Abstract
The 1,2,3-triazole-imidazole derivatives (TA-IM) were prepared as fluorescent probes for silver ions detection. The design principle is the incorporation of an intramolecular H-bond between the imidazole and triazole moiety that enables a co-planar conformation to achieve fluorescence emission in UV-blue range. Screening of different metal ions revealed excellent binding affinity of this new class of compounds toward silver ions in aqueous solution. The novel probe provided ultrafast detection (< 30 s) even for a very low concentration of silver ions (at nM range) with good linear correlation, making it a practical sensor for detection of silver ions.
Graphical Abstract
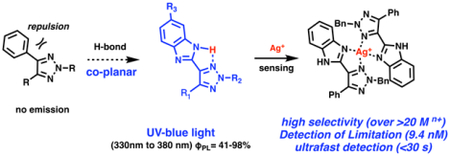
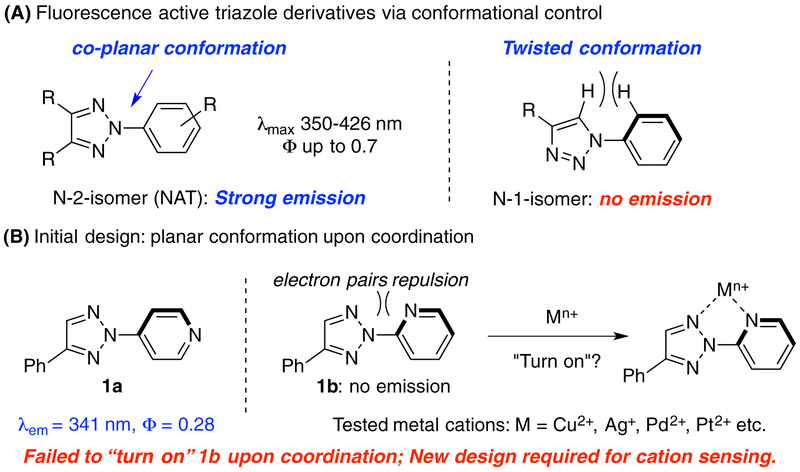
Fluorescence active molecules are of great importance in chemical,1 biological2 and medicinal research.3 New class of small organic molecules with good fluorescence emission could offer opportunity for interesting applications.4 Over the past decade, our group has been working on the development of functional 1,2,3-triazole (TA) as ligands for metal coordination.5 A series of triazole ligands have been identified to promote metal-catalyzed reactions.6 Upon synthesis of triazole derivatives,7 we found that N-2 aryl triazole (NAT) showed strong emission in UV-blue range (λmax between 380 nm and 430 nm). In contrast, the N-1 isomer exhibited almost no emission (Φ< 0.02). According to our structural analysis, strong fluorescence emission can be attributed to the co-planar conformation between triazole ring and N-2 aryl groups (Scheme 1A).8
Scheme 1.
Design of triazole-based fluorescent probes
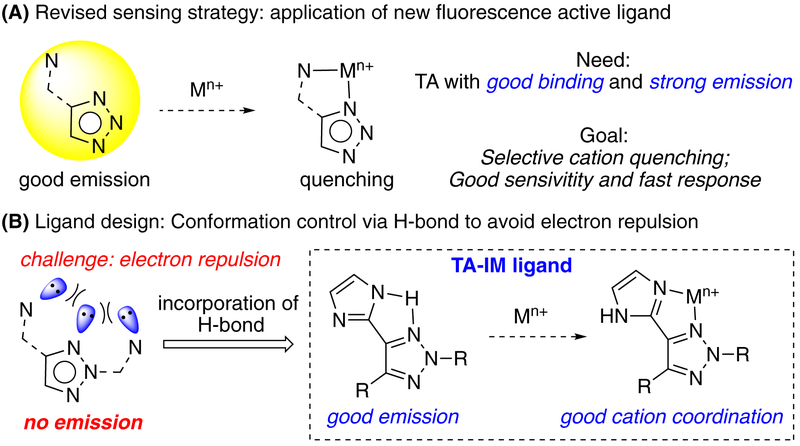
Based on these unique features,9 we wondered whether 1,2,3-triazole can be developed as novel fluorescence probe for metal cation detection upon coordination. Herein, we report imidazole substituted 1,2,3-triazole (TA-IM) as a new class of fluorescent active compounds with excellent selectivity toward Ag+ (over 20 other tested cations) in aqueous media. This new fluorescent probe also gave high sensitivity with linear concentration-emission correlation. Moreover, compared with other reported Ag+ detecting methods, TA-IM demonstrated ultrafast response time less than 30 seconds upon coordination. All these features make TA-IM a new practical sensor for Ag+ detection.
Our interest in developing triazole derivatives as metal cation sensor was originated from its conformational analysis of N-2-aryl triazole (NAT) fluorophore. As shown in Scheme 1B, we have previously demonstrated that 4-pyridyl triazole 1a showed good fluorescence emission (Φ = 0.28), while the 2-pyridyl isomer 1b was fluorescence-inactive (no emission). X-ray crystal structure of 1b revealed the twisted conformation between the two aromatic rings, likely due to repulsion between the lone pair electrons of nitrogen.8 Based on this analysis, we postulated that metal coordination between the two nitrogen atoms in 1b could force the two aromatic rings to adopt a co-planar conformation and, therefore, may “turn on” the fluorescence emission upon coordination. To confirm this idea, we performed the coordination experiments of 1b (TA-Py) with various metal cations (Cu2+, Ag+, Pd2+, Pt2+, Ni2+ etc.). Unfortunately, no fluorescence emission change was observed in all cases (see detail in SI, Figure S1). In spite of the possible co-planar conformation upon chelation, the results suggested that NAT exciting state was nevertheless quenched due to potential electron and/or energy transfer upon cation binding.10 As a result, the initial turn-on sensor hypothesis did not work, and we turned to design the turn-off sensor with similar framework.
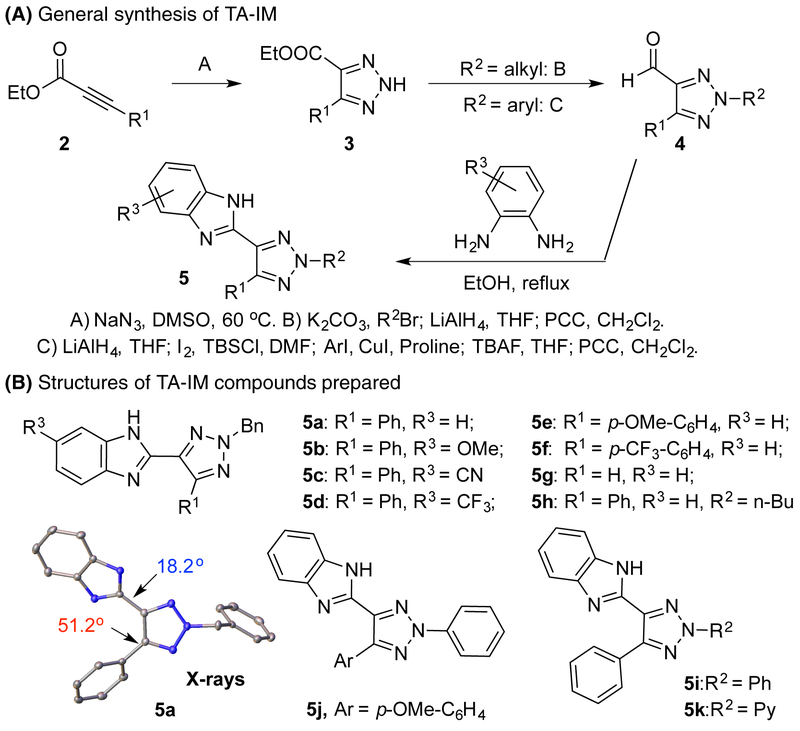
With the assumption that fluorescence quenching occurs upon metal coordination for TA-Py ligand 1b, we sought out to develop new triazole ligands with both good fluorescence emission and metal binding ability to achieve metal sensing (Scheme 2A).11 As discussed above, no fluorescence emission was observed for TA-Py ligand due to the nitrogen lone pair electron repulsion (Scheme 2B). To address this problem while keeping the bi-dentate binding nature, we proposed a new ligand system as triazole-imidazole (TA-IM). The key of our design is the incorporation of intramolecular H-bond to avoid lone pair electron repulsion so to enhance the formation of co-planar conformation for effective fluorescence emission while maintains the bi-dentate nitrogen binding mode. As shown in Figure 1A, a group of TA-IM compounds was successfully prepared. The structures of these TA-IM are summarized in Figure 1B.
Scheme 2.
Revised sensing strategy with TA-IM ligand
Figure 1.
General synthetic route for TA-IM ligands
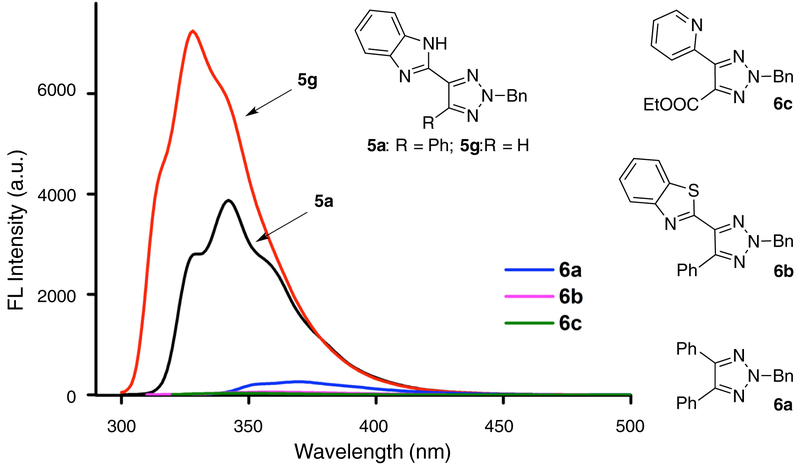
X-ray crystal structures of TA-IM were successfully obtained with several TA-IM compounds (5a, 5b, 5d, 5i and 5k), which verified the proposed N-2 isomer conformation. Moreover, as highlighted in Figure 1B, compared with C-5 phenyl ring, the C-4 imidazole ring had a much smaller dihedral angle (18.2°) with triazole ring, which suggested the formation of intramolecular H-bond. With the TA-IM successfully prepared and N-2-isomer identified, we evaluated their fluorescence emission to verify our initial design that intramolecular H-bond plays a crucial role in providing conformational control for effective fluorescence emission. The emission spectra of several representative TA-derivatives including TA-IM are shown in Figure 2
Figure 2.
Fluorescence emission of some TA-IM ligandsa
a Fluorescence emission of compound 5a-6c. Concentration: 20 μmol/L in EtOH.
As expected, TA-IM 5a and 5g displayed strong fluorescence emission with quantum yields (ΦPL) of 0.77 and 0.98 respectively. The stronger emission obtained with 5g over 5a is likely due to the reduced steric hindrance of C-5-H in 5g over C-5-Ph in 5a. It suggested that 5g favored the co-planar conformation. Switching imidazole to phenyl (6a), thiazole (6b) and pyridine (6c) almost shut down the fluorescence emission completely, which highlighted the crucial role of intramolecular H-bond in achieving the fluorescence-active TA-ligand. Importantly, fluorescence intensity of 5a in solution remained the same even after ten months (Figure S6), suggesting the excellent stability of the new TA-IM fluorophore. Detailed excitation and emission information of all TA-IM substrates 5 are summarized in Table 1.
Table 1.
Optical data of TA-IM ligandsa
| Ligand | Excitation λmax (nm) |
Emission λmax (nm) |
Stokes shift (nm) |
ΦPL (%) |
Intensity (a. u.) |
|---|---|---|---|---|---|
| 5a | 290 | 342 | 52 | 77 | 3837 |
| 5b | 310 | 364 | 54 | 64 | 2318 |
| 5c | 309 | 346 | 37 | 45 | 3154 |
| 5d | 293 | 329 | 36 | 41 | 2062 |
| 5e | 290 | 352 | 62 | 64 | 1836 |
| 5f | 293 | 349 | 56 | 86 | 2935 |
| 5g | 296 | 328 | 32 | 98 | 7246 |
| 5h | 292 | 341 | 49 | 72 | 3576 |
| 5i | 291 | 368 | 77 | 93 | 2780 |
| 5j | 309 | 378 | 69 | 66 | 2532 |
| 5k | 309 | 380 | 71 | 54 | 1717 |
| 6a | 317 | 370 | 53 | - | 258 |
| 6b | - | - | - | - | 47 |
| 6c | - | - | - | - | 31 |
Fluorescence emission of compound 5a-6c. Concentration: 20 μmol/L in EtOH.
To As shown in Table 1 all tested TA-IM 5 exhibited strong UV-blue fluorescence emission with λmax between 330 nm and 380 nm. Electron donating group on imidazole (5b) caused emission red-shift while electron withdrawing group resulted in slightly blue-shift (5d). No significant electronic effect was observed on the phenyl substitution (5e vs 5f). Large red-shift was obtained with conjugated N-2 aryl substrates (5i, 5j and 5k), similar to the previously reported N-2-aryl triazoles (NAT) system. Compared with NAT, TA-IM possessed similar UV-blue emission and comparable fluorescence intensity/efficiency, suggesting similar planar intramolecular charge transfer (PICT) mechanism as proposed. With the fluorescence-active ligand available, we tested whether metal cation coordination may influence TA-IM fluorescence emission.
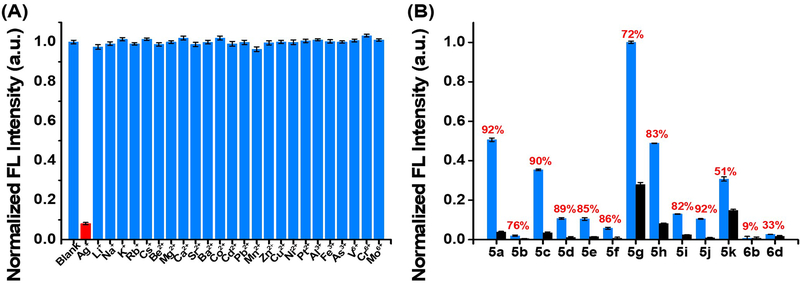
As shown in Figure 3A, treating a solution of 5a with various metal cations (24 cations tested) showed almost no fluorescence change except for Ag+ cation. Notably, this highly selective Ag+ induced fluorescence quenching is very efficient (92%) under mild conditions (room temperature). Impressively, this Ag+ sensing was very robust, showing almost no influence by other cations and anions (no significant change while combining Ag+ with more than 30 cations and anions, see details in Figure S7, S8). This result is interesting and suggests that TA-IM can be developed as a potential fluorescent sensor toward Ag+ detection. Since silver and silver ion-containing materials have been increasingly used in the industry12. A large amount of non-biodegradable silver ion from industrial wastes has been discharged into the environment.13 This could cause a severe harm to the environment14 and human health.15 Hence, establishment of an efficient and reliable sensor for the Ag+ detection is great demand.
Figure 3.
A) The high selectivity of the TA-IM for detection of Ag+. (B) Sensitivity of all TA-IM (5a-5k) in response to silver cation.a, b
a Concentration: 5a-5k, 2 μmol/L; metal ions, 2 μmol/L; EtOH: Hepes, v:v=1:99.
b The blue bar is the normalized FL intensity of 5a-6d; the blank bar is the FL intensity of 5a-6d after treating with Ag+; the red number is quenching ratio of FL.
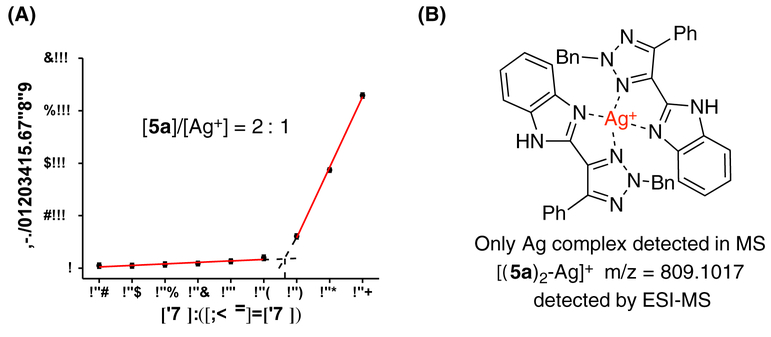
To identify the best TA-IM in Ag+ sensing, comparisons of quenching efficiency among all prepared TA-IMs (5a-5k) were performed. Compound 5a was identified as the optimal ligand with the highest sensitivity (92%) in response to Ag+ binding (Figure 3B). To explore Ag+/TA-IM coordination, standard titration was performed (Job’ method, Figure 4A). A 2:1 ligand/cation ratio was revealed. ESI-MS further confirmed the formation of [L2Ag]+ complex with the detection of m/z at 809.10 as the major complex peaks (Figure 4B, see detailed MS spectra in Figure S9).
Figure 4.
(A) Job’s plot: total concentration of 5a aand Ag+ was 10 μmol/L. (B) ESI-MS of [(5a)2-Ag]+ ion.
a EtOH: Hepes, v:v = 1:99.
Based on Benesi-Hildebrand equation, the binding association constant Ka was determined as 1.01×107 M−2, suggesting a strong coordination between ligand and Ag+ (Figure S10). The fluorescence lifetime of 5a was monitored (Figure S11), giving no changes in the absence and presence of Ag+. This result suggested that the observed silver cation sensing was attributed to static quenching as proposed.16
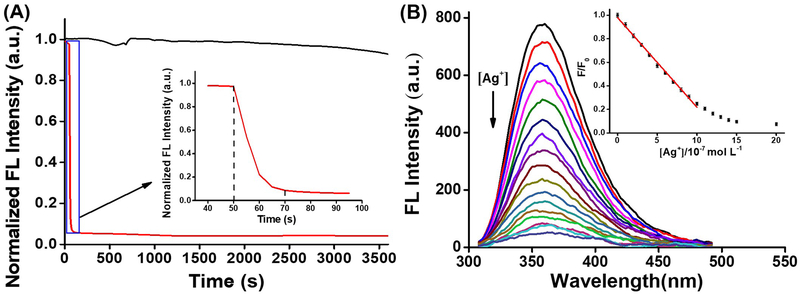
One important feature for any cation fluorescence probe is the response time. As shown in Figure 5A, solution fluorescence intensity decreased immediately upon treating with silver cation and reached an equilibrium within 20 seconds. To the best of our knowledge, this sensing response rate is faster than any previously reported Ag+ fluorescent probes.17 Moreover, FL emission intensity of 5a solution remained the stable even after 120 min irradiation treatment with xenon lamp (Figure S12). Detections within pH range from 4.0 to 9.0 were performed with no clear drop of sensitivity and stability (Figure S13). All these features (ultrafast response time, excellent stability and wide pH operating range in aqueous medium) warrant TA-IM a promising fluorescent sensor for Ag+ detection.
Figure 5.
(A) Time dependent titration of 5a (2 μmol/L) with Ag+ (2 μmol/L); (B) Linear correlation of [Ag]+ and FL emission.a
a EtOH: Hepes, v:v=1:99.
Finally, to establish the quantitative detection curve, titration of Ag+ with TA-IM ligand was performed (Figure 5B). Under the concentration from 1.0×10−7 to 1.0×10−6 mol/L, a good linear correlation between fluorescent intensity and Ag+ concentration was obtained with R2. = 0.9948. The detection limit (LOD) was calculated to be 9.4 nmol/L (based on S/N = 3, n = 20). To the best of our knowledge, this is much lower than previously reported fluorescent probes (see detailed comparison of TA-IM with reported Ag+ sensors in SI, Table S5).
Conclusions
In summary, we have successfully developed triazole-imidazole ligands as highly selective fluorescent probes for detection of silver ions. The introduction of intramolecular H-bond allows TA-IM to exhibit strong emission in both organic and aqueous solutions. This sensor can be used in aqueous media with ultrafast response time (< 30 s), which highlighted the practical advantages of this new cation probe and importance of these triazole-based fluorescence compounds in chemical and material research.
Supplementary Material
Acknowledgements
We are grateful to the NSF (CHE-1665122), NIH (1R01GM120240–01), NSFC (21629201) and the Development Project of the Pharmaceutical Industry of Jilin Province (20150311070YY; 20170307024YY) for financial support.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: Experimental procedures, characterization data, NMR spectra and X-ray data for 5a(CCDC 1835133), 5b(CCDC 1835134), 5d(CCDC 1835139), 5i(CCDC 1835140) and 5k (CCDC 1835141). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/x0xx00000x
Notes and references
- 1.(a) Hong Y, Lam J and Tang B, Chem. Commun, 2009, 4332–4353.; [DOI] [PubMed] [Google Scholar]; (b) Viles JH, Coord. Chem. Rev, 2012, 256, 2271–2284; [Google Scholar]; (c) Zhu H, Fan J, Wang B and Peng X, Chem. Soc. Rev, 2015, 44, 4337–4366; [DOI] [PubMed] [Google Scholar]; (d) Jung S and Chen X, Adv. Healthc. Mater, 2018, 7, 1800252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Guo Z, Park S, Yoon J and Shin I, Chem. Soc. Rev, 2014, 43, 16–29;. [DOI] [PubMed] [Google Scholar]; (b) Ding D, Li K, Liu B and Tang B, Accounts. chem. Res, 2013, 46, 2441–2453. [DOI] [PubMed] [Google Scholar]
- 3.(a) Qian X and Xu Z, Chem. Soc. Rev, 2015, 44, 4487–4493. [DOI] [PubMed] [Google Scholar]; (b) Cheng H, Yoon J and Tian H, Coord. Chem. Rev, 2018, 372, 66–84. [Google Scholar]; (c) Kobayashi H, Ogawa M, Alford R, Choyke PL and Urano Y, Chem. Rev, 2009, 110, 2620–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Chan J, Dodani S and Chang C, Nat. Chem, 2012, 4, 973–984.; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Formica M, Fusi V, Giorgi L, Micheloni M, Coord. Chem. Rev 2012, 256, 170–192; [Google Scholar]; (c) Hyman LM and Franz KJ, Coord. Chem. Rev, 2012, 256, 2333–2356; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gao M and Tang BZ, ACS sensors, 2017, 2, 1382–1399. [DOI] [PubMed] [Google Scholar]
- 5.(a) Aromí G, Barrios L and Roubeau O,; Gamez P, Coord. Chem. Rev, 2011, 255, 485–546. [Google Scholar]; (b) Huang D, Zhao P and Astruc D, Coord. Chem. Rev, 2014, 272, 145–165. [Google Scholar]
- 6.(a) Yan W, Ye X, Akhmedov NG, Petersen JL and Shi X, Org. Lett, 2012, 14, 2358–2361; [DOI] [PubMed] [Google Scholar]; (b) Cai R, Yan W, Bologna MG, de Silva K, Ma Z, Finklea HO, Petersen JL, Li M and Shi X, Org. Chem. Front, 2015, 2, 141–144; [Google Scholar]; (c) Hosseyni S, Ding S, Su Y, Akhmedov N and Shi X, Chem. Commun, 2016, 52, 296–299. [DOI] [PubMed] [Google Scholar]; (d) Cai R, Ye X, Sun Q, He, He Y, Ma Sand Shi X, ACS Catal, 2017, 7, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen Y, Yan W, Akhmedov N and Shi X, Org. Lett, 2010, 12, 344–347; [DOI] [PubMed] [Google Scholar]; (f) Wang D, Ye X and Shi X, Org. Lett, 2010, 12, 2088–2091. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chen Y, Liu Y, Petersen JL and Shi X, Chem. Commun, 2008, 3254–3256; [DOI] [PubMed] [Google Scholar]; (b) Sengupta S, Duan H, Lu W, Petersen JL and Shi X, Org. Lett, 2008, 10, 1493–1496. [DOI] [PubMed] [Google Scholar]
- 8.(a) Liu Y, Yan W, Chen Y, Petersen JL and Shi X, Org. Lett, 2008, 10, 5389–5392; [DOI] [PubMed] [Google Scholar]; (b) Yan W, Wang Q, Lin Q, Li M, Petersen JL and Shi X, Chem. Eur. J, 2011, 17, 5011–5018. [DOI] [PubMed] [Google Scholar]
- 9.(a) Duan H, Sengupta S, Petersen JL and Shi X, Organometallics, 2009, 28, 2352–2355; [Google Scholar]; (b) Liao W, Chen Y, Liu Y, Duan H, Petersen JL and Shi X, Chem. Commun, 2009, 6436–6438; [DOI] [PubMed] [Google Scholar]; (c) Chen Y, Wang D, Petersen JL, Akhmedov NG and Shi X, Chem. Commun, 2010, 46, 6147–6149; [DOI] [PubMed] [Google Scholar]; (d) Ye X, He Z, Ahmed T, Weise K, Akhmedov NG, Petersen JL and Shi X, Chem. Sci, 2013, 4, 3712–3716; [Google Scholar]; (e) Ghosh D, Rhodes S, Hawkins K, Winder D, Atkinson A, Ming W, Padgett C, Orvis J, Aiken K and Landge S, New J. Chem, 2015, 39, 295–303; [Google Scholar]; (f) Scattergood PA, Sinopoli A and Elliott PIP, Coord. Chem. Rev, 2017, 350, 136–154; [Google Scholar]; (j) Meisner QJ, Accardo JV, Hu G, Clark RJ, Jiang DE and Zhu L, J. Phys. Chem. A, 2018, 122, 2956–2973;. [DOI] [PubMed] [Google Scholar]
- 10.(a) Aromí G, Barrios LA, Roubeau O and Gamez P, Coord. Chem. Rev, 2011, 255, 485–546; [Google Scholar]; (b) Huang D, Zhao P and Astruc D, Coord. Chem. Rev, 2014, 272, 145–165; [Google Scholar]
- 11.For review of fluorescent sensor for meal detection:Jiang P and Guo Z, Coord. Chem. Rev, 2004, 248, 205–229;Xu Z, Chen X, Kim HN and Yoon J, Chem. Soc. Rev, 2010, 39, 127–137;Aragay G, Pons J and Merkoci A, Chem. Rev, 2011, 111, 3433–3458;Jeong Y and Yoon J, Inorg. Chim. Acta, 2012, 381, 2–14;Kim HN, Ren WX, Kim JS and Yoon J, Chem. Soc. Rev, 2012, 41, 3210–3244;Yin J, Hu Y and Yoon J, Chem. Soc. Rev, 2015, 44, 4619–4644;Kaur B, Kaur N and Kθar S, Coord. Chem. Rev, 2018, 358, 13–69:Sivaraman G, Iniya M, Anand T, Kotla NG, Sunnapu O, Singaravadivel S, Gulyani A and Chellappa D, Coord. Chem. Rev, 2018, 357, 50–104.
- 12.Wen Y, Xing F, He S, Song S, Wang L, Long Y, Li D and Fan C, Chem. Commun, 2010, 46, 2596–2598. [DOI] [PubMed] [Google Scholar]
- 13.Mao K, Wu Z, Chen Y, Zhou X, Shen A and Hu J, Talanta, 2015, 132, 658–663. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhou Y, Yoon J and Kim J, Chem. Soc. Rev, 2011, 40, 3416–3429. [DOI] [PubMed] [Google Scholar]
- 15.(a) Lansdown A, Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use, Royal Society of Chemistry, UK, 2010; [Google Scholar]; (b) Tupling R and Green H, J. Appl. Physiol,2002, 92, 1603–1610; [DOI] [PubMed] [Google Scholar]; (c) Gould G, Colyer J, East J, and Lee A, J. Biol. Chem, 1987, 262, 7676–7679. [PubMed] [Google Scholar]
- 16.(a) Kim I, Lee NE, Jeong YJ, Chung YH, Cho BK and Lee E, Chem. Commun, 2014, 50, 14006–14009; [DOI] [PubMed] [Google Scholar]; (b) Fang Y, Li C, Wu L, Bai B, Li X, Jia Y, Feng W and Yuan L, Dalton Trans, 2015, 44, 14584–14588. [DOI] [PubMed] [Google Scholar]
- 17.Methods have been applied for Ag(I) determination, such as atomic absorption spectrometry, see:Liang P, and Peng L, Microchimica Acta, 2010, 168, 45–50;Inductively coupled plasma mass spectrometry:Gautier C, Bourgeois M, Isnard H, Nonell A, Stadelmann G, and Goutelard F, J. Chromatogr. A, 2011, 31, 5241–5247;voltammetry:Wang F, Liu Q, Wu Y and Ye B, J. Electroanal. Chem, 2009, 630, 49–54;Fluorescence probe, see:Singha S, Kim D, Seo H, Cho SW and Ahn KH, Chem. Soc. Rev, 2015, 44, 4367–4399;Zhang JF, Zhou Y, Yoon J and Kim JS, Chem. Soc. Rev, 2011, 40, 3416–3429.Xu L, Xu Y, Zhu W, Yang C, Han L and Qian X, Dalton Trans, 2012, 41, 7212–7217;Zhang L, Jian Y, Wang J, He C, Li X, Liu T and Duan C, Dalton Trans, 2012, 41, 10153–10155;Sun J, Yue Y, Wang P, He H and Jin Y, J. Mater. Chem. C, 2013, 1, 908–913;Zhou YP, Liu EB, Wang J and Chao HY, Inorg. Chem, 2013, 52, 8629–8637;Anand T, Sivaraman G, Anandh P, Chellappa D and Govindarajan S, Tetrahedron Lett, 2014, 55, 671–675;Bian L, Ji X and Hu W, J. Agr. Food Chem, 2014, 62, 4870–4877;Zhang Y, Jiang H and Wang X, Anal Chim Acta, 2015, 870, 1–7;Kursunlu AN and Güler E, J. Mol. Struct, 2017, 1134, 345–349;Wu H, Jia J, Xu Y, Qian X and Zhu W, Sens. Actuators B, 2018, 265, 59–66;Upadhyay PK, Marpu SB, Benton EN, Williams CL, Telang A and Omary MA, Anal. Chem, 2018, 90, 4999–5006.Singha S, Kim D, Seo H, Cho SW and Ahn KH, Chem. Soc. Rev, 2015, 44, 4367–4399.Shi D, Wei X, Sheng Y, Zang Y, He X, Xie J, Liu G, Tang Y, Li J and Chen G, Sci. Rep, 4, 4252–4258.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.