Abstract
Background
Previous work has demonstrated that drugs can be delivered across the blood-brain barrier by exposing circulating microbubbles to a sequence of long ultrasound pulses. Although this sequence has successfully delivered drugs to the brain, concerns remain regarding potentially harmful effects from disrupting the brain vasculature.
Purpose
To determine whether a low-energy, rapid, short-pulse ultrasound sequence can efficiently and safely deliver drugs to the murine brain.
Materials and Methods
Twenty-eight female wild-type mice underwent focused ultrasound treatment after injections of microbubbles and a labeled model drug, while three control mice were not treated (May–November 2017). The left hippocampus of 14 mice was exposed to low-energy short pulses (1 MHz; five cycles; peak negative pressure, 0.35 MPa) of ultrasound emitted at a rapid rate (1.25 kHz) in bursts (0.5 Hz), and another 14 mice were exposed to standard long pulses (10 msec, 0.5 Hz) containing 150 times more acoustic energy. Mice were humanely killed at 0 (n = 5), 10 (n = 3), or 20 minutes (n = 3) after ultrasound treatment. Hematoxylin-eosin (H-E) staining was performed on three mice. The delivered drug dose and distribution were quantified with the normalized optical density and coefficient of variation. Safety was assessed by H-E staining, the amount of albumin released, and the duration of permeability change in the blood-brain barrier. Statistical analysis was performed by using the Student t test.
Results
The rapid short-pulse sequence delivered drugs uniformly throughout the parenchyma. The acoustic energy emitted from the microbubbles also predicted the delivered dose (r = 0.97). Disruption in the blood-brain barrier lasted less than 10 minutes and 3.4-fold less albumin was released into the brain than with long pulses. No vascular or tissue damage from rapid short-pulse exposure was observable using H-E staining.
Conclusion
The rapid short-pulse ultrasound sequence is a minimally disruptive and efficient drug delivery method that could improve the treatment, diagnosis, and study of neurologic diseases.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Klibanov and McDannold in this issue.
Summary
In this animal study, rapid short-pulse ultrasound sequences deliver drugs uniformly to the brain by disrupting the blood-brain barrier permeability for less than 10 minutes and with a low level of blood albumin release into the brain.
Key Points
■ In a mouse model, rapid short-pulse sequences show the potential to deliver drugs at a comparable dose to long pulses across the blood-brain barrier with a uniform distribution and into neurons.
■ These sequences also have the potential to deliver drugs with minimal damage to the blood-brain barrier function and the neuronal microenvironment as indicated by a permeability change of very short duration (< 10 min) and a 3.4-fold lower amount of albumin released into the brain parenchyma when compared with long pulses.
Introduction
Noninvasively applied pulses of ultrasound and microbubbles can locally deliver molecules to the brain (1,2). Ultrasound has been shown to shrink brain tumors and improve cognitive function in animal models of Alzheimer disease (3–5). However, despite promising results, there remain concerns about potentially harmful effects from permeability changes to the blood-brain barrier (6,7).
Focused ultrasound delivers molecules from the bloodstream to the brain by mechanically stimulating the vessels with acoustically active microbubbles. The microbubbles, composed of a lipid shell and stabilized gas core (1–10 µm), are administered intravenously (8). Pressure oscillations from the ultrasound drive the microbubbles to expand and contract, transporting molecules across the blood-brain barrier.
In animals and in clinical trials, the most common method to drive microbubbles to disrupt the blood-brain barrier is with long-pulse sequencing (9–11), characterized by long pulses emitted in a slow sequence (6,12–14). Although parameters within this regimen attain the best performance-safety balance through thorough optimization, limitations exist. First, treatment is unequal within the beam (12,15), resulting in drugs delivered in some, but not all, targeted areas. Second, unwanted biologic responses can be triggered, such as neuronal damage, red blood cell extravasation, and hemorrhage (6,16,17). A recent clinical study has shown the presence of potential microhemorrhages (shown as hypointense areas on T2 MRI scans) using pulses that last in the range of milliseconds in humans (18). Third, it takes 4–48 hours for the permeability of the blood-brain barrier to return to normal (14,19), allowing other blood molecules to enter the brain, including albumin (7,20), a potentially neurotoxic agent that may trigger neuroinflammation (7,21). Ideally, we want to reduce the duration of the permeability change in the blood-brain barrier and the amount of blood proteins entering the brain.
To mitigate the potential damage caused by long-pulse sequencing, we have developed a low-energy rapid short-pulse ultrasound sequence that delivers compounds to the brain with a high performance and safety profile. This rapid short-pulse sequence is based on an earlier sequence design (12) with modifications to produce more uniform microbubble activity in vitro than long pulses (22,23). We define the desired performance profile as providing uniform delivery of a molecule to specific tissue regions at a high enough dose. We define the desired safety profile as delivering molecules with a short permeability change in the blood-brain barrier and low leakage of blood proteins into the brain. We hypothesized that rapid short-pulse sequences can improve the efficacy and safety of drug delivery to the brain in vivo with low levels of disruption to the blood-brain barrier.
Materials and Methods
Animals
The UK Home Office and Imperial College London’s animal facility committee approved all experimental protocols. Thirty- one female C57bl/6 wild-type mice (8–10 weeks old, 19.2 g ± 0.95; Envigo, Huntingdon, England) were anesthetized with 1.5%–2.0% vaporized isoflurane (Zoetis UK, London, England) mixed with oxygen (0.8 L/min) by using an anesthesia vaporizer (Harvard Apparatus, Cambridge, England) between May and November 2017. Fourteen mice were treated with a rapid short-pulse ultrasound sequence and another 14, with a long-pulse sequence (Fig 1, A) (n = 5 humanely killed at 0 min, n = 3 at 10 min, n = 3 at 20 min, and another n = 3 at 0 min for hematoxylin-eosin [H-E] staining). Two controls were used in this study: (a) mice unexposed to ultrasound (n = 3), and (b) in all mice, the right hippocampus unexposed to ultrasound.
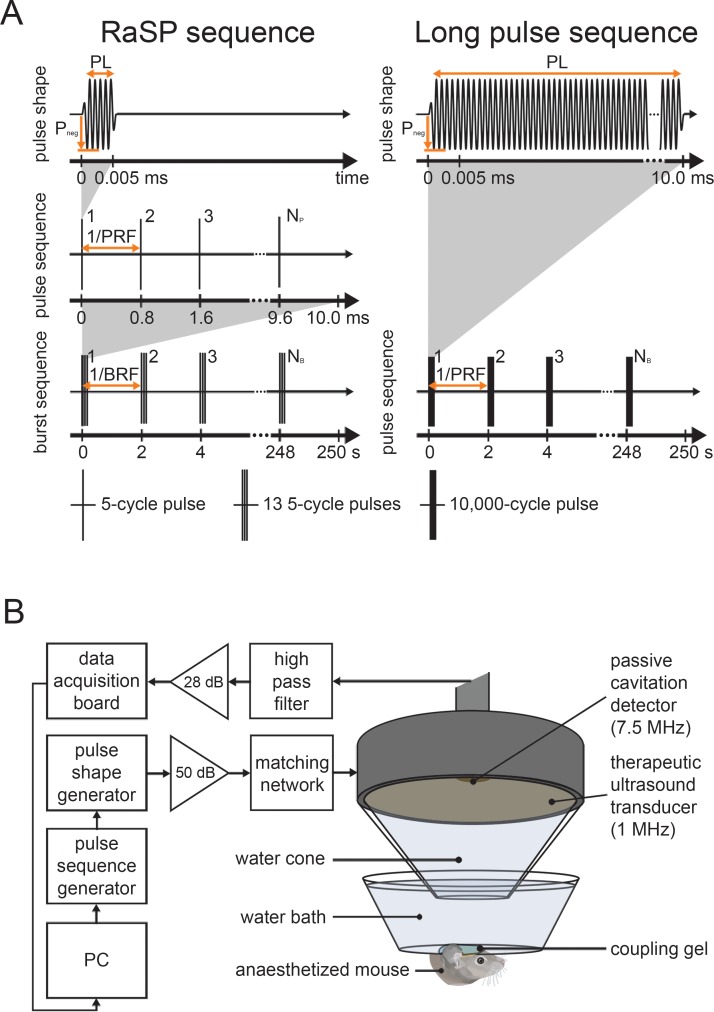
Figure 1:
Ultrasound sequence and experimental setup. A, The mouse brain was exposed to 1-MHz ultrasound using a rapid short-pulse (RaSP) or long-pulse sequence. The RaSP sequence was composed of short, low-energy pulses (pulse length [PL] = five cycles) emitted at a rapid rate (pulse repetition frequency [PRF ] = 1.25 kHz) and in a slow burst sequence (burst repetition frequency [BRF ] = 0.5 Hz), while the long-pulse sequence was composed of a long, high-energy pulse (PL = 10 000 cycles) emitted at a slow rate (BRF = 0.5 Hz). The pulses in both sequences had a peak negative pressure (Pneg) of 0.35 MPa. B, Ultrasound was applied through the intact scalp and skull. The pulses were emitted onto the left hippocampus while the right hippocampus acted as a control. A 7.5-MHz passive cavitation detector captured the acoustic emissions from the microbubbles. NB = number of bursts, PC = personal computer.
Pulse-Sequence Design
The rapid short-pulse sequence was designed to promote the microbubble stimulus that safely delivers molecules—stable cavitation—and to suppress the stimuli that damage tissue—high magnitude inertial cavitation, clustering, coalescence, rectified diffusion, microjetting, et cetera (24). We (S.V.M., PhD student; A.N.P., associate research scientist; J.J.C., senior lecturer) selected the pulse-shape parameters by reducing the pulse-shape energy (low peak rarefactional pressures and short pulse lengths) to the delivery threshold. We selected the pulse-sequence parameters to provide a time interval for microbubbles to move between pulses and not overstress any given capillary site. Our intention was that this combination of short pulses and rapid emission allowed each microbubble to be gently stimulated multiple times as it flowed through the vascular network. We selected the burst-sequence parameters to allow microbubbles to replenish the vasculature exposed to the ultrasound beam.
Ultrasound Setup and Targeting Method
Therapeutic ultrasound pulses were emitted from a single-element spherical-segment focused US transducer (center frequency [fc], 1 MHz; Sonic Concepts, Bothell, Wash) driven by two function generators (33500B Series; Agilent Technologies, Santa Clara, Calif) through a 50-dB power amplifier (Precision Acoustics, Dorchester, England). The first function generator set the pulse and burst sequence, while the second defined the pulse shape. A passive cavitation detector (PCD) (fc, 7.5 MHz; Olympus, Essex, England) was positioned through a central opening in the therapeutic transducer with foci overlapping. Acoustic emissions captured by the PCD were filtered (band-pass filter, 3–30 MHz; Mini Circuits, Brooklyn, NY), amplified by a 28-dB preamplifier (Stanford Research Systems, Sunnyvale, Calif), and recorded with an oscilloscope (Pico Technology, Cambridge, England). The therapeutic transducer had a mounted cone filled with distilled water and covered with an acoustically transparent parafilm membrane (Fig 1, B).
After removing the fur, we (S.V.M, T.G.C., and M.J.C., PhD students) placed the mouse head into a stereotaxic frame (World Precision Instruments, Hertfordshire, England). We placed a container with degassed water, covered by a transparent parafilm membrane, on the head and coupled with US gel. Using a three-dimensional positioning system (Velmex, Bloomfield, NY), the focal point of the ultrasound beam was placed 3 mm lateral of the sagittal suture and 0.5 mm anterior of the lambdoid suture onto the left hippocampus (Fig E1 [online]) (2).
Dextran and Microbubbles
We used lysine-fixable Texas Red 3 kDa dextran (Life Technologies, Paisley, England) diluted in 100 µL phosphate-buffered saline. SonoVue (Bracco, Milan, Italy) microbubbles were used (volume, 100 µL; concentration, 5 µL/g) and injected intravenously with a 30-gauge catheter through the tail vein over 30 seconds, following dextran administration and after the first 10 seconds of ultrasound emission.
Sonication Protocol
Ultrasound pulses at 1 MHz were administered to the intact scalp and skull. Two pulse sequences were applied: a rapid short-pulse sequence (pulse length, five cycles; pulse repetition frequency, 1.25 kHz; burst length, 10 msec or 13 pulses; burst repetition frequency, 0.5 Hz, 125 bursts; n = 14) or a long-pulse sequence (pulse length, 10 000 cycles; pulse repetition frequency, 0.5 Hz, 125 pulses; n = 14) (Fig 1, A). For both sequences, the peak negative pressure was 0.35 MPa because it is just above the acoustic pressure threshold for drug delivery (Fig E2 [online]). Dextran was injected immediately prior to the ultrasound emission (n = 5) or at 10 (n = 3) or 20 (n = 3) minutes after the end of the ultrasound treatment.
Histologic Staining
Mice were transcardially perfused with 10% formalin (PFA; Sigma Aldrich, St Louis, Mo). Fixed brains, after 6 hours in 15% sucrose and overnight in 30% sucrose, were embedded in optimum cutting temperature compound (OCT; Agar Scientific, Stansted, England) and sectioned into 30-µm horizontal slices (see Appendix E1 [online]). Immunostaining helped detect albumin with a rabbit antimouse serum albumin primary antibody (1:100 overnight; Ab19196; Abcam, Cambridge, England) and a donkey antirabbit IgG H&L Alexa Fluor 488 secondary antibody (1:200 for 2 h; Ab150073; Abcam). For H-E staining, fixed brains (n = 3) were paraffin embedded and sectioned horizontally at 6 µm.
Microscopy and Analysis
Images were acquired using fluorescence (5× and 10×; Zeiss Axio Observer, Oberkochen, Germany) and confocal microscopy (20×; Zeiss LSM-510 inverted, Oberkochen, Germany). Texas Red was excited at 562/40 nm with emissions filtered at 624/40 nm, and Alexa 488 was excited at 470/40 nm with emissions filtered at 525/50 nm. Regions of interest (ROIs) around the left and right hippocampus were selected by using MATLAB R2016b (Mathworks, Natick, Mass) after removing artifacts from these regions. The normalized optical density (NOD) was a normalized measurement of the detected dextran dose and extent of albumin extravasation (12). Pixels with intensities higher than twice the standard deviation of the control ROI pixel intensity were first summed in both ROIs. The sum in the targeted ROI was subtracted by that in the control ROI to obtain the NOD. Delivery was successful if the NOD was at least 1 standard deviation above the control mean. The drug distribution was quantified with the coefficient of variation (COV), that is, ratio of the standard deviation to the mean fluorescence pixel intensity in the targeted region. The COV was calculated for five sections for each brain treated with rapid short-pulse (n = 5) or long-pulse (n = 5) ultrasound.
Acoustic Signal Analysis
Acoustic emissions acquired with the PCD were processed in MATLAB. To obtain the energy, the time-varying voltage signal was squared, integrated over time, and corrected for electronic and experimental noise (23). The duration of emissions was determined with the t80 constant (time required for the energy emissions to reach 80% of the cumulative energy). The spectrum was calculated by using the Fourier transform (see Appendix E1 [online]).
Statistical Analysis
A two-sided Student t test determined whether the NOD, COV, and t80 values were significantly different (P < .01) between rapid short-pulse and long-pulse sequences. The relationship between drug dose and acoustic emissions was assessed with a least-squares linear regression and by calculating the correlation coefficient. All analysis was done by using MATLAB R2016b.
Results
Delivered Dose and Distribution
Ultrasound applied in a rapid short-pulse sequence showed a higher NOD in all mice evaluated, compared with the controls (Fig 2A). The higher NOD was confined to the targeted tissue volume and not observed in untargeted regions. With the long-pulse sequence (Fig 2, B), which deposited 150 times more energy into the tissue, the NOD was 1.2 times higher than with the rapid short-pulse sequence. However, the difference in NOD between the sequences was not significant (P > .01; Fig 2, C).
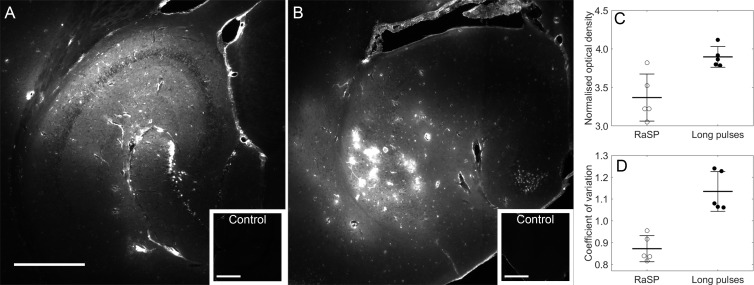
Figure 2:
Distribution and normalized dose of dextran delivered with rapid short-pulse (RaSP) and long-pulse sequences. Fluorescence images (10×) show the drug distribution in the left hippocampus using, A, RaSP and, B, long-pulse sequences. Corresponding right hippocampi, which were control unsonicated regions, are displayed in smaller white boxes on the bottom right of each image. No fluorescence was detectable in these regions. C, Normalized optical density (NOD) and, D, coefficient of variation (COV) of brains treated with both sequences quantify the dose and distribution at peak negative pressure of 0.35 MPa (n = 5), respectively. C, There was no difference in NOD (P > .01), a surrogate measurement of dose, between sequences, while, D, a significant difference in COV, a measure of distribution heterogeneity, was quantified between sequences (P < .001). The white scale bar in A indicates 500 µm.
The rapid short-pulse sequence delivered dextran in a uniform pattern in the ultrasound beam, providing complete coverage of the targeted tissue (Fig 2, A). This contrasted with the more heterogeneous pattern delivered with the long-pulse sequence, where regions with high levels of dextran on the order of square micrometers and regions with low or no detectable levels of dextran were observed within the same treated area (Fig 2, B). The difference in distribution was consistent across all mice tested as the heterogeneity, quantified by the COV, was lower for the rapid short-pulse sequence (P < .001; Fig 2, D).
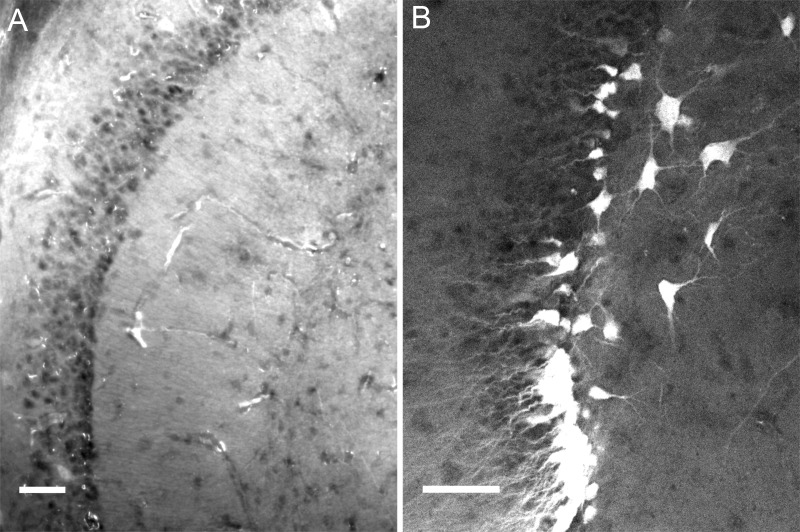
At the microscopic scale, the rapid short-pulse sequence delivered dextran to neurons and to the parenchyma in a uniform distribution (Fig 3, A and B; Fig E3, A–E [online]). Neuronal uptake occurred in multiple regions within the beam, such as in the granule cells of the dentate gyrus (Fig 3, B; Fig E3, D and E [online]). The distribution within the parenchyma was uniform, with less fluorescence only where the dextran was absent within blood vessels, cell bodies, and axons (Fig 3, B; Fig E3, B and C [online]). Dextran was also delivered in other patterns, with uptake observed, although rarely, in glial cells (Fig E3, F, and E4, A [online]). In comparison, the long-pulse sequence (Fig E3, G–L [online]) delivered dextran at high concentrations in some regions (Fig E5, A [online]), such as in and around vessels (Fig E3, H, and E6, B [online]), while there were multiple regions with less or no dextran. In all brains tested, regions of high fluorescence were also detected within glial cells (Fig E3, L and A [online]), such as astrocytes and microglia, and within neurons (Fig E3, J, and E5, B [online]). These data reveal the focused and uniform distribution of drug delivered with the rapid short-pulse sequence.
Figure 3:
Dextran delivery distribution throughout the parenchyma and in neurons when using a rapid short-pulse sequence. Confocal microscopy images (20×) of mouse brain regions exposed to ultrasound after intravenous injection of microbubbles and dextran reveal, A, dextran delivered homogeneously throughout the parenchyma and, B, into neuronal cell bodies and their axons. The white scale bars indicate 50 µm.
Time Window for Molecular Delivery
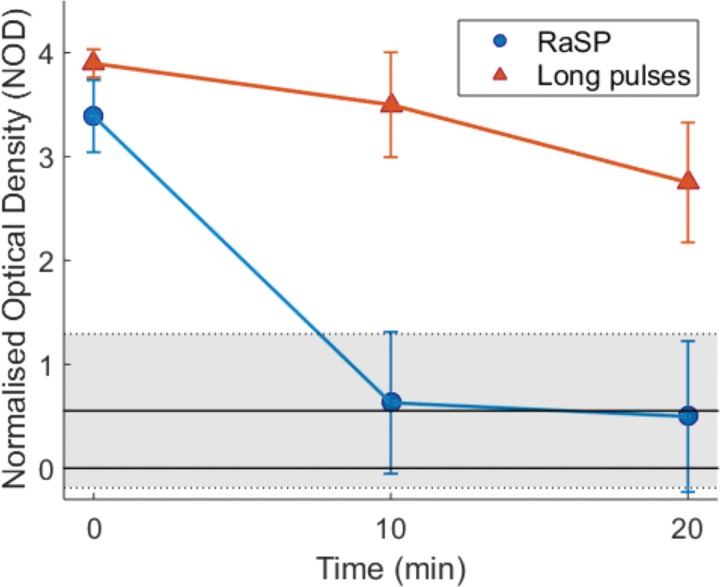
To determine the period of time in which the permeability of the blood-brain barrier increased following a rapid short-pulse sequence, we (S.V.M.) injected dextran at 0 (during), 10, and 20 minutes after ultrasound exposure. When we injected dextran during ultrasound exposure, we detected dextran in the brain with fluorescence microscopy. When dextran was administered 10 or 20 minutes after ultrasound exposure with a rapid short-pulse sequence, we (S.V.M.) did not detect dextran in the brain (Fig 4; Fig E7 [online]). This result suggests that the drug delivery effect with the rapid short-pulse sequence occurs either during or within 10 minutes of the ultrasound exposure. With the long-pulse sequence, we confirmed previous reports that show a prolonged increase in permeability of the blood-brain barrier after ultrasound exposure; in these experiments, the permeability increase was still present after 20 minutes. Thus, the increase in permeability of the blood-brain barrier is much shorter following a rapid short-pulse sequence than a long-pulse sequence.
Figure 4:
Blood-brain barrier closing timeline with rapid short-pulse (RaSP) and long-pulse sequences. With RaSP sequences, the blood-brain barrier closed within 10 minutes (blue line), while the blood-brain barrier remained open at 20 minutes with long pulses (orange line). The shaded region denotes the mean ± standard deviation normalized optical density (NOD) of nonsonicated mice used as a control.
Safety and Predictability
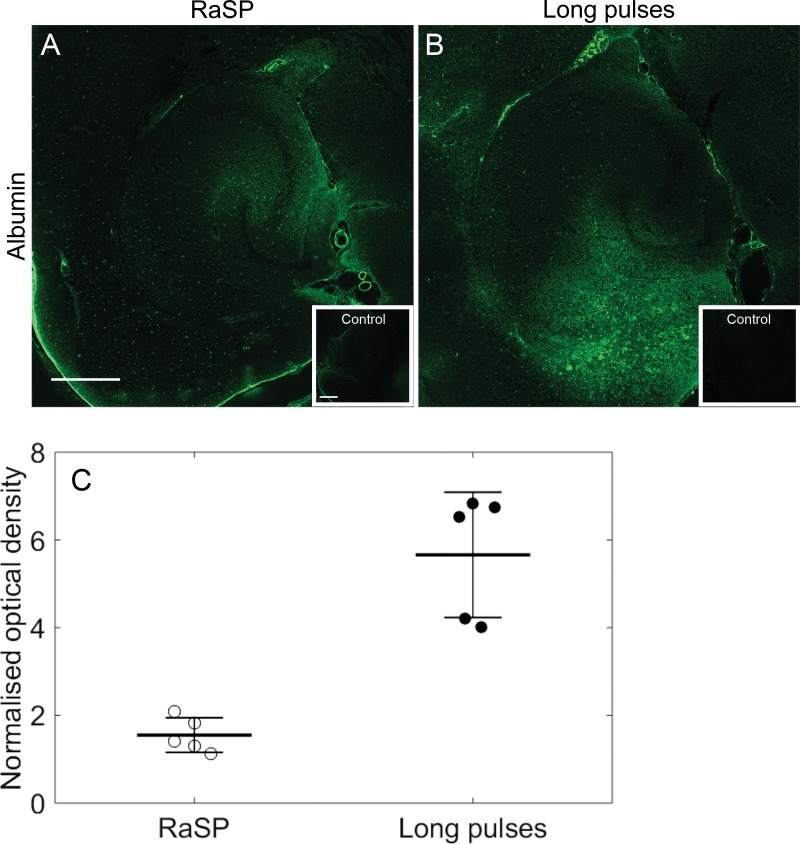
We investigated the relative damage caused by the two sequences by measuring the amount of blood albumin extravasating into the brain by imaging fluorescently labeled antibodies bound to the albumin (Fig 5, A and B). The NOD of albumin-stained fluorescence was 3.7 times lower for rapid short pulses than for long pulses (P < .001; Fig 5 C). Although the distribution of albumin (Fig 5, A and B) was similar to the distribution of dextran (Fig E8, C, and D [online]), dextran was also present where albumin was not. This suggests that the ultrasound delivery pattern may be dependent on the molecule. In addition, H-E staining showed no damage with the rapid short-pulse sequence (Fig E9 [online]). Furthermore, the acoustic emissions generated by the microbubbles exposed to ultrasound using the rapid short-pulse sequence correlated with the relative dose of molecular delivery (r = 0.97) (Fig E10, A [online]), which instead was a weak correlation when long pulses were used (r = 0.21). Thus, the damage caused by ultrasound was mitigated using the rapid short-pulse sequence, which also provided a predictable method of delivery.
Figure 5:
Albumin extravasation with rapid short-pulse (RaSP) and long-pulse sequences. Fluorescence images (10×) show the extravasation of albumin using, A, RaSP and, B, long-pulse sequences with the control regions (small boxes). Albumin staining was performed by immunostaining for serum albumin antibodies. C, A reduction in albumin extravasation was observed with the RaSP sequence compared with the long-pulse sequence (P < .001, n = 5). The white scale bar in A indicates 500 µm.
Acoustic Emissions Analysis
We (S.V.M. and J.J.C.) investigated the acoustic emissions generated by the microbubbles to gain mechanistic insight into the microbubble dynamics that were stimulated in the brain. First, the rapid short-pulse sequence was more efficient at delivering molecules to the brain than the long-pulse sequence. Although the long-pulse sequence deposited 150 times more acoustic energy into the brain, only 70 times more acoustic energy returned to our passive cavitation detection system. Thus, an average cycle in a rapid short-pulse sequence was more efficient, producing 2.2 times more acoustic energy. Second, rapid short-pulse sequences produced acoustic emissions for longer durations than the long-pulse sequences (Fig E10, B–D [online]). The time needed for the energy to reach 80% of its cumulative value (t80 constant) was twice as long (P < .001; Fig E10, B [online]) and the energy remained above the noise at the end of sonication. This may be due to a reduced level of microbubble destruction (Fig E10, C [online]). Third, the type of cavitation generated with the rapid short-pulse sequence was milder than with long-pulse sequences. Whereas long pulses produced broadband emissions and high magnitudes of high-order harmonics and ultraharmonics, which are indicative of high-magnitude inertial cavitation, rapid short-pulse sequences did not have these features (Fig E10, E and F [online]). The rapid short-pulse sequence produced low-magnitude harmonic emissions and very low-magnitude ultraharmonic emissions. The harmonic emissions were broader than observed with the long-pulse sequence because of the shorter pulse lengths.
Discussion
We have shown that ultrasound emitted in a rapid short-pulse sequence, rather than in long pulses, delivers drugs more uniformly into the brain parenchyma (P < .001). With a rapid short-pulse sequence, the delivered dose could be predicted from the acoustic energy emitted from the stimulated microbubbles (r = 0.97). The permeability of the blood-brain barrier returned to control levels in less than 10 minutes and a limited amount of blood albumin was released into the brain, 3.4-fold lower than with long pulses (P < .001). In addition, no vascular or tissue damage from rapid short-pulse exposure was observable from H-E staining.
The improved performance and safety profile of rapid short-pulse ultrasound may have positive implications for the treatment of neurologic diseases, such as Alzheimer disease, Parkinson disease, and brain tumors. These diseases are difficult to treat because they are colocated with healthy tissue and have large regions protected by the blood-brain barrier. The uniform delivery pattern obtained with rapid short-pulse ultrasound enables the drug to cover the intended region in full. With rapid short-pulse ultrasound, the blood-brain barrier also returns to its normal permeability within minutes and only allows a limited amount of albumin into the brain, reducing the likelihood of long-term side effects. For chronic disorders that require repeated drug delivery sessions, like Alzheimer disease, minimizing these side effects is essential. For brain tumors that spread alongside healthy neurons and whose margins are protected by an intact blood-brain barrier, use of rapid short-pulse ultrasound could avoid collateral damage.
The drug delivery improvements with rapid short-pulse ultrasound are thought to be due to the stimulation of better cavitation distribution and a reduction of the cavitation magnitude and diversity (22,23,25). Short pulses alone have not been enough to produce better drug delivery because they required high pressures or delivered a low drug dose (15,26–29). Rapid emission of short pulses has been shown to deliver drugs in a uniform pattern (12), but was achieved with high pressures (0.51 MPa) while no safety benefits nor direct comparison between the two regimes were reported. The designed sequence emits low-energy pulses at a carefully selected pulse repetition frequency to reduce microbubble destruction and allow microbubbles to move between excitations. In addition, these rapid short pulses are thought to cause less microbubble aggregation due to shorter periods of secondary radiation forces. In previous in vitro work, we showed that long pulses increased the number and size of microbubble clusters (24), which may explain why long pulses produce greater vascular effects than those observed with short pulses.
The acoustic emissions of the rapid short-pulse sequence lacked broadband features and persisted across all pulses as intended. Short pulses also reduced the diversity and range of cavitation, which may be why the microbubble emissions with rapid short-pulse ultrasound were a good predictor of drug dose. Thus, to achieve better drug delivery, we had to not only carefully select parameters but also design a pulse shape and sequence structure.
The rapid short-pulse sequence delivered dextran with a safety profile previously unachievable using ultrasound. First, with rapid short-pulse ultrasound, the blood-brain barrier returned to its normal permeability within 10 minutes, the fastest reported so far. With long pulses, the blood-brain barrier remains open for several hours (4–48 hours), depending on the parameters and sensitivity of the permeability assessment method (19,30). Second, the reduction in albumin extravasation with rapid short-pulse ultrasound could mean that similar compounds would also not cross the blood-brain barrier, reducing exposure to foreign substances. Third, less dextran was taken up by glial cells with the rapid short-pulse sequence, which could be linked to a tissue response to damage but also specifically to albumin extravasation. The clearance of albumin following focused ultrasound treatment is mediated by glial rather than neuronal cells (20). The higher extravasation of albumin with the long pulses could be a reason for greater glial cell involvement and dextran uptake within these cells. Lastly, no signs of red blood cell extravasation or morphologic changes to the brain tissue with the rapid short-pulse sequence confirmed an improved safety profile of this sequence compared with long pulses.
Despite the improvements of rapid short-pulse sequences, the possibility of improving these features further remains. Altering the permeability of the blood-brain barrier, even for a short duration, releases not only the drug of interest into the brain, but also unwanted blood-derived compounds (eg, albumin). A possible explanation is that both the dextran (3 kDa) and albumin (67 kDa) are delivered by the same pathway, and that the delivery of the drug will always be accompanied by the release of albumin. However, our data showed that the drug and albumin were delivered with different distributions and that the differences varied throughout the ultrasound beam. This suggests that the opening may have a molecular size threshold and/or a different transfer rate. These opening properties could also vary throughout the ultrasound beam and could depend on the specific cavitation types, magnitudes, durations, and distributions. We are not yet able to conclude whether the drug and albumin are delivered by the same pathway or whether there are multiple pathways of delivery. Another possible limitation is that over the duration of hours, rapid short-pulse sequences may be delivering lower drug transfer yields than long-pulse sequences due to the short duration of permeability change in the blood-brain barrier. However, it may be too early to conclude this because rapid short-pulse sequence parameters, such as center frequency, pressure, pulse length, pulse-repetition frequency, and the total number of pulses emitted, have not yet been optimized for delivery dose. One could also produce a higher delivery yield by sonicating for a longer duration.
In future work, we will seek to understand how a rapid short-pulse sequence delivers drugs across the blood-brain barrier and how this differs from delivery using long pulses. We will also characterize the long-term effects of rapid short-pulse–mediated drug delivery, such as whether microglial cells are activated or inflammation is triggered—responses which have been observed with long pulses. We plan to optimize rapid short-pulse sequences for drug delivery, modifying not only the pulse shape and sequences but also the microbubbles and the protocol of microbubble administration. For example, it may be beneficial to infuse the microbubbles continuously rather than inject them as a bolus. Finally, we plan to explore the use of rapid short-pulse sequences in other organs and diseases and to explore new pulse-sequence designs.
We have introduced a rapid short-pulse ultrasound sequence as a noninvasive and localized method to deliver drugs in a uniform pattern throughout the brain parenchyma with a high rate of neuronal uptake. We achieved high delivery efficiency while altering the permeability of the blood-brain barrier for less than 10 minutes and minimizing the level of blood albumin released into the brain. We achieved this improvement with 150 times less acoustic energy deposited into the brain, suggesting that raising acoustic energies does not always improve performance. Instead, the structure of the ultrasound sequence must be considered in concert with the tissue structure, its vascular flow, and ultrasound beam shape. We anticipate that the design of rapid short-pulse sequences can be optimized for different purposes (eg, sonoporation, sonothrombolysis). As a molecular delivery technique for the brain, rapid short-pulse sequences may potentially be used to deliver compounds for the treatment, diagnosis, and study of neurologic diseases.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgements
The authors thank Magdalena Sastre and Steve Gentleman for discussions and the identification of cells. S.V.M. also wishes to acknowledge Javier Cudeiro Blanco and Dr El Ghamrawy for their help.
S.V.M., T.G.C., and M.J.C. were supported by Engineering and Physical Sciences Research Council (EP/L015226/1; EP/L016737/1). This study was supported by Biotechnology and Biological Sciences Research Council (BB/L015129/1), Wellcome Trust (104931/Z/14/Z), and Alzheimer's Research UK (ARUK-IRG2017A-7). This study used equipment from the Facility for Imaging by Light Microscopy (FILM) at Imperial College London, which is supported by funding from the Wellcome Trust (grant 104931/Z/14/Z) and BBSRC (grant BB/L015129/1).
Disclosures of Conflicts of Interest: S.V.M. disclosed no relevant relationships. A.N.P. disclosed no relevant relationships. T.G.C. disclosed no relevant relationships. M.J.C. disclosed no relevant relationships. J.L. disclosed no relevant relationships. N.J.L. disclosed no relevant relationships. J.J.C. Activities related to the present article: disclosed that this study was funded by the Alzheimer’s Research UK (ARUK-IRG2017A-7) and that the PhD studentships for S.V.M., T.G.C., and M.J.C. were funded by the King’s and Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) and the EPSRC Centre for Neurotechnology (EP/L016737/1). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed receipt of payment for patent US20110295105A1.
Abbreviations:
- COV
- coefficient of variation
- fC
- center frequency
- H-E
- hematoxylin-eosin
- NOD
- normalized optical density
- PCD
- passive cavitation detector
- ROI
- region of interest
References
- 1.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001; 220(3):640–646. [DOI] [PubMed] [Google Scholar]
- 2.Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultra-β plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One 2010;5(5):e10549.20485502 [Google Scholar]
- 3.Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One 2010;5(5):e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 2012;38(10):1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess A, Dubey S, Yeung S, et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014;273(3):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baseri B, Choi JJ, Tung YS, Konofagou EE. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol 2010;36(9):1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs ZI, Kim S, Jikaria N, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A 2017;114(1):E75–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 2004;3(6):527–532. [DOI] [PubMed] [Google Scholar]
- 9.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with inertial cavitation. IEEE Ultrason Symp 2005;2:1249–1252. [DOI] [PubMed] [Google Scholar]
- 10.Downs ME, Buch A, Sierra C, et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One 2015;10(5):e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier A, Canney M, Vignot A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 2016;8(343):343re2. [DOI] [PubMed] [Google Scholar]
- 12.Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci U S A 2011;108(40):16539–16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys Med Biol 2007;52(18):5509–5530. [DOI] [PubMed] [Google Scholar]
- 14.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol 2008;34(7):1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JJ, Selert K, Gao Z, Samiotaki G, Baseri B, Konofagou EE. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cereb Blood Flow Metab 2011;31(2):725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin J, Kong C, Cho JS, et al. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus 2018;44(2):E15. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006;103(31):11719–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 2018;9(1):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control 2013;60(11):2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso A, Reinz E, Fatar M, Hennerici MG, Meairs S. Clearance of albumin following ultrasound-induced blood-brain barrier opening is mediated by glial but not neuronal cells. Brain Res 2011;1411:9–16. [DOI] [PubMed] [Google Scholar]
- 21.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 2010;6(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouliopoulos AN, Li C, Tinguely M, Garbin V, Tang MX, Choi JJ. Rapid short-pulse sequences enhance the spatiotemporal uniformity of acoustically driven microbubble activity during flow conditions. J Acoust Soc Am 2016;140(4):2469–2480. [DOI] [PubMed] [Google Scholar]
- 23.Pouliopoulos AN, Bonaccorsi S, Choi JJ. Exploiting flow to control the in vitro spatiotemporal distribution of microbubble-seeded acoustic cavitation activity in ultrasound therapy. Phys Med Biol 2014;59(22):6941–6957. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus C, Pouliopoulos AN, Tinguely M, Garbin V, Choi JJ. Clustering dynamics of microbubbles exposed to low-pressure 1-MHz ultrasound. J Acoust Soc Am 2017;142(5):3135–3146. [DOI] [PubMed] [Google Scholar]
- 25.Choi JJ, Coussios CC. Spatiotemporal evolution of cavitation dynamics exhibited by flowing microbubbles during ultrasound exposure. J Acoust Soc Am 2012;132(5):3538–3549. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused-ultrasound disruption of the blood-brain barrier using closely-timed short pulses: influence of sonication parameters and injection rate. Ultrasound Med Biol 2011;37(4):587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bing KF, Howles GP, Qi Y, Palmeri ML, Nightingale KR. Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in Mice. Ultrasound Med Biol 2009;35(8):1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynynen K, McDannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison). Ultrasound Med Biol 2003;29(3):473–481. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, Chen Y, Liu J, et al. Blood-brain barrier disruption induced by diagnostic ultrasound combined with microbubbles in mice. Oncotarget 2017;9(4):4897–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med 2012;67(3):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.