of stroke survivors [3–5]. Compared with traditional techniques, modified constraint-induced movement therapy (mCIMT), a recently developed intervention, has shown to substantially improve the motor function of the upper limbs of hemiparetic patients because of its ability to accelerate cortical map reorganization [6]. Although several studies have evaluated the effectiveness of mCIMT, its effect on spasticity is still unclear [7].
Spasticity is a common post-stroke complication, which can restrict the voluntary movements of and reduce rehabilitation efficacy in stroke survivors [8]. Botulinum toxin type A (BTX-A) injection, which is a focal, reversible, selective, cost-effective, safe, and efficient treatment, effectively reduces muscular spasticity by blocking synaptic transmission and inhibiting the acetylcholine effect, thus improving motor function recovery [9–11].
Several studies have attempted to boost the efficacy of BTX injection by administering it in combination with strategies such as functional electrical stimulation, kinesiotaping, serial casting, and mCIMT [12–14]. Although the combination of BTX injection with mCIMT (BTX-mCIMT) and that of BTX injection with intensive conventional therapy (BTX-ICT) are used in clinical practice, their therapeutic effects have not been compared to date.
Sun et al. compared the effect of BTX-mCIMT and BTX injection combined with conventional therapy (BTX-CT) on the recovery of upper limb motor function in stroke survivors for 6 months [14]. They observed that improvement in motor functions and reduction of spasticity were higher with BTX-mCIMT than with BTX-CT. However, this comparison was insufficient because of a difference in the duration of the two treatments.
2. Objectives
This randomized controlled trial (RCT) was designed based on previous supporting studies assessing the effect of BTX-mCIMT on UE rehabilitation in stroke survivors. By examining Fugl–Meyer assessment (FMA) and Barthel index (BI) assessment scores, respectively, this study compared the effectiveness of BTX-mCIMT with that of BTX-ICT for improving the motor function of stroke survivors and for increasing the patient’s ability to perform the activities of daily living (ADLs).
3. Materials and methods
3.1. Study design
This randomized, controlled, and evaluator-blinded trial was based on the Consolidated Standards of Reporting Trials Statement (CONSORT) for Randomized Trials of Non-Pharmacologic Treatment.
3.2. Subjects
This study recruited 116 patients who visited the physical medicine and rehabilitation department of the First Hospital of Jilin University between February 2015 and November 2017. Patients were included in the study if they (1) were a patient with unilateral stroke within one year from the stroke onset; (2) were aged 10–70 years at the time of enrollment; (3) had the ability to actively extend their wrist joint by 20° and their metacarpophalangeal and interphalangeal joints by ≥10°; and (4) had a modified Ashworth scale (MAS) score of ≥1 for the fingers, wrist, or elbow flexors. Patients were excluded from the study if they (1) showed a severe balance impairment (defined as a score less than 40 on the Berg balance scale), (2) were undergoing treatments that were not compatible with the rehabilitation approaches used in the present study, (3) had fixed contractures in the UE (maximum passive movement of less than 10°), (4) had undergone an upper limb surgery (e.g. tendon lengthening) for correcting spasticity, (5) had cognitive deficits (mini-mental status examination score of <24), (6) had previously received any neurolytic agents in the UE, and (7) had preexisting neuromuscular diseases.
None of the recruited patients participated in any other ongoing experimental research. The RCT was approved by the ethics committees of the First Hospital of Jilin University after a strict evaluation, and informed consent was obtained from all subjects or, if subjects were under 18, from a parent and/or legal guardian.
3.2.1. Patient recruitment
Of the 116 patients who were admitted to the physical medicine and rehabilitation department, 64 met the inclusion criteria and were enrolled in the study. In all, 52 patients were excluded from the study, of which 42 did not meet the inclusion criteria and 10 met the exclusion criteria. The 64 patients who met all the inclusion criteria were randomly allocated to two groups (BTX-mCIMT and BTX-ICT groups), with 32 patients in each group, by a third-party person who was blinded to the study details.
3.2.2. Blinding
Because of the recognized nature of the treatment approaches, the patients and therapists were not blinded to the treatments assigned to each group. However, the patients were blind to RCT aspects. Outcome measures were defined by an experienced physician who was blinded to the treatment approaches assigned to each group.
3.3. Treatments
3.3.1. BTX injection
The BTX injection (BOTOX®) was administered to all subjects using ultrasound-guided, single dose injection according to WHO guidelines (Level A evidence) [15,16]. The botulinum toxin is packaged in 100-unit vials as a vacuum-dried powder, and the powder was added to 0.9% sterile normal saline (SNS) in the concentration required. A trained physician chose the muscles that required BTX injection and determined the appropriate procedures to use. The biceps brachii were injected in two sites with 200 units each, while the flexor carpi radialis, flexor carpi ulnaris, flexor digitorum profundus, and flexor digitorum superficialis muscles were injected in one site with a dose of 150 units/point. Subjects were treated with either mCIMT or intensive conventional therapy one day after receiving the BTX injection.
3.3.2. The BTX-mCIMT group
The rehabilitation procedure for the BTX-mCIMT group consisted of wearing a restraint instrument and providing massed practice to the paralytic UE.
3.3.2.1. Restraint instrument
Patients in this group were asked to wear a padded mitten (glove) on the healthy UE for restraint and to use it for approximately 3 h daily, including during the therapeutic session and the home program. This glove was designed to prevent the patients from using their sound arm and thus encourage the use of the paralytic UE to perform the ADLs. The total time spent wearing the glove was recorded daily.
3.3.2.2. Massed practice
Practiced tasks included reaching, placing, lifting, and grasping (Figure 1). The task difficulty was determined by the baseline assessment, and the assigned task was followed by a continually increased challenge. Patients received mCIMT massed practice for 1 h per day, 6 times per week, for 4 weeks.
Figure 1.
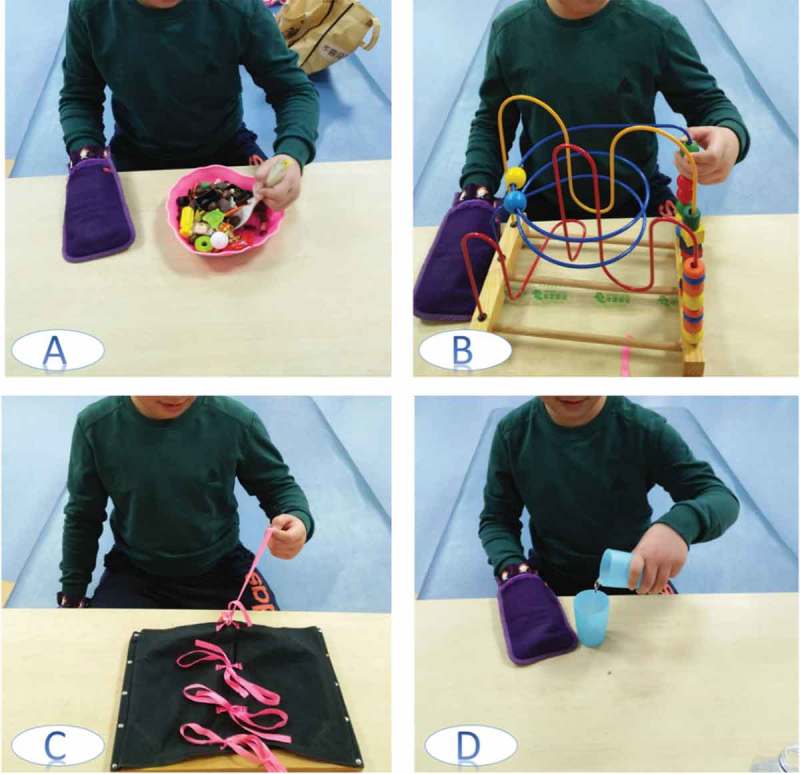
CIMT tasks practicing, use of the unaffected limb is restricted by a padded mitt.
(A) Picking up and placing things using a spoon, (B) playing a game, (C) tie and untie the knots, (D) pouring liquids.
3.3.3. The BTX-ICT group
Subjects in this group received individually intensive conventional treatment plans. The interventions were mainly dedicated to managing muscle hypertonia and restoring movement patterns and dexterity. The therapeutic plans mainly consisted of neurodevelopmental techniques, such as Bobath’s and Brunnstrom’s methods, in addition to other rehabilitation approaches, such as muscle strengthening, stretching, functional tasks (when applicable), manual dexterity exercises, and training on the activity of daily living (ADL). The treatment duration for upper limbs was 1 h per day, 6 times per week, for 4 weeks.
3.4. Assessments
3.4.1. Modified Ashworth scale (MAS)
Utilizing an established and reliable spasticity measuring tool is a cornerstone for achieving a sufficiently precise assessment. MAS is the most widely utilized scale in clinical practice and research, not only due to its convenience and availability at no cost, but also because it possesses a relatively high inter-rater reliability, especially in wrist and elbow flexors [17,18]. MAS is a 6-grade criterion ordinal scale that ranges from 0 to 4. For statistical analysis, we used numbers (0, 1, 2, 3, 4, and 5) as a corresponding score for MAS grades.
3.4.2. Fugl-Meyer assessment (FMA)
We used the Fugl–Meyer assessment (FMA), which assesses the upper and lower extremities, to reveal and measure the improvement of motor function after stroke. The FMA scale has a remarkable test–retest reliability (subtests 0.87–1.00) and possesses construct validity and inter-rater reliability [19]. In this case, we used the upper extremity scale, which has a total score of 66 points and can consider numerous dimensions of impairment by using a 3-point ordinal scale that reveals the ability to perform the test (0: cannot perform the test; 1: can partially perform; 2: can perform fully).
3.4.3. Barthel index (BI)
BI is an ordinal scale consisting of 10 variables, and a range score from 0 to 100 points, allowing to assess the ADLs, the higher scores means less affected functions [20,21]. Although there are other scales possessing higher sensitivity toward tiny improvements in functional independence, BI emerged as the best activities of daily living (ADL) assessment tool. It is commonly used in clinical and research fields, especially as a trial outcome measuring instrument for stroke patients, to measure baseline skills and consequential improvements.
3.5. Statistical analysis
A comparison of the assessments before and after the treatment within individual groups and between separate groups was conducted with the statistical package for the social sciences software version 20.0 (SPSS) using Mann Whitney U, Fisher and t tests.
The sample size was statistically calculated to offer 90% power at α = 0.05, a sample size of 32 subjects would participate to reach 90% power and avoid the dropout rate.
4. Results
4.1. Demographic data and clinical features
Among 64 screened subjects, 10 were excluded due to their inability to continue the therapeutic protocol; thus, 54 (84.3%) patients completed the treatment interventions and all outcomes assessments. 77.8% of the patients were males, and the mean of the time from stroke onset was 4.45 months. Patients with intracerebral hemorrhage have more motor impairment than patients with cerebral infarction, thus, following this pattern, 66.7% of patients had, 66.7% of patients had suffered stroke due to Intracerebral cerebral hemorrhage [22]. Both groups were comparable at the baseline with respect to their demographic data and clinical features.
4.2. Assessment outcomes
No significant differences were detected between the groups in terms of their MAS, BI, and FMA scores at the baseline assessment. Interestingly, all patients experienced an improvement in motor function, ADL performance, and spasticity reduction.
4.2.1. Modified Ashworth scale outcomes
Both groups showed a relatively significant improvement for all involved muscles after 4 weeks of treatment in MAS. However, finger flexors showed the best improvement with P= 0.001 in both groups.
Remarkably, the outcomes illustrated the significantly high effectiveness of both methods on decreasing spasticity. The results showed no statistically significant differences between the two groups (Table 1).
Table 1.
MAS mean scores, the variance between pre and post-treatment in two groups ( ± s).
| Group | Joint flexors | Baseline (Mean ±SD) |
Post treatment (Mean ±SD) |
Variance (Mean ±SD) |
P value within groups |
P value between groups |
|---|---|---|---|---|---|---|
| BTX-mCIMT | Elbow | 2.4 ± 0.8 | 1.2 ± 0.5 | −1.2 ± 0.7 | 0.003 | 0.2 |
| BTX-ICT | 2.7 ± 0.6 | 1.1 ± 0.3 | −1.5 ± 0.5 | 0.001 | ||
| BTX-mCIMT | Wrist | 2.3 ± 0.6 | 1.0 ± 0 | −1.3 ± 0.6 | 0.002 | 0.1 |
| BTX-ICT | 3.3 ± 0.6 | 1.5 ± 0.5 | −1.8 ± 0.5 | 0.001 | ||
| BTX-mCIMT | Fingers | 2.3 ± 0.6 | 0.9 ± 0.2 | −1.4 ± 0.5 | 0.001 | 0.5 |
| BTX-ICT | 2.9 ± 0.6 | 1.5 ± 0.5 | −1.3 ± 0.6 | 0.001 |
BTX-mCIMT: Botulinum toxin type A injection combined with modified constraint-induced movement therapy; BTX-ICT: Botulinum toxin type A injection combined with intensive conventional rehabilitation; SD: standard deviation.
4.2.2. Barthel index outcomes
Although both groups exhibited a relatively high substantial improvement, the BI score changes showed a greater improvement in the BTX-mCIMT group than the BTX-ICT group after 4 weeks of treatment (mean score 77.6 9.7 VS 70 5.6; P = 0.02) (Table 2).
Table 2.
BI and FMA mean scores of pre/post treatment in two groups ( ± s).
| Scale | Group | Baseline (Mean ±SD) |
Post treatment (Mean ±SD) |
P value Within groups | P value Between groups |
|---|---|---|---|---|---|
| BI | BTX-ICT | 58.9 ± 6.5 | 70 ± 5.8 | 0.02 | 0.02 |
| BTX-mCIMT | 56.9 ± 16.5 | 77.6 ± 10.1 | 0.01 | ||
| FMA | BTX-ICT | 30.7 ± 5.3 | 37.5 ± 5.2 | P < 0.01 | 0.01 |
| BTX-mCIMT | 32.2 ± 6.2 | 52 ± 7.5 | P < 0.01 |
BTX-mCIMT: Botulinum toxin type A injection combined with modified constraint-induced movement therapy; BTX-ICT: Botulinum toxin type A injection combined with intensive conventional rehabilitation; SD: standard deviation; BI: Barthel index; FMA: Fugl–Meyer assessment.
4.2.3. Fugl–Meyer assessment outcomes
Both methods displayed a noteworthy improvement to motor functions. However, the results revealed that the BTX-mCIMT method was significantly more effective than the BTX-ICT method in motor function restoration (mean score 52, 37.5, respectively) (Table 2).
5. Discussion
The results revealed that the BTX-mCIMT combination was superior to BTX-ICT in improving UE motor functions and reducing the dependence on performing ADLs. The findings further verified the distinct effectiveness of BTX-mCIMT and BTX-ICT. Before the treatment, all the subjects suffered from a relatively high level of dependence on performing ADLs, in addition to the motor impairment shown by the baseline scores of BI and FMA.
This noticeable improvement in UE motor functions could be attributed to the ability of BTX to reduce spasticity, which consequently facilitated performing the repetitive practice of mCIMT without spasticity hindrance. The treatment mCIMT mainly takes effect by increasing the functional usage of impaired UE in ADL and brain functional activation. In these ways, mCIMT improved the functional ability of upper limbs for stroke patients, especially in performing ADL.
Furthermore, the MAS score was the primary tool for the evaluation of spasticity, and a shift of one point on the scale was considered a significant change [23,24]. In this study, both groups experienced a highly significant improvement after 4 weeks of interventions. However, no significant difference was observed between the BTX-mCIMT and BTX-ICT groups. This indicated that neither mCIMT nor intensive conventional therapy was superior for the decrease in spasticity of stroke patients during 4 weeks of treatment. Nonetheless, Sun et al., in their randomized controlled study, compared BTX-mCIMT with conventional treatment [14]. Their outcomes recommended the use of the BTX-mCIMT combination as a more effective approach than conventional treatment for reducing spasticity. In this study, we used therapeutic sessions with the same duration, chose a larger age range, and selected the patients that were more likely to improve.
The absence of significant differences in the MAS score between the groups was not consistent with Sun’s study [14]. However, these findings were consistent with earlier studies that demonstrated that performing contractile activities of the BTX-injected muscles could augment and prolong the effectiveness of BTX [25,26] Our study results provided more insight into the correlation between spending more time on interventions and boosting the effectiveness of BTX, which consequently resulted in decrease of spasticity.
There are some limitations in the present study. First, a small study population was used to elucidate the benefit of BTX-mCIMT intervention. Second, we could not define the specific criteria to identify stroke patients that could have a higher improvement with the BTX-mCIMT combination. Finally, the BTX dose was not fixed for all patients; individual differences were considered in order to use the optimal dose for each patient.
6. Conclusion
Based on the results of this randomized controlled trial, both BTX-mCIMT and BTX-ICT methods show a relatively high effectiveness in managing spasticity, enhancing motor function recovery, and further decreasing the dependency of performing ADLs after stroke. However, the BTX-mCIMT combination has a greater effect on improving motor function than BTX-ICT and acts as a promising treatment method. Lastly, since decisive evidence is still limited, more attention should be dedicated to study the combination of BTX-mCIMT and BTX-ICT, both theoretically and experimentally. Future researchers must clarify the optimal combination protocol with consideration for cost-effectiveness, improving quality of life, and achieving a higher level of patient satisfaction.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Santisteban L, Térémetz M, Bleton J-P, et al. Upper limb outcome measures used in stroke rehabilitation studies: a systematic literature review. PLoS One. 2016;11(5):e0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duncan PW, Min Lai S.. Stroke recovery. Top Stroke Rehabil. 1997;4(3):51–6. [DOI] [PubMed] [Google Scholar]
- [3].Zhu Z, Cui L, Yin M, et al. Hydrotherapy vs. conventional land-based exercise for improving walking and balance after stroke: a randomized controlled trial. Clin Rehabil. 2016;30(6):587–593. [DOI] [PubMed] [Google Scholar]
- [4].Dutta Anirban, Lahiri Uttama, Das Abhijit, Nitsche Michael A., and Guiraud David. Post-stroke balance rehabilitation under multi-level electrotherapy: a conceptual review. Front Neurosci-Switz. 2014;8:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tashani O, Johnson MI.. Transcutaneous Electrical Nerve Stimulation (TENS) a possible aid for pain relief in developing countries? Libyan J Med. 2008;4(2):62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin K-C, Chung H-Y, Wu C-Y, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehab. 2010;89(3):177–185. [DOI] [PubMed] [Google Scholar]
- [7].Rostami HR, Malamiri RA. Effect of treatment environment on modified constraint-induced movement therapy results in children with spastic hemiplegic cerebral palsy: a randomized controlled trial. Disabil Rehabil. 2012;34(1):40–44. [DOI] [PubMed] [Google Scholar]
- [8].Wade DT. Stroke: rehabilitation and long-term care. Lancet. 1992;339(8796):791–793. [DOI] [PubMed] [Google Scholar]
- [9].Gilio F, Curra A, Lorenzano C, et al. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol. 2000;48(1):20–26. [PubMed] [Google Scholar]
- [10].Simpson DM, Gracies JM, Sahrmann S, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review). Neurology. 2009;73(9):736. [PubMed] [Google Scholar]
- [11].Ward A, Roberts G, Warner J, et al. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med. 2005;37(4):252–257. [DOI] [PubMed] [Google Scholar]
- [12].Pieber K, Herceg M, Wick F, et al. Functional electrical stimulation combined with botulinum toxin type A to improve hand function in children with spastic hemiparesis–a pilot study. Wien Klin Wochenschr. 2011;123(3–4):100–105. [DOI] [PubMed] [Google Scholar]
- [13].Karadag-Saygi E, Cubukcu-Aydoseli K, Kablan N, et al. The role of kinesiotaping combined with botulinum toxin to reduce plantar flexors spasticity after stroke. Top Stroke Rehabil. 2010;17(4):318–322. [DOI] [PubMed] [Google Scholar]
- [14].Sun S-F, Hsu C-W, Sun H-P, et al. Combined botulinum toxin type A with modified constraint-induced movement therapy for chronic stroke patients with upper extremity spasticity: a randomized controlled study. Neurorehab Neural Re. 2010;24(1):34–41. [DOI] [PubMed] [Google Scholar]
- [15].Simpson DM, Hallett M, Ashman EJ, et al. Practice e guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the guideline development subcommittee of the American academy of neurology. Neurology. 2016;86:1818–1826. DOI: 10.1212/WNL.0000000000002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Royal College of Physicians, British Society of Rehabilitation Medicine, Chartered Society of Physiotherapy, Association of Chartered Physiotherapists Interested in Neurology. Spasticity in adults: management using botulinum toxin. National guidelines. London: RCP; 2018. [Google Scholar]
- [17].Ansari NN, Naghdi S, Moammeri H, et al. Ashworth scales are unreliable for the assessment of muscle spasticity. Physiother Theor PR. 2006;22(3):119–125. [DOI] [PubMed] [Google Scholar]
- [18].Naghdi S, Nakhostin Ansari N, Azarnia S, et al. Interrater reliability of the Modified Modified Ashworth Scale (MMAS) for patients with wrist flexor muscle spasticity. Physiother Theor PR. 2008;24(5):372–379. [DOI] [PubMed] [Google Scholar]
- [19].Woytowicz EJ, Rietschel JC, Goodman RN, et al. determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehab. 2016;98(3):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahoney FI, Barthel DW. Functional evaluation: the barthel index, Maryland State. Med J. 1965;14:16–65. [PubMed] [Google Scholar]
- [21].Ashburn A. methods of assessing the physical disabilities of stroke patients. Physiother Pract. 1986;2(2):59–62. [Google Scholar]
- [22].Kelly PJ, Furie KL, Shafqat S, et al. Functional recovery following rehabilitation after hemorrhagic and ischemic stroke. Arch Phys Med Rehab. 2003;84(7):968–972. [DOI] [PubMed] [Google Scholar]
- [23].Albright AL, Barron WB, Fasick MP, et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270(20):2475–2477. [PubMed] [Google Scholar]
- [24].Simpson DM, Alexander DN, O’Brien CF, et al. Botulinum toxin type A in the treatment of upper extremity spasticity A randomized, double‐blind, placebo‐controlled trial. Neurology. 1996;46(5):1306–1312. [DOI] [PubMed] [Google Scholar]
- [25].Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Mov Disord. 1997;12(1):89–94. [DOI] [PubMed] [Google Scholar]
- [26].Hesse S, Jahnke MT, Luecke D, et al. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995;201(1):37–40. [DOI] [PubMed] [Google Scholar]


