ABSTRACT
Background: The umbrella term ATMPs (Advanced Therapy Medicinal Products) comprises cell therapies, gene therapeutics and tissue engineered products. After implementation of the Regulation 1394/2007, only a couple of products have obtained a centralized European marketing authorisation.
Objectives: The aim of the presented study is to give an overview on ATMPs available within the European Union either via centralized marketing authorisation or via national Hospital exemption. Additionally, a forecast on innovative ATMPs in the process of EMA approval as well as in phase III and IV clinical trial is provided.
Methods: Systematic literature search including ‘grey literature’ and database reviews as well as manual search following pre-defined search terms.
Results: 8 ATMPs are currently available via centralized marketing authorisation. 6 new product launches are expected before 2020. At least 32 additional ATMPs are available in individual European Union member states via Hospital exemption. Another 31 potential ATMP candidates could be identified in industry-driven phase III research projects.
Conclusion: Advanced therapeutic medicinal therapies are still in their early days, but constantly evolving. By 2020, innovative therapies targeting retinal dystrophy, ß-thalassemia, scleroderma, sickle-cell anaemia, adrenoleukodystrophy and leukaemia shall be available on the market.
KEYWORDS: ATMP, Advanced Therapeutic Medicinal Product, Hospital exemption, phase III clinical trial, marketing authorisation
Introduction
Recent advancements in biological therapies have initiated a shift from the traditional ‘one-size fits all’ approach towards personalized medicinal strategies. Advanced Therapy Medicinal Products (ATMPs) are at the forefront of this new tendency. ATMP is the umbrella term for three drug product classes: Somatic cell therapies, gene therapeutics and tissue engineered products as well as a combination of these technologies with a medicinal product. All ATMP classes contain either living cells or viral vectors and are therefore characterized by a high degree of complexity. Cells are usually derived from a patient or an allogeneic donor, processed in the laboratory (e.g. expanded in vitro or genetically engineered) and (re-) administered to the patient in a hospital. Gene therapy is designed to introduce genetic material into living cells to compensate for abnormal genes or express a beneficial protein.
On 30 December 2008, the Regulation 1394/2007 amending Directive 2001/83/EC on Advanced Therapy Medicinal Products entered into force and the first European Union wide regulatory framework for ATMPs was established [1]. This framework changed the code of regulatory practices, as a central marketing authorisation issued by the European Medicinal Agency (EMA) was required from now on. Previously, registration was not required for autologous products and pivotal clinical trials were not mandatory [2]. Not all ATMPs target high prevalence indications. In case of orphan diseases with a prevalence not more than 5 in 10.000, the ATMP regulation poses complex challenges to the design of clinical trials [2,3].
In recognition of the small scale and developmental nature of some intra-hospital ATMP applications, the regulation 2001/83/EC includes a ‘Hospital exemption’ for products not intended to be marketed. ATMPs applied via Hospital exemption must be prepared on a non-routine basis in a non-industrial manner and used as a custom made product for an individual patient [4]. However, the meanings of ‘non-routine basis’, ‘industrial manner’ and ‘custom made’ are not specified by the regulation and interpretations differ among different European countries [5]. ATMPs without a centralized European marketing authorisation can therefore still be approved in individual member states.
The aim of the presented study is to give an overview on ATMPs currently available within the European Union either via a centralized marketing authorisation or via national approval in an individual member state. ATMPs withdrawn from the market will be identified and the reasons for withdrawal analysed. Additionally, a forecast on innovative ATMPs in the process of EMA approval as well as products in phase III and IV clinical trial will be presented.
Methods
Search strategy
A systematic database review was conducted to identify published studies from Ovid MEDLINE, Ovid EMBASE, the Cochrane Library and clinicaltrials.gov. Details on clinical trials were also collected from clinicaltrialsregister.eu. Additional information was gathered from the homepage of the European Medicines Agency (www.ema.europa.eu) as well as from the webpages of the national competent authorities. A manual search for grey literature was performed following pre-defined search terms. Additionally, the national competent authorities were contacted to obtain information on ATMPs licensed via Hospital exemption.
Key words
ATMP, advanced therapeutic medicinal product, cell therapy, stem cell, stem cell transplantation, umbilical cord, cord blood, bone marrow, bone marrow transplantation, cancer vaccine, tissue engineering, mesenchymal stem cell, somatic cell, allogeneic cell, viable cell, tissue engineering, gene therapy, recombinant nucleic acid, recombinant DNA, nucleic acid therapy, gene transfer, virus delivery, cancer immunotherapy, RNA therapy, tumor vaccine, plasmid DNA, oligonucleotide, transgenic microorganism, genetically modified microorganism, transformed cell line, genetically modified cell line, gene vector, vector
Eligibility criteria
Publications targeting an ATMP approved by the EMA or in the process of an EMA approval as well as manuscripts targeting phase III and IV ATMP clinical trials were included in this review. Additionally, publications on ATMPs administered to patients via Hospital exemption were included. Products were excluded from evaluation if their ATMP status could not be clearly assessed, e.g. in case of cancer immunotherapeutics which had neither been submitted to the EMAs Committee for Advanced Therapies (CAT) for classification nor declared as ATMP in the reference literature. Further inclusion and exclusion criteria are detailed in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
Data extraction
The following clinical trial data were extracted using MS Excel 2011: ATMP, registration number, manufacturer, indication, clinical trial status and eventual marketing authorisation status. Duplicates with the same registration number were removed as well as all pre-clinical, phase I and II studies, observational studies, studies performed outside the European Union and studies with an unclear phase assignment. Clinical trials not targeting an ATMP as well as generic conference abstracts not containing concrete clinical data were also excluded.
Results
Search results
The literature search yielded 2.613 publications. After removal of duplicates, pre-clinical, phase I and II studies, observational studies and studies with an unclear phase assignment 502 full text records were considered for evaluation. 91 did not meet the inclusion criteria (mostly studies performed outside of the European Union). Finally, we identified 412 studies for investigation.
The clinical trials database search yielded 1.946 entries in European Union member states. In 1.516 trials, the interventional drug was not an ATMP. 430 clinical trials were included in the evaluation. After manual removal of duplicates resulting from multi-centre international clinical trials, 160 phase III and IV studies were reviewed. 20 did not meet the inclusion criteria (either erroneously reported as phase III in the database or not targeting an ATMP). Finally, 141 clinical trials were included in the evaluation. A flow chart of clinical trial identification and inclusion is presented in Figure 1.
Figure 1.
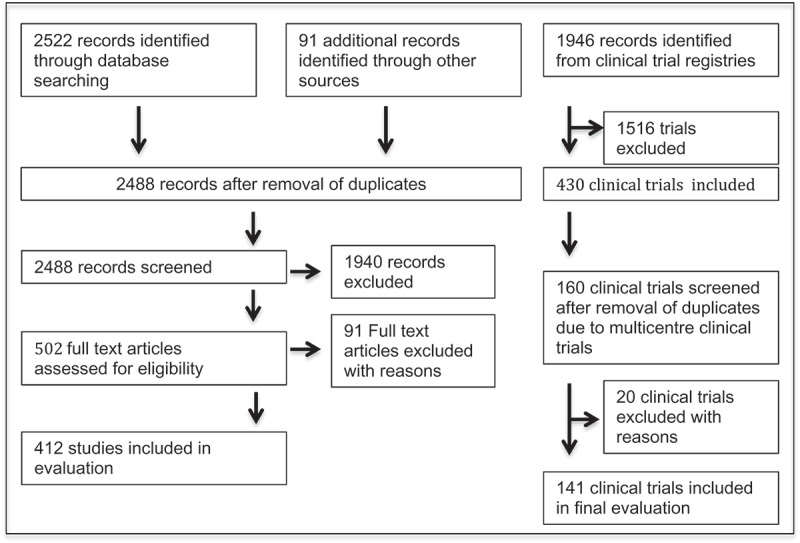
Flow chart of clinical trial identification and inclusion.
ATMPs with a valid central European marketing authorisation
Since approval of the first ATMP in 2009, 12 products have obtained a central European marketing authorisation by the European Medicines Agency. An overview on these products is presented in Table 2. By the end of August 2018, 4 licensed ATMPs had retired from the market. Currently 8 ATMPS are available within the European Union: The gene therapies Imlygic®, Strimvelis®, and Zalmoxis® and the cell based therapies Holoclar®, Spherox® and Alofisel®. In August 2018, the European Medicines Agency recommended the first two marketing authorisations for chimeric antigen receptor (CAR) T-cells medicines, Kymriah® (tisagenlecleucel) and Yescarta® (axicabtagene ciloleucel). Both substances belong to a new generation of individualized cancer immunotherapies based on the modification of the patients‘ immune cells for cancer treatment [6]. Details on ATMPs with a valid central European marketing authorisation are summarized in Table 3.
Table 2.
Overview on ATMPs with past/present marketing authorisation.
| Name | Authorisation holder | Indication | Authorisation number | Approval date | Status |
|---|---|---|---|---|---|
| Yescarta® | Kite Pharma | B-cell lymphoma | EMEA/H/C/004480 | 08/2018 | APPROVED |
| Kymriah® | Novartis | ALL, DLBCL | EMEA/H/C/004090 | 08/2018 | APPROVED |
| Alofisel® | TiGenix | Perianal fistulas in Crohn´s disease | EMEA/H/C/004258 | 03/2018 | APPROVED |
| Spherox® | CO.DON | Cartilage defects in the knee joint | EU/1/17/1181 | 05/2017 | APPROVED |
| Zalmoxis® | MolMed | Stem cell transplantation in high-risk blood cancer | EMEA/H/C/002801 | 06/2016 | APPROVED |
| Strimvelis® | GSK | ADA-SCID | EU/1/16/1097 | 04/2015 | APPROVED |
| Imlygic® | Amgen | Melanoma | EU/1/15/1064 | 09/2015 | APPROVED |
| Holoclar® | Chiesi | Severe limbal stem cell deficiency in the eye | EU/1/14/987 | 03/2015 | APPROVED |
| Provenge® | Dendreon | Metastatic prostate cancer | EMEA/H/C/002513 | 10/2013 | withdrawn in 2015 |
| MACI | Vericel | Cartilage defects in the knee joint | EU/1/13/847 | 07/2013 | withdrawn in 2014 |
| Glybera® | Uniqure | Lipoprotein Lipase Deficiency | EU/1/12/791/001 | 11/2012 | withdrawn in 2017 |
|
Chondro Celect® |
TiGenix | Cartilage defects | EMEA/H/C/000878 | 11/2009 | withdrawn in 2016 |
ALL … Acute Lymphoblastic Leukaemia
DLBCL … Diffuse Large B Cell Lymphoma
ADA-SCID … Adenosine Deaminase Severe Combined Immunodeficiency
Table 3.
ATMPs with a valid central European marketing authorisation by August 2018.
| Holoclar® | Holoclar® was the first stem cell based ATMP approved by the European Union. The product is based on ex vivo expanded autologous human corneal epithelial cells [10]. The cells are isolated from a limbus tissue biopsy, expanded in vitro and cryopreserved for alignment with the patient´s medical care. After thawing, the cells are seeded onto a fibrin matrix for transplantation [11]. |
| Imlygic® | Imlygic®was the first oncologic gene therapy reaching EMA approval. The product is based on a genetically modified oncolytic virus replicating within the tumoral tissue to produce granulocyte-macrophage colony stimulating factor (GM-CSF). Intratumoral application leads to tumor cell lysis and the release of tumor-derived antigens, which – in combination with GM-CFS – amplify the body´s anti-tumoral immune response [12]. |
| Strimvelis® | Strimvelis® is designed to treat severe combined immunodeficiency (SCID) due to Adenosin desaminase deficiency (ADA-SCID) in patients who cannot be treated with a bone marrow transplant due to lack of a suitable donor [13]. The product is based on autologous CD 34+ cells transduced with a retroviral vector encoding for the human ADA cDNA sequence [10]. |
| Zalmoxis® | Zalmoxis® is a patient specific immunogenic therapy serving as adjunctive treatment in haplo-identical haematopoietic stem cell transplantation in patients with leukaemia and high-risk haematological malignancies [14]. Data from 45 patients treated with Zalmoxis showed a survival rate of 49% after one year. Survival in the control group was 37% [15]. |
| Spherox® | Spherox® are spheroids of human autologous matrix-associated chondrocytes for treatment of cartilage defects in the knee joint [16]. Data of 30 patients after an average follow-up of 3 years demonstrate a significant increase in quality of life, pain reduction and an improvement of joint function [17]. |
| Alofisel® | Alofisel® consists of adipose tissue derived allogeneic mesenchymal stem cells for injection into the perianal fistula tract in Cohn’s disease [18]. Local application of Alofisel® in conjunction with surgical preparation of the fistula tract has been shown to induce and maintain fistula closure, but a high placebo effect due to background therapies was noted in the phase III clinical trial [19]. |
| Kymriah® | Kymriah® (CTL019/tisagenlecleucel) is intended for children and young adults with relapsed or refractory B-cell acute lymphoblastic leukaemia and for adult patients with diffuse large B-cell lymphoma who are ineligible for stem cell transplantation. In paediatric patients, an overall remission rate of 81% was achieved in the ELIANA trial [20]. Adult patients with diffuse large B-cell lymphoma achieved an overall response rate of 52% [21]. Treatment related adverse events occurred in 95% of the patients, mostly as cytokine release syndrome. |
| Yescarta® | Yescarta® (axicabtagene ciloleucel) is a chimeric antigen receptor T-cell therapy to treat aggressive non-Hodgkin´s lymphomas. In patients with large B-cell lymphoma, primary mediastinal B-cell lymphoma and transformed follicular lymphoma, the overall response rate was 71%. Complete remission was achieved in 57% (5/7) of the patients [22]. Adverse events include anaemia, neutropenia and decreased white blood cell count. Grade III or higher cytokine release syndrome is observed in 13% and neurologic events in 28% of the patients [23]. 71% of the patients treated for relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL) responded to the treatment either as complete response or complete response with incomplete hematologic recovery [24]. However, one patient experienced a fatal cytokine release syndrome [25]. |
ATMPs withdrawn from the market
Provenge®, MACI, Glybera® and ChondroCelect® have been withdrawn from the market. Provenge® (Sipuleucel-T) was a cellular immunotherapy for treatment of metastatic castration resistant prostate cancer. The substance was able to prolong median patient survival by 4,1 months. After a 3 years follow up, the proportion of patients alive in the vaccine group was 50% higher than in the control group [7]. Provenge® was approved by the EMA in 2012 and priced $93.000 per treatment [8]. Supply chain conditions were highly complex: Within a cooled, insulated container, shelf live was only 18 hours [9]. Due to the high price, a highly complex way of administration and reimbursement issues the product failed on the market and the manufacturer filed bankruptcy in 2015 [8].
MACI was on the market since 1998 in individual EU countries according to national procedures [10]. In 2013, the product was granted a central marketing authorisation for repair of cartilage defects in the knee joint. Due to commercial reasons, the company closed the European manufacturing site in 2014. Consequently, the marketing authorisation was suspended and expired during the suspension period [11].
Glybera® was an adenoassociated viral vector for treatment of lipoprotein lipase deficiency (LLD), a ultra-rare disease affecting only 1 in a million people [12]. The product was authorised under exceptional circumstances based on data received from 3 phase III trials enrolling a total of 27 patients [13]. Despite the clinical success, the product was a commercial failure. Four and a half years after making history for obtaining EMA approval as the first gene therapy in a regulated market, the manufacturer did not renew marketing approval and Glybera® was withdrawn in October 2017. In fact, only one patient had been treated with the commercial form of the LLD therapy, which was priced at 1,1 Million € [14].
ChondroCelect® (characterized, viable autologous cartilage cells expanded ex vivo expressing specific marker proteins) was approved in October 2009. The pivotal clinical trial demonstrated a superior structural cartilage repair when compared to standard Microfracture treatment [15]. Despite positive results, the product was withdrawn from the market in 2016 due to a lack of reimbursement in key European countries [9].
ATMPs in the process of EMA approval (planned launch before 2020)
Until 2020, 6 new ATMPs shall be launched on the European market. Developing companies, indications and clinical/regulatory status are summarized in Table 4. The Committee for Medicinal products for Human Use (CHMP) has already recommended the granting of a marketing authorisation for Luxturna™, a gene therapy for treatment of inherited retinal dystrophy [16]. LentiGlobin™ is a potential gene therapy for correction of transfusion dependant thalassemia and sickle cell disease. Published data from the phase I/II study report that 4 out of 7 patients remained transfusion free for more than 90 days [17]. Lenti-D™ is another gene therapy for treatment of childhood cerebral adrenoleukodystrophy, a genetic disease causing progressive damage to the brain [18]. The cell therapeutic product Habeo™ is an injection of adipose-derived regenerative cells to treat hand involvement in systemic sclerosis [19]. Despite not reaching significance in the phase III trial, clinically meaningful improvements in hand function were achieved in a subgroup of patients with diffuse cutaneous scleroderma [20]. A managed access programme is currently being established to provide access for patients in advance of the full marketing authorisation [21]. Neocart® is an autologous chondrocyte-based tissue implant. In contrast to the promising phase I and II results, the primary efficacy endpoints were not met in the subsequent phase III clinical trials [22]. As the data are still being analysed, eventual consequences for the European market launch are currently unclear. ATIR101 is a cell based immunotherapeutic product containing T-lymphocyte enriched leukocytes. The product is intended to restore lymphocyte levels in patients undergoing stem cell transplantation from a partially matched (haploidentical) family donor. Conditional approval is expected for Q1 2019.
Table 4.
ATMPs in the process of marketing authorisation or with a planned marketing launch.
| Product | Developer | Indication | Regulatory/ Clinical status |
|---|---|---|---|
| LuxturnaTM | Novartis | Biallelic RPE65-mediated retinal dystrophy | MAA submitted EMA: CHMP pos. opinion 09/2018 Phase III NCT00999609 Open-label, randomized controlled trial At least 24 patients planned Estimated study completion date 2029 |
| LentiGlobinTM | BlueBird Bio | Transfusion dependant ß-thalassemia, sickle cell disease | MAA submitted EMA: accelerated approval granted Phase III NCT02906202 Single arm, multi site, single dose study Approx. 23 patients planned Estimated study completion date 2020 |
| HabeoTM | Cytori Therapeutics | Hand dysfunction due to scleroderma | EMA: Orphan drug designation granted Phase n.a. NCT02396238 prospective, randomized, multi-center device trial 88 patients enrolled Study completion date 2018 |
| Lenti-DTM | BlueBird Bio | Cerebral adrenoleukodystrophy | Phase II/III NCT01896102 Single arm open label 30 patients planned Estimated study completion date 2021 |
| Neocart® | Histogenics corporation | Cartilage repair | Phase III NCT01066702 245 participants enrolled Randomized, open label Estimated study completion date 2020 |
| ATIR101 | Kiadis Pharma | AML, ALL or myelodysplastic syndrome | MAA submitted response to EMA submitted 03/2018 Phase III NCT02999854 Randomized controlled multicenter open-label study 250 participants planned Estimated study completion date 2021 |
| JCAR 017 | Celgene | DLBCL | EMA: PRIME Phase III NCT03575351 Randomized open label study 182 participants planned Estimated study completion date 2023 |
| bb2121 | Celgene | Multiple myeloma | EMA: PRIME eligibility 11/2017 Phase III NCT03651128 Multicenter randomized open label 381 participants planned Estimated study completion date 2025 |
| Tab-celTM | Atara Biotherapeutics | EBV associated post-transplant lymphoproliferative disorder | Phase III NCT03392142 Multi-center, single arm, open label 33 participants planned Estimated study completion date 2020 |
| Lenadogene nolparvovec | GenSight Biologics SA | Vision loss from Leber hereditary optic neuropathy | Phase III NCT02652767 Randomized, double-masked, sham-controlled clinical trial 36 participants planned Estimated study completion date 2019 |
| REX-001 | Rexgenero | Critical limb ischemia | EMA: Certificate for manufacturing and non-clinical data 01/2018 Phase III NCT03174522 Randomized, double-blind, controlled clinical trial 78 participants planned Estimated study completion date 2021 |
| Multistem | Athersys | Ischemic stroke | Phase III NCT03545607 Randomized, quadruple-masked clinical trial 300 participants planned Estimated study completion date 2021 |
| PLX-PAD | Pluristem therapeutics | Critical limb ischemia | Phase III NCT03006770 Multicenter randomized controlled clinical trials 246 participants planned Estimated study completion date 2020 |
EMA … European Medicines Agency
MAA … Marketing Authorisation Application
CHMP … Committee for Medicinal products for Human Use
AML … Acute Myeloid Leukaemia
ALL … Acute Lymphoblastic Leukaemia
DLBCL … Diffuse Large B Cell Lymphoma
EBV …. Epstein Barr Virus
PRIME …PRIority MEdicines scheme
ATMPs with a planned launch in or after 2020
Two further CAR T-cell therapies currently in phase III clinical trial are planned for a centralized European market approval: JCAR017 (lisocabtagene maraleucel, liso-cel) is a treatment for aggressive B-cell non-Hodgkin´s lymphoma. Data from the phase I study demonstrated an overall response rate of 66% with 50% of the patients achieving complete response at three months [23]. bb2121 is intended to treat multiple myeloma. Published efficacy data from the phase I trial report an overall treatment response of 95,5% [24]. Filing of a marketing authorisation application is anticipated for 2019 [25].
Tab-celTM is an allogeneic T-cell immunotherapy to treat Epstein Barr Virus (EBV) associated post-transplant lymphoproliferative disorder and other EBV associated tumors [26]. The product was accepted into the EMA Priority Medicines regulatory pathway and is available to eligible patients through a multicentre expanded access protocol [27]. Evaluation of the expanded access programme demonstrates a response rate of 80% after hematopoietic stem cell transplantation and 83% after solid organ transplantation at a medium follow up of 3,3, months. Overall survival at 1 year among all patients treated was 90,3% [28].
Lenadogene nolparvovec (GS-010) is a gene therapy for treatment of Leber’s hereditary optic neuropathy (LHON), a genetic disorder leading to a rapid loss of bilateral central vision [29]. REX-001 (Rexmylocel) are autologous bone marrow mononuclear cells administered through an intra-arterial catheter to treat critical limb ischemia [30,31]. Multistem® is an off the shelf cell therapy product applicable for treatment of multiple distinct diseases. Multistem cells are currently in phase III clinical trials for treatment of ischaemic stroke and phase II for ulcerative colitis [32,33]. Phase I studies for acute myocardial infarction and Graft vs. Host Disease have been completed [34,35].
PLX-PAD PLacental eXpanded cells are mesenchymal-like stromal cells applicable without tissue or genetic matching. The cell-released cytokines, chemokines and growth factors are supposed to facilitate tissue regeneration [36]. PLX-PAD was granted FDA fast track approval and was accepted into the EMA Adaptive Regulatory Pathway [36] . Data of all ATMPs with a planned launch in or after 2020 are summarized in Table 4.
ATMPS available in individual European member states via hospital exemption
A survey performed by the Pharmaceutical Committee of the European Commission in 2012 reported that 37% of the responding European member states had ATMPs legally on the market and 22% had issued Hospital exemptions for ATMP products [37]. In 2018, 47% of the responding countries reported to have issued Hospital exemptions. Data are summarized in Table 5.
Table 5.
Hospital exemption for ATMPs.
| Country | Hospital exemption issued in 2012 | Hospital exemption issued in 2018 | Cell types applied via Hospital exemption |
|---|---|---|---|
| Austria | no | no | |
| Belgium | yes | no | |
| Bulgaria | ? | ? | |
| Croatia | no | ? | |
| Cyprus | no | ? | |
| Czech Republic | no | yes | Chondrocytes |
| Denmark | yes | ? | |
| Estonia | no | no | |
| Finland | no | ? | |
| France | yes | ? | |
| Germany | yes | yes | Cytokine induced killer cells, dendritic cells, chondrocytes, mesenchymal stroma cells, engineered oral mucosa, bone marrow derived progenitor cells |
| Greece | no | ? | |
| Hungary | no | no | |
| Iceland | no | no | |
| Ireland | no | yes | Limbal stem cells |
| Italy | no | ? | |
| Latvia | no | no | |
| Liechtenstein | ? | ? | |
| Lithuania | no | yes | Dendritic cells, cytokine activated killer cells, |
| T-cells, stromal vascular fraction cells | |||
| Luxembourg | no | ? | |
| Malta | no | ? | |
| Netherlands | yes | yes | Lymphocytes, mesenchymal stem cells, mononuclear cells, T-cells |
| Norway | ? | yes | Chondrocytes, autologous T-cells, autologous dendritic cells, skin cells |
| Poland | no | ? | |
| Portugal | no | no | |
| Romania | no | ? | |
| Slovakia | no | ? | |
| Slovenia | no | ? | |
| Spain | yes | ? | |
| Sweden | no | yes | Chondrocytes, mesenchymal stem cells, mesenchymal stromal cells, fetal stem cells, keratinocytes |
| UK | no | no |
The national competent authorities of Germany, Czech Republic, Ireland, Lithuania, Norway, Sweden, Italy and the Netherlands stated to have national approvals for ATMPs. No national approvals are currently issued in Austria, Belgium, Estonia, Hungary, Iceland, Latvia, Portugal and the UK. Tumor vaccines and autologous chondrocytes for restoration of cartilage defects are the most commonly used ATMP products under the Hospital exemption. Other cell types applied are oral mucosa cells, skin cells, bone marrow derived and mesenchymal stem cells as well as limbal stem cells (Figure 2).
Figure 2.
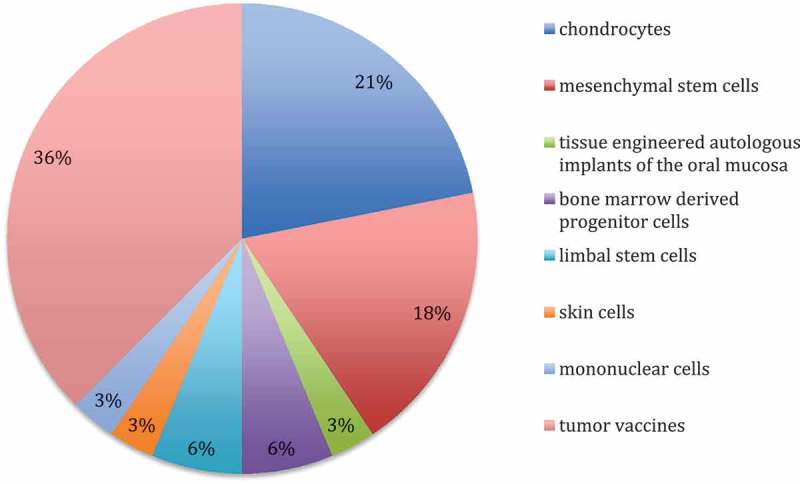
ATMP products applied in individual European member states via Hospital exemption.
ATMPS in phase III or IV clinical trial
Apart from ATMPs following the centralized marketing authorisation pathway, 141 phase III and four phase IV clinical trials investigating potential ATMPs were identified. The majority (74%) of these are academic trials without an industrial sponsor. The remaining 26% are industry-driven research projects examining 31 different ATMP candidates. Indications are coronary artery disease, urinary stress incontinence, critical limb ischemia and chronic leg ulcers as well as cartilage restoration, oncological indications, mucopolysaccharidosis and spinal muscular atrophy. Most ATMP candidates in the industrial pipeline are cancer vaccines (29%), followed by gene (25%) and stem cell therapies (23%). Details are presented in Figure 3. 13 studies are still active at the time of this report and have no results published. 3 studies with published results failed to demonstrate clinical efficacy in phase III. The marketing authorisation application of Cerepro® (Ark Therapeutics) was withdrawn for this reason [38]. Data on potential ATMPs in the industrial pipeline are summarized in Table 6.
Figure 3.
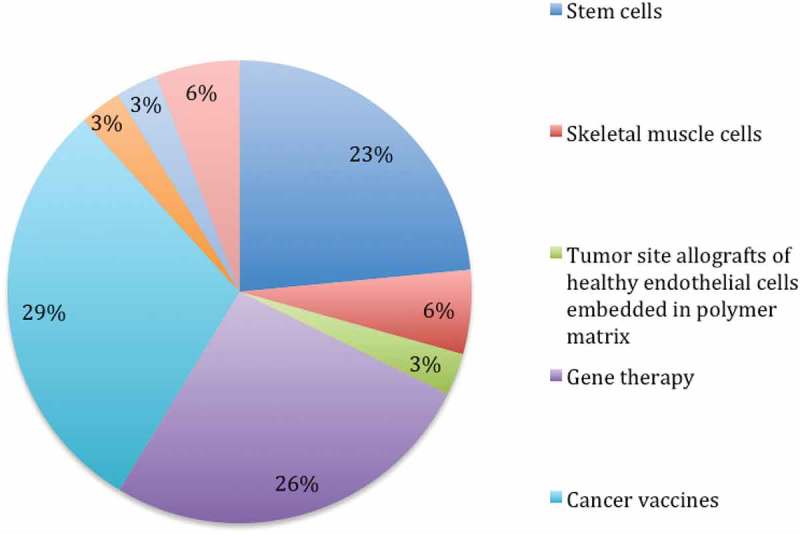
Potential ATMP candidates in industry-driven phase III clinical trials.
Table 6.
ATMP candidates in the industrial pipeline in phase III/IV clinical trial.
| ATMP candidate | Clinical trial identifier | Sponsor | Indication | Study State |
|---|---|---|---|---|
| Autologous CD133+ bone marrow stem cells | NCT00950274 | Miltenyi Biotec GmbH | Chronic ischemic coronary artery disease | Terminated (slow recruitment) |
| Skeletal muscle derived cells | 2014–001656-34 | Innovacell Biotechnologie AG | Stress urinary incontinence | Completed |
| Tumor site allografts of healthy endothelial cells embedded in polymer matrix | Unknown | Shire (Pervasis) | Treatment/ Prevention of metastatic cancer |
Unknown |
| Bone marrow derived mononuclear cells | NCT01285297 | Cardiogenesis | Transmyocardial revascularisation | Completed |
| C-CURE (bone marrow derived cardiopoietic cells) | NCT01768702 | Celyad (formerly named Cardio3 BioSciences) | Ischaemic heart failure | Completed |
| Generx (FGF-4 gene therapy) | NCT02928094 | Angionetics Inc. | Coronary artery disease | Completed |
| Riferminogene pecaplasmid (Gene therapy) | NCT00566657 | Sanofi | Critical limb ischemia | Completed (failure to detect efficacy) |
| Cerepro (cancer vaccine) | EUDRACT2004-000464–28. | Ark Therapeutics | Operable high-grade glioma | Completed; MAA withdrawn (unable to demonstrate a clinically meaningful benefit) |
| Autologous chondrocytes | EUDRACT2016-002817–22 | TETEC Tissue Engineering Technologies – AG | Cartilage damage | Active |
| Keratinocytes | EUDRACT2012-003286–18 | Smith & Nephew | Chronic leg ulcer | Terminated (failure to detect efficacy) |
| Autologous dendritic cells | EUDRACT2012-000871–17 | Argos Therapeutics, Inc. | Renal cell carcinoma stage IV | Active |
| Autologous dendritic cells | NCT02111577 | Sotio a.s. | Metastatic castration resistant prostate cancer | Active |
| Cancer vaccine | NCT01383148 | Transgene | Non small cell lung cancer | Terminated (reason unclear) |
| Pexa-Vec (Cancer vaccine) | NCT02562755 | SillaJen, Inc. | Hepatocellular Carcinoma | Recruiting |
| AVXS-101 (Gene Therapy) | NCT03461289 | AveXis, Inc. | Spinal Muscle Atrophy | Recruiting |
| CER-001 (Gene Therapy) | NCT02697136 | Cerenis Therapeutics, SA | Primary Hypoalphalipoproteinemia | Recruiting |
| Autologous fat enhanced with regenerative cells | NCT00616135 | Cytori Therapeutics | Cosmetic breast deformities | Completed |
| AMG0001 (Gene Therapy) | NCT02144610 | AnGes USA, Inc. | Critical limb ischemia | Terminated (strategy amendment) |
| Autologous Muscle Derived Cells | NCT01893138 | Cook MyoSite|Cook Group Incorporated | Female Urinary Sphincter Repair | Active, not recruiting |
| Cancer vaccine | NCT01817738 | CureVac AG | Prostate cancer | Terminated |
| Valoctocogene Roxaparvovec (Gene Therapy) | NCT03392974 | BioMarin Pharmaceutical | Hemophilia A | Recruiting |
| AAVrh10-h.SGSH Gene Therapy | NCT03612869 | LYSOGENE | Mucopoly-saccharidosis | Not yet recruiting |
| Bone marrow stem cells | NCT00462774 | Miltenyi Biotec GmbH | Ischaemic heart failure | Completed |
| Progenitor Cells | NCT00279175 | Eli Lilly and Company | Acute Myocardial Infarction | Completed |
| Cancer vaccine | NCT00676507 | NovaRx Corporation | Non-small Cell Lung Cancer | Completed |
| GSK2696274 (Gene Therapy) | NCT03392987 | GlaxoSmithKline | Metachromatic Leukodystrophy | Recruiting |
| Mesenchymal Stem Cells | NCT00366145 | Osiris Therapeutics | Acute Graft Versus Host Disease | Completed |
| NiCord® cord blood stem cells | NCT02730299 | Gamida Cell ltd | Hematologic malignancies | Recruiting |
| TG4010 (Cancer vaccine) | NCT00415818 | Transgene | Non-Small Cell Lung Cancer | Completed |
| DCVax®-L (Cancer vaccine) | NCT00045968 | Northwest Biotherapeutics | Glioblastoma | Unknown status |
| AAV2-REP1(Gene Therapy) | NCT03496012 | Nightstar Therapeutics | Choroideremia | Recruiting |
The majority of clinical trials performed in an academic setting target stem cell transplantation for oncological indications (68%). Most of them are not embraced by the ATMP definition, as autologous stem cells for transplantation after chemotherapy are usually neither substantially manipulated nor intended to be used for a different essential function. Other indications are myocardial infarction and heart failure (9%), critical limb ischemia (6%), stroke, burns and infertility. Except for one tumor vaccine, all academic clinical trials investigate cell therapeutic products (Figure 4). Details on ATMP candidates developed in academic settings are presented in Table 7.
Figure 4.
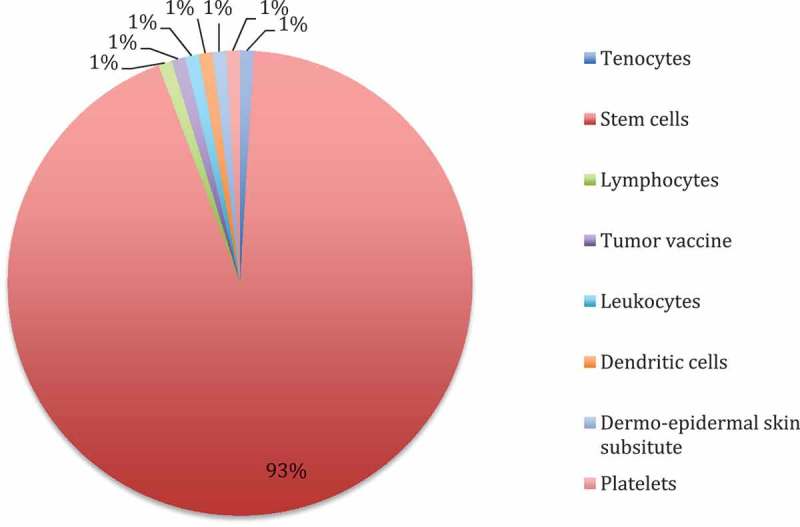
Stem cell therapies and potential ATMP candidates applied in academic phase III and IV clinical trials.
Table 7.
Overview on academic phase III/IV ATMP clinical trials (Stem cell transplantation for haematological malignancies not included).
| Trial Identifier | Phase | Sponsor | Product/Procedure | Indication |
|---|---|---|---|---|
| NCT00434616 | III | Franziskus-Krankenhaus | Autologous bone marrow cells | Critical limb ischemia |
| NCT01803347 | III | Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz | Autologous expanded adipose-derived stem cells | Anal fistula |
| NCT01569178 | III | Queen Mary University of London | Bone marrow derived mononuclear cells | Acute myocardia infarction |
| NCT03477500 | III | NCT03477500 Haukeland University Hospital | Autologous stem cells | Multiple sclerosis |
| ISRCTN54371254 | III | EBMT Central Office | Autologous hematopoietic stem cells | Diffuse cutaneous systemic sclerosis |
| EUDRACT2015-000431–32 | III | Universidad Autónoma de Madrid (U.A.M.) | Autologous human bone marrow-derived expanded mesenchymal stromal cells | Diaphyseal metaphyseal fracture and non union |
| n/a | III | Unknown | Renal cell tumor vaccine | Renal carcinoma |
| NCT00297193 | III | European Group for Blood and Marrow Transplantation | The Broad Foundation | Autologous Stem Cells | Cohn’s Disease |
| NCT02437708 | III | Universitaire Ziekenhuizen Leuven | Stem cells | Periapical bone healing in infected immature primary teeth |
| NCT01818310 | II|III | University Hospital Ostrava|Ministry of Health, Czech Republic|Regional Council of the Moravian-Silesian region, KU MSK | Autologous Bone Marrow Aspirate Concentrate | No-Option Critical Limb Ischemia |
| NCT02849613 | II|III | University Hospital, Grenoble | Regenerative Stem Cell Therapy | Stroke |
| NCT00904501 | III | CHU de Reims |Etablissement Francais du Sang | Bone Marrow Autograft | Limb Ischemia |
| NCT03325504 | III | Universidad Autonoma de Madrid | Mesenchymal stem cells + Biomaterial | Bone Healing in Non-Union |
| NCT01489501 | III | CellSeed France S.A.R.L.|FGK Clinical Research GmbH | Oral mucosal epithelial cell sheet | Limbal Stem Cell Deficiency |
| NCT00938847 | III | Asklepios proresearch Cordis Corporation | Bone Marrow Derived Mononuclear | Myocardial Regeneration |
| NCT01983748 | III | University Hospital Erlangen | Dendritic Cells Plus Autologous Tumor RNA | Uveal Melanoma |
| NCT01693042 | II|III | Johann Wolfgang Goethe University Hospital | Autologous Bone Marrow-derived Mononuclear Cells | Chronic Post-infarction Heart Failure |
| NCT01753440 | II|III | AHEPA University Hospital | Allogeneic Stem Cells Implantation Combined With Coronary Bypass Grafting | Ischemic Cardiomyopathy |
| NCT01759212 | II|III | AHEPA University Hospital | Left Ventricular Assist Device + Allogeneic Mesenchymal Stem Cells Implantation | End-stage Heart Failure |
| NCT03112122 | IV | Istituto Ortopedico Rizzoli | Bone Marrow Concentrate | Bone Marrow Edema |
| NCT03110679 | IV | Istituto Ortopedico Rizzoli | Autologous Bone Marrow Concentrate | Osteoarthritis |
| NCT02454231 | II|III | University of Florence|Tuscany Region | Stem Cells | Life Threatening Limbs Arteriopathy |
| NCT00539266 | II|III | Leiden University Medical Centre | Autologous Bone Marrow-derived Mononuclear Cells | Limb Ischemia |
| NCT03042572 | II|III | The Netherlands Organisation for Health Research and Development|UMC Utrecht | Allogeneic Mesenchymal Stromal Cells | No-option Ischemic Limbs |
| NCT01343836 | II|III | Erasmus Medical Center | Autologous Tenocyte Implantation | Chronic Achilles Tendinopathy |
| NCT03229564 | II|III | University of Zurich|ETH Zurich (Switzerland)|Julius Clinical, The Netherlands | Autologous Dermo-epidermal Skin Substitute | Treatment of Burns in Children |
| NCT02323620 | III | American Heart of Poland | Bone marrow derived mononuclear cells | Myocardial infarction |
| NCT03404063 | II|III | John Paul II Hospital, Krakow|KCRI|National Center for Research and Development, Poland | CardioCell (Wharton´s Jelly derived mesenchymal stem cells) | Acute Myocardial Infarction |
| NCT03423732 | II|III | John Paul II Hospital, Krakow|KCRI|National Center for Research and Development, Poland | CardioCell (Wharton´s Jelly derived mesenchymal stem cells) | No-option Critical Limb Ischemia |
| NCT02248532 | II|III | University Medical Centre Ljubljana | CD34+ Cells | Dilated Cardiomyopathy |
| NCT02144987 | IV | Instituto Valenciano de Infertilidad, IVI VALENCIA | Bone Marrow Stem Cells | Asherman’s Syndrome and Endometrial Atrophy |
| NCT03535480 | IV | Instituto de Investigacion Sanitaria La Fe | Autologous Bone Marrow Stem Cells | Premature Ovarian Failure |
| NCT02389010 | III | Centro Nazionale Sangue|Italian National Cord Blood Network | Platelet Gel From Cord Blood | Diabetic Foot Ulcers |
| NCT00747708 | II|III | Barts & The London NHS Trust | Bone Marrow Derived Adult Stem | Chronic Heart Failure |
Conclusion
Advanced therapeutic medicinal therapies are still in their early days, but constantly evolving. Until 2017, more than 900 ATMPs have been examined in clinical trials worldwide [39]. Despite this impressive number of projects, the number of ATMPs on the market is still considerably low, and some of them were withdrawn only a couple of years after their market launch. Up to date, there are 8 ATMPs available via a centralized European marketing authorisation. Information on their commercial success is still very limited. GlaxoSmithKline, for example, has announced the first reimbursement of Strimvelis® for its first patient in March 2017 despite being approved under a full performance-based reimbursement scheme since 2016 [40].
7 European Union member states reported providing additional ATMPs outside of clinical trials via Hospital exemption regulation. Due to a poor return rate, the data of the 2018 survey are of limited significance. However, combining the actual data with the results of the 1012 report published by the European Commission [37], there are still only 8 countries having issued Hospital exemptions. At the 26th Annual EuroMeeting in Vienna, concerns were raised that European member states might consider the Hospital exemption as an opportunity for early clinical development prior to clinical trials [41]. ATMPs that had been legally on the market before 2008 might avoid the complex authorisation procedure by evading under the Hospital exemption regulation. However, considering the actual survey result in combination with the 2012 data, these concerns have not been verified on a large scale.
6 new ATMPs shall be launched until 2020 and offer new treatment modalities for retinal dystrophy, ß-thalassemia, scleroderma, sickle-cell anaemia, adrenoleukodystrophy and leukaemia. Luxturna™, LentiGlobin™ and ATIR101 have already submitted a central marketing authorization application. For Luxturna™, the Committee for Medicinal products for Human Use has issued a positive opinion recommending approval [42]. LentiGlobin™ has been granted accelerated assessment by the EMA and ATIR101 expects conditional approval in 2019 [43,44]. Habeo™ and Neocart® did not reach significance in the primary efficacy endpoints in their respective phase III clinical trials, and eventual consequences for their marketing launch are unclear. For LentiD™, a modified paediatric investigation plan was accepted by the EMA in September 2018 [45]. 7 additional ATMPs currently in phase III clinical development are planned for a marketing authorisation application in or after 2020.
131 phase III clinical trials with ATMPs could be identified apart from the centralized marketing authorisation procedure. 31 ATMP candidates are industrial research projects with an assumptive interest in obtaining a marketing authorisation. However, reaching phase III stage does not guarantee a roadmap to successful clinical translation: Out of 19 finalized clinical trials, 26% were terminated prematurely and 23% of the ATMP candidates finally failed to demonstrate efficacy when evaluated against the current standard of care.
Funding Statement
This work was supported by the Ludwig Boltzmann Institute for Health Technology Assessment.
Acknowledgments
The authors would like to thank Mr. Tarquin Mittermayr, BA, for performing the systematic literature search and Mr. Florian Prammer for contacting the notified bodies to obtain information on the national application of the Hospital exemption as well as performing additional research on the marketing authorisation status of ATMPs in the industrial pipeline.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].European Commission Commission directive 2009/120/EC of 14 December 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use as regards advanced therapy medicinal products. Off J Eur Union. L242/3–12. [Google Scholar]
- [2].Buljovcic Z.European marketing authorisation: a long process. Experiences of small biotech companies with the ATMP regulation. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54(7):831–13. [DOI] [PubMed] [Google Scholar]
- [3].European Medicines Agency Orphan designation: overview. [cited2018November21]: Available from: https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation-overview.
- [4].gov.uk, GUIDANCE ON THE UK’S ARRANGEMENTS UNDER THE HOSPITAL EXEMPTION SCHEME [cited201821November]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/397738/Guidance_on_the_UK_s_arrangements_under_the_hospital_exemption_scheme.pdf
- [5].Pellegrini G, Rama P, Di Rocco A, et al. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32(1):26–34. [DOI] [PubMed] [Google Scholar]
- [6].European Medicines Agency First two CAR-T cell medicines recommended for approval in the European Union. [cited201821November]. Available from: https://www.ema.europa.eu/en/news/first-two-car-t-cell-medicines-recommended-approval-european-union
- [7].Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. [DOI] [PubMed] [Google Scholar]
- [8].Jaroslawski S, Caban A, Toumi M. Sipuleucel-T (Provenge(R)): autopsy of an innovative change of paradigm in cancer treatment. Value Health. 2015;18(7):A479. [DOI] [PubMed] [Google Scholar]
- [9].Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell. 2016;19(3):293–297. [DOI] [PubMed] [Google Scholar]
- [10].European Medicines Agency., MACI 2014. [cited 2018 November21] Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Maci/human_referral_000380.jsp&mid=WC0b01ac05805c516f
- [11].European Medicines Agency Closure of EU manufacturing site for MACI. 2014. [cited 2018 November21] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Maci_20/WC500173680.pdf
- [12].Ylä-Herttuala S. The need for increased clarity and transparency in the regulatory pathway for gene medicines in the European Union. Mol Ther. 2012;20(3):471–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glybera (alipogene tiparvovec) for Treatment of Lipoprotein Lipase Deficiency (LPLD) [cited201818August]. Available from: https://www.drugdevelopment-technology.com/projects/glybera-alipogene-tiparvovec-treatment-lipoprotein-lipase-deficiency-lpld/. [DOI] [PubMed]
- [14].World´s fist gene therapy to be withdrawn in (sic!) from market in Europe 2017[cited 2018 August16] Available from: https://sciencebusiness.net/news/80248/World’s-first-gene-therapy-to-be-withdrawn-in-from-market-in-Europe.
- [15].Yano Kazuo WN, Kenichiro T, Taisuke I, et al. Regulatory approval for autologous human cells and tissue products in the USA, the European Union, and Japan. Regener Ther. 2015;1:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].European Medicines Agency , CHMP summary of positive opinion for Luxturna in Opinion. 2018. [cited 2018 August18] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004451/WC500255715.pdf
- [17].Walters M., Hongeng S., Kwiatkowski J., et al. Update of Results from the Northstar Study (HGB-204): A Phase 1/2 study of gene therapy for beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex-vivo with a lentiviral beta AT87Q-globin vector (LentiGlobin BB305 Drug Product). In: 57th Annual Meeting of the Anerican Society of Hematology Orlando, FL; 2015. [Google Scholar]
- [18].bluebirdbio , bluebird bio presents updated data from phase 2/3 starbeam study of investigational lenti-D™ gene therapy for CALD and initial data from observational study ALD-103 of allogeneic hematopoietic stem cell transplant in CALD at 2018 SSIEM. 2018. [cited 2018 August18] Available from: https://www.businesswire.com/news/home/20180905005434/en/bluebird-bio-Presents-Updated-Data-Phase-23
- [19].Cytori , Enrollment completed in randomized clinical trial of Habeo™ cell therapy for scleroderma and impaired hand function.[cited201818August]. Available from: http://ir.cytori.com/investor-relations/news/news-details/2018/Enrollment-Completed-in-Randomized-Clinical-Trial-of-Habeo-Cell-Therapy-for-Scleroderma-and-Impaired-Hand-Function/default.aspx
- [20].Cytori , Cytori announces top-line 24- and 48-Week Results from the STAR Trial of Habeo™ cell therapy in patients with scleroderma. [cited 2018 August18] Available from: http://ir.cytori.com/investor-relations/news/news-details/2017/Cytori-Announces-Top-Line-24–and-48-Week-Results-from-the-STAR-Trial-of-Habeo-Cell-Therapy-in-Patients-with-Scleroderma/default.aspx
- [21].Cytori launches managed access programme for ECCS-50 for scleroderma. 2016[cited 2018 September27] Available from: https://www.europeanpharmaceuticalreview.com/news/37778/cytori-launches-managed-access-programme-eccs-50-scleroderma/.
- [22].Histogenics , Neocart phase III clinical trial results call. 2018. Presented 2018 September5
- [23].BusinessWire Juno Therapeutics Presents Updated TRANSCEND NHL 001 trial data demonstrating high durable response rates in patients with relapsed or refractory CD19+ Aggressive non-hodgkin lymphoma. 2017[cited 2018 October1] Available from: https://www.businesswire.com/news/home/20170605005425/en/Juno-Therapeutics-Presents-Updated-TRANSCEND-NHL-001.
- [24].bb2121: is CAR T-cell therapy moving to Myeloma? 2018[cited 2018 October2] Available from: https://www.ashclinicalnews.org/on-location/bb2121-car-t-cell-therapy-moving-myeloma/.
- [25].bio, b Making hope a reality – bluebird style. 2018[cited 2018 October2] Available from: http://investor.bluebirdbio.com/encrypt/files?file=nasdaq_kms/assets/2018/03/07/11-53-56/BLUECompanyOverviewJanuary2018.pdf&file_alias=10561.
- [26].Prockop S, Baiocchi R, Baiocchi R, et al. Efficacy and Safety of ATA129, partially matched allogeneic third-party epstein-barr virus-targeted cytotoxic t lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Biol Blood Marrow Transplant. 2018;24(3):S41–S42. [Google Scholar]
- [27].Biotherapeutics A, Expanded access protocol for tabelecleucel in subjects with EBV-associated viremia or malignancies. 2016. [cited 2018 October2] Available from: https://clinicaltrials.gov/ct2/show/NCT02822495?term=ATA129&rank=3
- [28].Sharon W.High objective response rate, OS seen with ATA129 in PTLD. in Hematology News. 2018. [cited 2018 October2] Available from: https://www.mdedge.com/hematologynews/article/159375/aggressive-lymphomas/high-objective-response-rate-os-seen-ata129-ptld
- [29].Meyerson C, Van Stavern G, McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin Ophthalmol. 2015;9:1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rexgenero, REX-001 [cited2October2018] Available from: http://www.rexgenero.com/rex-001/
- [31].Rexgenero Ltd. , The efficacy and safety of REX-001 to treat ischemic ulcers in subjects with CLI rutherford category 5 and DM. 2017. [cited 2018 October2] Available from: https://clinicaltrials.gov/ct2/show/NCT03174522
- [32].Athersys inc , Athersys announces enrollment of first patient in masters-2 phase 3 study of multistem® treatment for ischemic stroke. 2018, [cited 2018 October2] Available from: http://www.athersys.com/news-releases/news-release-details/athersys-announces-enrollment-first-patient-masters-2-phase-3
- [33].Athersys inc. , Athersys announces results from phase 2 study of multistem(R) cell therapy for ulcerative colitis. 2014. [cited 2018 October2] Available from: http://www.athersys.com/news-releases/news-release-details/athersys-announces-results-phase-2-study-multistemr-cell-therapy
- [34].Athersys inc. , Athersys announces positive results from phase I study of multistem(R) in Heart Attack Patients. 2010. [cited 2018 October2] Available from: http://www.athersys.com/news-releases/news-release-details/athersys-announces-positive-results-phase-i-study-multistemr
- [35].Athersys inc. , A., athersys announces positive results of multistem(r) clinical trial for hematopoietic stem cell transplant support and prevention of graft-versus-host disease. 2012. [cited 2018 October2] Available from: http://www.athersys.com/news-releases/news-release-details/athersys-announces-positive-results-multistemr-clinical-trial
- [36].Pluristem PLX products. [cited2October2018]; Available from: http://www.pluristem.com/placental-expanded-plx-products/.
- [37].European Commission. , Hospital exemption for ATMPs (implementation of Art 28 (2)of Regulation 1394/2007): update on feedback received by the Commission, in PHARM 608. European Commission.
- [38].European Medicines Agency Cerepro: withdrawal of the marketing authorisation application. 2010[cited 2018 November21] Available from: https://www.ema.europa.eu/documents/other/withdrawal-letter-cerepro_en-0.pdf.
- [39].Seimetz D. What can we learn from case studies to address development and approval challenges of ATMPs? Hum Gene Ther. 2017;28(12):A61–A62. [Google Scholar]
- [40].Macaulay R. Advanced therapy medicinal products-transformational patient benefits but destined for commercial failure? Value Health. 2017;20(9):A702. [Google Scholar]
- [41].Salmikangas P. The hospital exemption for advanced therapies: the regulator´s view. In: 26th annual EuroMeeting Vienna; 2014. [Google Scholar]
- [42].Novartis , Novartis announces positive CHMP opinion for one-time gene therapy Luxturna® to treat children and adults with rare inherited retinal disease. 2018. [cited 2018 March10] Available from: https://www.novartis.com/news/media-releases/novartis-announces-positive-chmp-opinion-one-time-gene-therapy-luxturna-treat-children-and-adults-rare-inherited-retinal-disease
- [43].bluebirdbio , bluebird bio Announces European medicines agency’s acceptance of marketing authorization application for lentiglobin™ gene therapy for the treatment of transfusion-dependent β-thalassemia. 2018. [cited 2019 March10] Available from: http://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-european-medicines-agencys-acceptance
- [44].Pharma K, Kiadis Pharma on track with European regulatory review for ATIR101. 2018. [cited 2019 March10] Available from: https://www.kiadis.com/kiadis-pharma-on-track-with-european-regulatory-review-for-atir101/
- [45].European Medicines Agency , European Medicines Agency decision P/0290/2018. [cited10March2019]Available from: https://www.ema.europa.eu/en/documents/pip-decision/p/0290/2018-ema-decision-12-september-2018-acceptance-modification-agreed-paediatric-investigation-plan_en.pdf


