Abstract
Co-infection with HIV and hepatitis C virus (HCV) results in a three-fold increase in progression to end stage liver disease and cirrhosis compared to HCV alone. Although curative treatments exist, less than one quarter of people with HCV are linked to care and even fewer have received treatment. The Care2Cure study is a single-blinded, randomized controlled trial to improve the HCV care continuum among people co-infected with HIV. This ongoing study tests whether a nurse case management intervention can 1) improve linkage to HCV care and 2) decrease time to HCV treatment initiation among 70 adults co-infected with HIV who are not engaged in HCV care. The intervention is informed by the Andersen Behavioral Model of Health Services Use and consists of nurse-initiated referral, strengths-based education, patient navigation, appointment reminders, and care coordination for drug-drug interactions in the setting of HIV primary care. Validated instruments measure participant characteristics including HCV knowledge, substance use, and depression. The primary outcome is linkage to HCV care (yes/no) within 60 days. This protocol paper describes the first clinical trial to examine the effects of a nurse case management intervention to improve the HCV care continuum among people co-infected with HIV/HCV in the era of all-oral HCV treatment. We describe our work in progress, challenges encountered, and strategies to engage this hard-to-reach population.
Keywords: AIDS, health care delivery, nursing care/interventions, randomized controlled trials, social and economic aspects of illness
Chronic infection with hepatitis C virus (HCV) is a leading cause of liver cancer and the most common non-AIDS-related cause of death among persons living with HIV (PLWH) (Centers for Disease Control and Prevention, 2016; Ly et al., 2012). One in four people with HIV are co-infected with HCV, and 80% of injection drug users who have HIV also have HCV (Centers for Disease Control and Prevention, 2016). HIV increases relative risk of HCV-related progression to cirrhosis and end stage liver disease three-fold (Deng, Gui, Zhang, Gao, & Yang, 2009; Graham et al., 2001; Lo Re et al., 2014).
Prior to 2014, HCV treatment for PLWH was less effective and highly toxic with interferon injections, but contemporary all-oral direct acting antivirals (DAAs) are exceptionally effective, shorter in duration and much less toxic in this population (Thomas, 2014). With new advances in treatment, we have the opportunity to cure HCV in at least 94– 97% of people, including PLWH (Naggie et al., 2015; Sulkowski et al., 2015; D. Wyles et al., 2017; D. L. Wyles et al., 2015). However, medication availability and the promise of HCV cure alone are not sufficient to engage all people in care. Challenges across the HCV continuum of care persist, especially in the HIV-co-infected population, indicating a need for interventions to minimize barriers to linking HIV/HCV co-infected patients into HCV care and providing support for this population to succeed in treatment (Cachay et al., 2014).
The greatest gap in the HCV care cascade in the United States falls between diagnosis and treatment initiation (Cachay et al., 2014; Linas et al., 2014; Yehia, Schranz, Umscheid, & Lo Re, 2014). As few as half of those with an HCV diagnosis are linked to HCV care (Yehia et al., 2014), while in a multi-site study of 1,303 HIV/HCV co-infected patients linked to care, researchers found that only half were prescribed DAAs and 43% started a DAA between 2014 and 2017 (Jayaweera et al., 2018). Some of this gap in linkage to care and treatment initiation may be attributed to the low perceived threat of HCV and, as such, a low perceived need for HCV treatment and limited motivation (M. Harris & Rhodes, 2013; Yap et al., 2014). Likewise, U.S. adults with HCV have reported difficulty in accessing HCV-treating providers and navigating the healthcare system (Zickmund, Campbell, Tirado, Zook, & Weinrieb, 2012). In particular, with few overt symptoms, HCV is a “silent epidemic,” often taking a lower priority among PLWH and their providers compared to other comorbidities (Alavi et al., 2013; Reiberger et al., 2011; C. Treloar, Newland, Rance, & Hopwood, 2010), whereas HIV poses a greater perceived threat and a more imminent one (Munoz-Plaza et al., 2008; Wagner et al., 2009).
The low perceived threat of HCV is exacerbated by a lack of knowledge about HCV and available therapies (Coupland, Day, Levy, & Maher, 2009; Carla Treloar, Hull, Dore, & Grebely, 2012; Zickmund et al., 2012). While HCV treatment initiation is associated with greater HCV knowledge (Grebely et al., 2011), lack of knowledge is a self-reported barrier to starting HCV treatment in up to 78 percent of patients (Alavi et al., 2013; Mehta et al., 2008). It is important to note that peer networks facilitate an exchange of 42% of HCV-related information (Watson et al., 2007), yet this peer pipeline can also focus on the negative effects of treatment, many of which have been eliminated with newer DAA-based regimens (North, Devereaux, Pollio, Hong, & Jain, 2014; Carla Treloar et al., 2012; Zickmund et al., 2012). Clearly, interventions to increase HCV knowledge, motivation, and access are needed to improve linkage to HCV care.
Prior interventions to promote the care continuum have included a variety of approaches with some success. For example, brief HCV education alone was shown to significantly increase knowledge and linkage to HCV care by 14–25% (Chen et al., 2013; Gupta, Romney, Briggs, & Benker, 2007; Surjadi, Torruellas, Ayala, Yee, & Khalili, 2011; Tyler et al., 2014). Similarly, nurse case management and care coordination have also been shown to improve linkage to HIV or HCV care by up to 30% (Craw et al., 2008; Gardner et al., 2005; Tyler et al., 2014). Reminder systems using text and phone messages have also been effective strategies in increasing linkage to HIV care and substance use services in combination with other interventions, but have not been tested in the context of HCV care (Farmer, Brook, McSorley, Murphy, & Mohamed, 2014; Finitsis, Pellowski, & Johnson, 2014; van Velthoven, Brusamento, Majeed, & Car, 2013). Yet, a critical gap in the existing approaches is that few linkage to care studies target HCV; the majority of evidence comes from similar populations, including PLWH and substance use disorders. Those that are targeted towards HCV were conducted prior to the era of DAAs, often excluding persons with mental illness, substance use, or HIV co-infection.
In addition, HIV-HCV drug interactions will exist for up to 88% of PLWH (Patel et al., 2015). Because cure rates are now similar between HCV mono- and HIV/HCV co-infected individuals, the only difference in management of HCV in PLWH is the need to address drug interactions (American Association for the Study of Liver Diseases & Infectious Diseases Society of America, 2018). A large proportion of PLWH who are initiating HCV treatment will have to modify their HIV treatment regimen to accommodate these drug-drug interactions, but modifying HIV treatment regimens add a further delay and deter PLWH from starting HCV treatment, as well as negatively impact quality of life and increase HIV symptom burden (Sherr et al., 2007). Moreover, HCV cure rates may be lower in persons who switch antiretroviral therapy (ART) to accommodate DAAs (Falade-Nwulia et al., 2017). The addition of care coordination for ART modification may address this important new barrier for PLWH. To our knowledge, no published interventions to date have sought to support ART modifications to accommodate HIV/HCV drug interactions.
The Care2Cure intervention was designed to address these gaps by testing comprehensive, multi-component nurse case management to improve the HCV care continuum among vulnerable HIV co-infected adults with HCV. The aims of the Care2Cure Study are to: 1) test whether a nurse case management intervention will increase linkage to HCV care among persons with HIV/HCV co-infection compared to usual care; and 2) determine if a nurse case management intervention will decrease time to HCV treatment initiation among persons with HIV/HCV co-infection compared to enhanced usual care. This paper describes the protocol of the randomized, single blinded, controlled trial. The SPIRIT Statement checklist was adhered to in reporting this intervention protocol.
Methods
Study Aim and Design
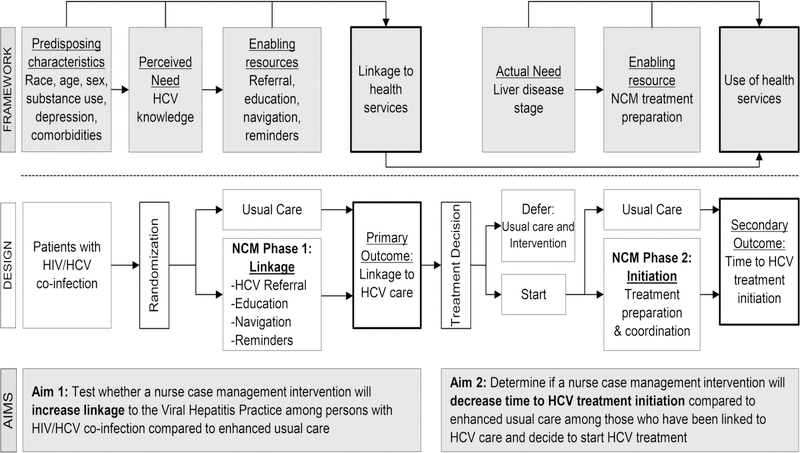
The Care2Cure study is designed to evaluate the preliminary efficacy of a standardized nurse case management intervention to improve the HCV care continuum among PLWH in a randomized controlled trial. PLWH who are co-infected with chronic HCV and not engaged in HCV care are randomized to enhanced usual care or usual care plus HCV nurse case management (NCM) and followed for six months. After baseline assessment, participants are randomly assigned to the intervention arm in a 1:1 allocation using a block randomization scheme. Participants are evaluated at 60 days for linkage to care and 6 months for time to treatment initiation. We hypothesize that a) a higher proportion of participants in the intervention arm will attend an appointment for HCV care within 60 days, and b) participants in the intervention arm will have a decreased time to HCV treatment initiation compared to participants in the usual care arm. This study protocol has been approved by the IRB of Johns Hopkins Medicine and is registered on ClinicalTrials.gov (NCT02707991). An integrated study flow and conceptual framework is detailed in Figure 1.
Figure 1.
Integrated study flow and conceptual framework. This figure illustrates the Care2Cure study design in the context of the Andersen Behavioral Model of Health Services Use. HCV= chronic hepatitis C virus; NCM= nurse case management.
The NCM intervention development was guided by Andersen’s Behavioral Model of Health Services Use. Initially developed in the 1960s, this model explains the conditions that facilitate or impede utilization of health care (Andersen, 1995). The model posits that a person’s use of health services is a function of predisposing factors, perceived and actual need for care, and enabling or impeding resources. Predisposing characteristics, such as age, sex, race, ethnicity, substance use, and comorbidities are not easily modified. But perceived need, according to Andersen, can be changed through education; the greater the perceived and actual threat, the more likely one is to use services. Andersen also suggests that enabling resources can be influenced by interventions at the system, provider, and patient level. The more enabling resources one has, the greater the likelihood of healthcare utilization. We hypothesize that enabling resources for the uptake of HCV care among PLWH may include patient navigation, appointment reminders, and ART modification support, all addressed by nurse case management in the Care2Cure intervention.
Andersen’s model has been applied previously to explain HCV and HIV health care utilization patterns, and informs the intervention components of the Care2Cure study (Henry, Goetz, & Asch, 2012; Holtzman et al., 2015; Mehta et al., 2005; Rapp et al., 2008). Interventions that increase perceived need, minimize barriers, and maximize resources have the potential to improve the HCV care cascade for PLWH.
Study Setting
The Care2Cure Study is being conducted at the Johns Hopkins Hospital HIV clinic which is part of the Bartlett Specialty Practice in Baltimore, Maryland. This is a hospital-based outpatient infectious disease clinic that provides both HIV primary care and HCV specialty care. The HIV practice cares for approximately 3,500 patients per year. The clinic serves a primarily adult, urban population, with approximately 75% of patients being African American. The average age of clinic patients is 48 years (Lesko, Lau, Chander, & Moore, 2018; Moore, 1998). Approximately 50% of patients with HIV are co-infected with HCV. The Bartlett Viral Hepatitis Practice, staffed with hepatologists and viral hepatitis-focused infectious disease physicians and nurse practitioners, is also part of the broader Bartlett Specialty Practice.
Sample Size and Eligibility Criteria
Seventy participants will be enrolled, with 35 in each treatment arm. In prior studies testing the intervention components in similar populations, including PLWH and/or substance use disorders, researchers found an improvement in linkage to care of 18–30% (Craw et al., 2008; Gardner et al., 2005; Masson et al., 2013; Rapp et al., 2008). At the time of study initiation, 40% of new HCV Practice appointments were attended by patients (i.e., 60% no-show rate). We assume that we will find an improvement in linkage to care of at least 30% in the context of HCV (i.e., 70% attendance [0.70] in the NCM group compared to 40% attendance [0.40] in the enhanced usual care group). Using these figures and an alpha of 0.05, an estimate of the total sample size required to achieve 80% power is 33 per group, or a total of 66 participants (G*Power 3.1.9.2). Therefore, a sample size of 35 participants in each group should be adequate to detect the difference of interest in proportion of linkage to care for the primary aim.
Participants are included in this study if they 1) are HIV positive; 2) are current patients of the Bartlett HIV Practice, defined as having a visit with an HIV provider in the past 12 months; 3) have chronic HCV infection with a detectable plasma HCV RNA; 4) are 18 years of age or older; 5) speak English; and 6) did not attend an appointment for HCV care in the past 12 months. Participants are excluded from participation in this study if they are pregnant or unable to independently provide informed consent.
Recruitment and Enrollment
Patients either 1) self-refer by contacting the research team after seeing flyers and brochures posted in the clinic; 2) are referred by their HIV provider in clinic; 3) self-refer after receiving a targeted recruitment letter in the mail; or 4) self-refer after finding the study on clinicaltrials.gov, Trials@Hopkins (an institutional database of research studies), or referral from the Johns Hopkins Center for AIDS Research Study-Finder Hotline. The clinic schedule is screened weekly by the study team to identify eligible patients. Letters are mailed to eligible patients indicating that they may qualify to participate in a research study while at their next clinic appointment with information about how to contact the study team. HIV providers are also notified by the study team if they have eligible patients scheduled each day and are asked to refer those patients to the study at the end of the visit. After the patient has contacted our study team, a member of the study team provides information regarding the study, answers questions, and obtains informed consent. The consent form was developed at a 5th grade reading level. Participants in both arms are compensated $20 for their time at the end of the baseline visit.
Study Interventions
Control group – enhanced usual care.
After randomization into the enhanced usual care arm, or the “fact sheet group,” the study team member provides participants with a CDC HCV Fact Sheet which contains basic educational information about HCV transmission, signs and symptoms, treatment, and prevention (Centers for Disease Control and Prevention, 2015). Participants are then referred to the usual clinic appointment check-out process. It is important to note that the clinic relocated during the course of this study, slightly changing the check-out process. For the first 9 months of enrollment, patients in the clinic returned to the front desk or check out area with a billing page and printed appointment referrals if applicable. The administrative staff closed the appointment encounter and gave the patient a phone number for the Johns Hopkins Hospital central scheduling to schedule referral-based appointments. Patients were responsible for calling central scheduling to set up the specialty appointment. In the new clinic, all encounters are handled electronically; the patient still goes to a check out area to end an appointment and the clinic staff reviews referrals and closes the appointment. The clinic procedure is to assist the patient to schedule any follow-up appointments indicated in the electronic record, although specialty referral-based appointments are often left unscheduled and therefore the patient is responsible for calling central scheduling to set up a specialty appointment. When an appointment is scheduled at this health system, all patients receive an automated appointment reminder call 2 days before the scheduled appointment.
Patients at the Bartlett Specialty Practice have access to HIV nurse case managers and Ryan White-funded social workers, similar to many HIV care settings. Participants in the enhanced usual care arm will continue to have usual access to these services. Per the Bartlett Specialty Practice standard care protocol, once a prescription is written for HCV treatment, the assigned HIV nurse case manager will work with the patient to coordinate HCV care; however, HIV nurse case managers are not directly involved in HCV care until after prescription for HCV treatment is written, which is the gap our NCM intervention seeks to fill.
Experimental group – usual care plus HCV NCM.
The NCM intervention components per intervention phase (Phase 1: Linkage to care and Phase 2: Treatment initiation) are outlined in Table 1.
Table 1.
Hepatitis C Nurse Case Management Intervention
| Component | Description | Process Measures |
|---|---|---|
| Nurse-Initiated Referral | Verify insurance and need for referral | Baseline presence of referral (y/n) |
| Request referral from PCP | Referral requested (y/n) | |
| Confirm referral entrance in Epic | Referral obtained (y/n) | |
| Time spent obtaining referral | ||
| Strengths-Based Education | Handout-guided education on HCV symptoms, transmission, treatment, liver health | Baseline HCV knowledge scale |
| Time spent on education & content | ||
| Assessment of strengths and barriers related to HCV care | Referrals made to other services (i.e. social work, support group, psych) | |
| Set goals for engagement in liver health/HCV care | ||
| Patient Navigation | Make appointment with HCV provider per patient’s request and needs | Appointment scheduled (y/n) |
| Number of days to first appt. | ||
| Reschedule as needed | Number of appointments. scheduled & attended in 6 months | |
| Time spent on navigation | ||
| Appointment reminders | 1-week and 1-day appointment reminders | Number of encounters with participant |
| Phone, text, or email | Time spent on reminders |
PCP= primary care provider; y/n= yes or no; HCV= hepatitis C virus
Phase 1: Linkage to care.
This phase of the intervention is hypothesized to increase the proportion of participants who link to HCV care through NCM consisting of nurse-initiated referral, strengths-based HCV education, patient navigation, and HCV appointment reminders.
Nurse-initiated referral.
The nurse case manager initiates an HCV referral for participants randomized to the intervention group via the electronic health record or in-person conversation with the provider. This cues the HIV provider to submit the referral to HCV specialty care and minimizes the barrier of non-referral by the provider.
Strengths-based education.
Participants receive brief strengths-based HCV education (Gottlieb, 2014). Strengths-based education can improve knowledge and motivation to achieve health-related goals among PLWH (Craw et al., 2008; Gardner et al., 2005; Gottlieb, 2014). The nurse case manager helps participants identify their strengths within the context of engaging in HCV care, including social support and engagement in HIV primary care (Fusfeld et al., 2013; Grebely et al., 2011). HCV education topics include transmission, symptoms, treatment, and risk reduction, with a focus on the fact that there is a cure. Education is standardized and guided by a study-developed “Hepatitis C Basics” patient education handout.
Study participants also identify barriers to linkage to care and form a plan with the nurse case manager to minimize these barriers; this may include referrals to benefits counseling, substance use or mental health services, and/or an HCV support group called the “Cure Club” (Masson et al., 2013).
Patient navigation and clinical coordination.
Because appointment scheduling is a known barrier to linkage to care, and specialty appointments are often left for patients to schedule themselves, patient navigation includes scheduling an appointment for the HCV Practice with the participant (Coupland et al., 2009; Carla Treloar et al., 2012; Zickmund et al., 2012). After receiving permission from the patient as well as preferred dates and appointment times, the nurse case manager calls the central scheduling office during the baseline research visit to navigate participants through the HCV Practice scheduling process. The nurse case manager also assists participants in the NCM arm with rescheduling HCV Practice appointments and coordinating access to hepatitis C-treating research studies currently recruiting at the clinic.
Appointment reminders.
Participants in the NCM arm receive personalized HCV appointment reminders in addition to the automated phone reminder that all patients receive through the Johns Hopkins Hospital usual care system. A plan for contacting participants for personalized appointment reminders is made, including the best mode of contact (phone, text, or email) and time of day. Participants are contacted by the nurse case manager both one week and one day before their scheduled HCV Practice appointment for an appointment reminder (Gardner et al., 2014). During this reminder, participants are also given the opportunity to ask the nurse case manager questions about HCV or their care and the nurse case manager again emphasizes that HCV can be cured.
Phase 2: Time to treatment initiation.
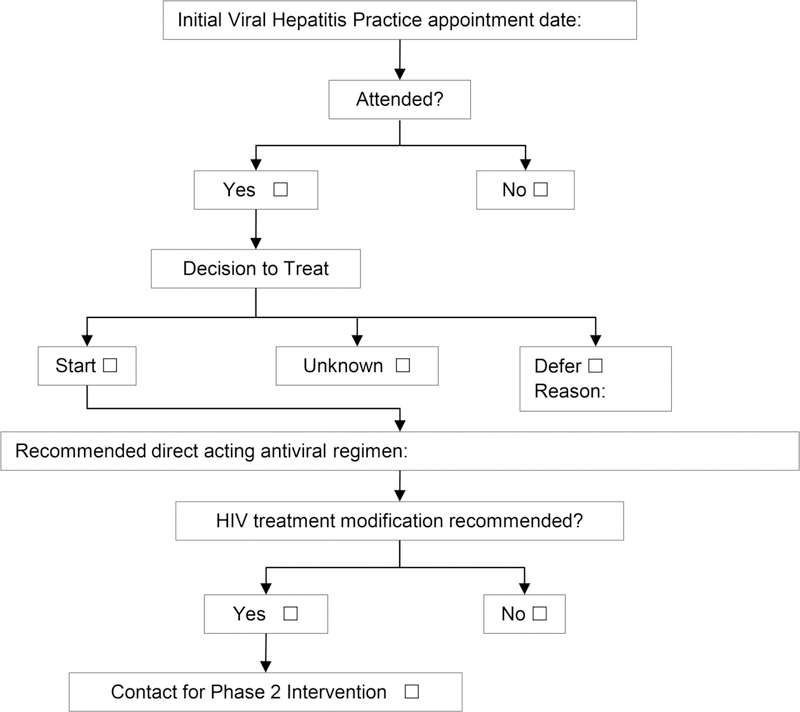
This phase of the intervention is hypothesized to decrease time to HCV treatment initiation by using a nurse case manager to coordinate communication about ART modifications between the patient and HIV provider, based on need identified in the HCV provider’s documentation. Participants who are enrolled in another clinical trial are not included in this phase because participants in HCV-treating clinical trials receive an intervention to initiate HCV treatment within those trials. These participants are expected to follow a different timeline than clinical patients and face different challenges. Therefore, this phase includes a subgroup of consented Care2Cure study participants who link to an appointment at the HCV Practice through a clinical process. An algorithm is used to guide eligibility determination for Phase 2 (Figure 2).
Figure 2.
Nurse Case Management Phase 2 Algorithm. This figure illustrates the decision algorithm for determining participant eligibility for Phase 2 of the nurse case management intervention (time to treatment initiation).
After the participant attends an HCV Practice appointment, the research nurse case manager reviews the HCV provider’s note in the electronic medical record to determine what decision was made about initiating HCV treatment (defer or start). This phase is single-blinded; no direct communication occurs between the research team and the HCV provider to avoid influencing the HCV management of the patient. Instead, the intervention supports the HIV provider and patient without influencing the HCV provider’s decisions. Using the Phase 2 Algorithm, the research nurse case manager identifies participants who have a decision to start treatment indicated in the HCV visit note. The anticipated HCV therapy regimen in the clinic note is assessed for potential drug-drug interactions with the participants’ current ART regimen. If a modification in ART is indicated because a contraindicated drug-drug interaction exists, the research nurse case manager contacts the participant via his/her preferred contact method to schedule a follow-up NCM visit. At this visit, the participant is given an investigator-developed one-page drug interaction sheet tailored to his/her ART regimen. The research nurse case manager also sends a secure email to the participant’s HIV provider notifying him/her of the potential need for ART modification. This email includes the latest DHHS “Concomitant Use of Selected HIV Drugs and FDA-Approved HCV Drugs for Treatment of HCV in HIV-Infected Adults” Table 12 (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2017). The table serves as a decision-making aid for the HIV provider to modify the ART regimen if needed. The research nurse case manager coordinates an ART modification appointment with the participant’s primary HIV provider as needed so the patient does not wait the usual 3 to 6 months until his or her next regularly scheduled HIV primary care visit.
Fidelity.
Several mechanisms are in place to ensure the intervention is delivered as planned. A detailed protocol and standard operating procedure manual that clearly specify the intervention components are available to the interventionist. Checklists have been developed for each interaction with a study participant to ensure consistency and handouts are used to guide the intervention components. All study team members were trained and observed for adherence to the protocol. To minimize potential bias with the interventionist’s involvement in every step of the research, a study team member who is blinded to randomization collects medical record data for the outcome variables at 60 days and 6 months.
Measures
Three types of study assessments are collected: 1) baseline data (including a self-report questionnaire and medical record review); 2) monthly healthcare utilization logs (via the medical record); and 3) outcome data (at 60 days and 6 months via the medical record). All study assessments are conducted by trained study staff. The baseline assessment is collected in the clinic after consent and before the participant is randomized to a study arm. Sixty-day and 6-month assessments are collected from the participants’ electronic medical record by study staff who are blind to treatment allocation.
Baseline characteristics.
The baseline characteristics questionnaire used in the Care2Cure study includes demographics (age, race, ethnicity, gender), education and employment status, income and financial strain, self-reported health history, and substance use in the past 6 and 12 months. The current study team has used this questionnaire for a prior study in the same setting (Farley et al., 2017). Questions have been added or removed as applicable to this study and patient population. In addition to these baseline characteristics, validated instruments to assess HCV knowledge, depression, and alcohol use are also administered at baseline.
Hepatitis C knowledge.
The 19-item Brief Hepatitis C Knowledge Scale (Cronbach’s α=0.87) is administered to measure HCV knowledge (Balfour et al., 2009). The Brief HCV Knowledge Scale is a unidimensional measure comprising a comprehensive list of items that address the main aspects of HCV knowledge: prevention, risk reduction, transmission, and treatment. It was designed and tested on a diverse sample of patients, health care workers, and students with varying socioeconomic and demographic backgrounds. It uses a simple true/false scoring system with a total score range of 0–19.
Depression.
The Patient Health Questionnaire (PHQ-9) is used to measure depression, which can be associated with engagement in care (Cronbach’s α=0.89) (Kroenke, Spitzer, & Williams, 2001). The PHQ-9 is scored immediately. Participants scoring 10–19, indicating moderate depression, are referred to their primary care provider for further follow up after the baseline study visit is complete per a standard protocol. Those with scores of 20 or greater (severe depression) are immediately referred to their primary provider, or, if the primary provider is not available, the covering urgent provider of the day, for further evaluation before the study visit is completed.
Alcohol use.
The Alcohol Use Disorders Identification Test (AUDIT) is administered to any participant who reports any alcohol use in the past 6 months on the baseline characteristics questionnaire (Babor, Higgins-Biddle, Saunders, Monteiro, & Dependence, 2001). The AUDIT consists of 10 items about recent alcohol use, alcohol dependence, symptoms, and alcohol-related problems. It was first developed in 1989 and has been validated in diverse international samples. The internal consistency reliability has been reported at 0.83 (Hays, Merz, & Nicholas, 1995) to the mid-0.90s (Babor et al., 2001). In a primary care setting, the AUDIT is intended to be administered by a nurse or social worker (Babor et al., 2001). It is administered by a registered nurse in the Care2Cure study. The research nurse case manager counsels participants about alcohol use according to the recommended actions of the World Health Organization based on the individual AUDIT score. Participants scoring in Zone IV, suggesting alcohol dependence, are referred to their Bartlett Specialty Practice social worker to discuss further counseling and treatment options.
Medical record review.
At the end of each baseline visit the research team reviews the patient’s medical record to verify specific pieces of the medical history. These variables of interest include CD4+ T cell count, CD4 nadir (i.e. lowest historical CD4 count), HIV viral load, prescribed ART, year of HCV diagnosis, HCV treatment history, HCV viral load, liver fibrosis level, HCV genotype, HCV appointment history, comorbidities, urine toxicology screening results if available, and health insurance status.
Health care utilization.
In addition to baseline and outcome data collection, monthly activity logs are completed for each study participant, independent of allocated study arm. These data are collected from the study participant’s electronic medical record and the research nurse case manager’s notes. The type, quantity, and content of all encounters with a study participant in the Johns Hopkins system are recorded each month, including nurse visits. This will define usual care for Bartlett Specialty Practice patients. The type, quantity, and content of all Care2Cure study visits are also recorded to define the dose of the intervention.
Outcomes.
Study outcomes (linkage to care and time to treatment initiation) are assessed using objective data extracted from the participants’ medical records.
Linkage to care.
For the primary outcome of linkage to care, the medical record is reviewed 60 days after the baseline visit to verify whether the participant attended one or more HCV Practice appointment since randomization (yes/no). All provider visits at the HCV Practice are registered in the electronic medical record, so absence of a registered appointment during the study period is considered non-attendance for the primary outcome variable.
Time to treatment initiation.
To measure our secondary outcome of time to treatment initiation, the electronic medical record is reviewed 6 months after the baseline visit. The study team collects multiple variables related to treatment initiation from the medical record, including whether a change in ART was made during the study period (yes/no), if a prescription for HCV therapy was written during the study period (yes/no and the drug(s) prescribed), if and when the participant started taking the prescribed DAA, and the number of days between the baseline visit and the date HCV therapy was started. The pharmacy at the Specialty Practice documents in the electronic medical record when DAAs are dispensed and the first dose of DAAs, which, per clinic protocol, is directly observed by a clinic nurse or provider. The 6-month period for final data collection should be adequate to account for the various steps in HCV treatment preparation among PLWH: 1) linkage to care; 2) ART modification as needed; 3) rechecking the HIV viral load 4 to 8 weeks after modification to ensure viral suppression on new regimen (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2017); 4) HCV-related blood tests and imaging, and 5) uncontrollable factors such as appointment cancellations, rescheduling, and payer reimbursement.
Participant characteristics obtained in the baseline medical record abstraction are recollected at 6 months as well. This includes HCV RNA, which will indicate whether a participant achieved sustained virologic response (SVR) during the study period. Additional items to record specific barriers to HCV treatment referral, appointment scheduling, appointment attendance, and treatment initiation are also added to the 6-month medical record review.
Data Storage and Monitoring
All data are collected and managed using REDCap electronic data capture tools hosted at Johns Hopkins University (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. Randomization occurs directly in REDCap to ensure that the study team has no a priori knowledge of group assignment when enrolling and randomizing study participants.
Statistical Analysis
Exploratory and descriptive analyses will be completed for all study variables. Variables will be inspected for normality and examined with means and standard deviations or medians and interquartile ranges accordingly. Baseline characteristics of the two groups (enhanced usual care vs. NCM intervention) will be assessed. Any differences between the groups will be adjusted for in further analysis. The significance level will be set at 0.05. The primary (outcome) analysis will follow the principle of intention-to-treat.
The primary outcome is linkage to care, measured by attendance at the HCV Practice within 60 days of randomization (yes/no). The group difference in the primary outcome will be tested using a two-sample z-test for the difference in proportions comparing the NCM intervention group to the enhanced usual care group. Binary logistic regression will be used to determine an effect on linkage to care controlling for differences in age, sex, race, HCV knowledge, substance use, liver fibrosis level, CD4+ T cell count, HIV viral load, and health insurance type.
The secondary outcome is time to HCV treatment initiation, measured by the number of days from randomization to the date the participant starts taking DAAs in the medical record. Kaplan Meier estimates will be conducted to compare time to HCV treatment initiation between participants in the NCM intervention group and the enhanced usual care group, and a log-rank test will be used to examine the difference. Potential covariates (age, sex, race, HCV knowledge, substance use, liver fibrosis level, CD4+ T cell count, HIV viral load, and health insurance type) will be included in the Cox regression model. To account for a time to treatment initiation greater than the observed time period of 6 months, right censoring will be implemented for participants who have not initiated HCV treatment at the end of the 6-month period (Hosmer, Lemeshow, & May, 1999).
Discussion
To our knowledge, this is the first clinical trial to examine the effects of a nurse case management intervention protocol to improve the HCV care continuum among people co-infected with HIV/HCV in the era of all-oral DAAs. Given the acceleration of liver disease among people co-infected with HIV/HCV, timely treatment is essential, and DAAs make this possible (Bhattacharya et al., 2017; Lo Re et al., 2014). The Care2Cure study attempts to increase the proportion of HIV/HCV co-infected adults who link to HCV care and decrease time to DAA initiation. The findings from this study will inform efforts to ensure that the most effective linkage to care and treatment approach is integrated into care of this population.
The Care2Cure study attends to the needs of hard-to-reach patient populations. At the time this study was rolled out, the clinic had already linked the majority of its HIV/HCV co-infected patients to HCV care and achieved a cure in 43%, indicating that many patients who were immediately ready for HCV therapy and the ideal treatment candidates had already received HCV treatment in the first few years of DAA availability. The remaining population may be less engaged in their healthcare and/or more complex to treat, including those who inject drugs, drink alcohol, have mental health diagnoses, and may not be well engaged in HIV care, including patients not virally suppressed on ART. Our HCV NCM intervention is designed to improve care for these populations and the sample enrolled is expected to reflect the real-world challenges of improving the HCV care continuum. This study will also help us to identify characteristics associated with, and barriers to, engaging in the HCV care continuum among a complex HIV co-infected population impacted by multiple barriers influenced by their social determinants of health.
Challenges
The target population for the Care2Cure study introduces many challenges. Recruitment of patients who are not well engaged in health care into research is difficult, and many strategies have been employed in response to this challenge. We obtained IRB approval to mail letters to potentially eligible clinic patients who were scheduled for an HIV appointment each week to encourage them to attend their HIV appointment and meet with research staff on the same day. We engaged providers in the clinic, who have trusting relationships with their patients, to refer eligible participants to our study. To attract more participants, we also increased compensation for the baseline study visit from $10 to $20. With these adjustments, we have enrolled 68 study participants and are continuing to collect data and implement the intervention.
Barriers to successful implementation of the intervention occur at the provider and payer levels. At the HIV provider level, hesitation to provide an HCV care referral for participants who have a detectable HIV viral load, are currently injecting drugs, or frequently miss HIV primary care appointments has led our research team to collaborate with the HCV Practice on a campaign to stress the ability to cure every patient, regardless of these stigmatizing factors. Insurance approval for DAAs has slowed time to treatment initiation and overall treatment initiation abilities, but our study team has worked with the pharmacy prior authorization team to minimize this barrier and refers participants to HCV-treating research studies to work around insurance approval.
Strengths and Limitations
Despite overcoming these challenges, the Care2Cure study has a few limitations worth noting. Self-reported assessments in the baseline questionnaire may be subject to desirability bias. However, the items and instruments chosen, including the AUDIT, for example, are widely used and regarded as valid and reliable measures of the concepts of interest. We also verify important health-related measures, including medications and lab values, in the medical record. We are unable to control for external factors that may affect study results, such as change in clinic processes, change in state Medicaid reimbursement policies, FDA approval of new DAAs, and changes in HIV and HCV treatment guidelines. The HIV clinic moved nine months into study recruitment and became co-located with the HCV Practice, which was expected to improve linkage to HCV care naturally within usual care. In addition to randomization, which ensures external factors are applied to both groups equally, we are documenting these contextual factors to help with interpretation of findings. Finally, given the fortunate and fairly unique situation of having a co-located HIV and HCV clinic, HIV patients in this setting may generally do better in engaging in HCV care than settings that do not have HCV resources, limiting the generalizability of the Care2Cure study. However, the NCM protocol is not specific to a co-located setting and can be translated to other settings for future studies.
This study does have several strengths as well. The intervention is theory-driven and its components are based on prior effective interventions with similar populations (Andersen, 1995; Craw et al., 2008; Gardner et al., 2005). The RCT design minimizes bias and baseline differences between groups, while also allowing causal relationships to be tested. Standard operating procedure manuals have been developed to maintain intervention fidelity and protect human subjects, including procedures for participants reporting depressive symptoms and alcohol dependence. Our outcome variables are objective measures and collected from the medical record by a study team member blinded to study arm assignment. Our primary outcome of linkage to care will have minimal to no missing data because HCV Practice visits are required to be entered in the electronic medical record. The study does not require extensive follow up with participants, which will enhance retention and feasibility. The NCM intervention is fairly low-intensity and is intended to be easily integrated into HIV primary care settings that already have nurse case managers.
Conclusions
This study of HCV nurse case management will provide much-needed evidence for the feasibility and usefulness of this novel intervention. Results of this trial will be important in informing clinical care and future research to improve the care continuum for people living with HIV and HCV, particularly as we reach to achieve the World Health Organization’s goal of HCV elimination by 2030 (World Health Organization, 2016).
Acknowledgment of financial and other support:
Research supported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number F31NR016200, a Sigma Theta Tau International/Association of Nurses in AIDS Care Grant, and a Council for the Advancement of Nursing Science/Southern Nursing Research Society Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Contributor Information
Laura E. Starbird, Department of Community and Public Health, Johns Hopkins University School of Nursing.
Hae-Ra Han, Department of Community and Public Health, Johns Hopkins University School of Nursing.
Mark S. Sulkowski, Divisions of Infectious Diseases and Gastroenterology/Hepatology, Johns Hopkins University School of Medicine.
Chakra Budhathoki, Department of Acute and Chronic Care, Johns Hopkins University School of Nursing.
Nancy R. Reynolds, Department of Community and Public Health, Johns Hopkins University School of Nursing.
Jason E. Farley, Department of Community and Public Health, Johns Hopkins University School of Nursing.
References
- Alavi M, Grebely J, Micallef M, Dunlop AJ, Balcomb AC, Day CA, … Enhancing Treatment for Hepatitis C in Opioid Substitution Settings (ETHOS) Study Group. (2013). Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 57 Suppl 2, S62–69. 10.1093/cid/cit305 [DOI] [PubMed] [Google Scholar]
- American Association for the Study of Liver Diseases & Infectious Diseases Society of America. (2018). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Retrieved March 14, 2018, from https://www.hcvguidelines.org/
- Andersen RM (1995). Revisiting the behavioral model and access to medical care: does it matter? Journal of Health and Social Behavior, 36(1), 1–10. [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, & Dependence, W. H. O. D. of M. H. and S. (2001). AUDIT : the Alcohol Use Disorders Identification Test : guidelines for use in primary health care. Retrieved from http://www.who.int/iris/handle/10665/67205
- Balfour L, Kowal J, Corace KM, Tasca GA, Krysanski V, Cooper CL, & Garber G (2009). Increasing public awareness about hepatitis C: development and validation of the brief hepatitis C knowledge scale. Scandinavian Journal of Caring Sciences, 23(4), 801–808. 10.1111/j.1471-6712.2008.00668.x [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, & Backus LI (2017). Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 64(12), 1711–1720. 10.1093/cid/cix111 [DOI] [PubMed] [Google Scholar]
- Cachay ER, Hill L, Wyles D, Colwell B, Ballard C, Torriani F, & Mathews WC (2014). The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PloS One, 9(7), e102883 10.1371/journal.pone.0102883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). Hepatitis C General Information. Centers for Disease Control and Prevention. (2016, June). HIV and Viral Hepatitis. Retrieved from https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf
- Chen EY, North CS, Fatunde O, Bernstein I, Salari S, Day B, & Jain MK (2013). Knowledge and attitudes about hepatitis C virus (HCV) infection and its treatment in HCV mono-infected and HCV/HIV co-infected adults. Journal of Viral Hepatitis, 20(10), 708–714. 10.1111/jvh.12095 [DOI] [PubMed] [Google Scholar]
- Coupland H, Day C, Levy MT, & Maher L (2009). Promoting equitable access to hepatitis C treatment for Indo-Chinese injecting drug users. Health Promotion Journal of Australia: Official Journal of Australian Association of Health Promotion Professionals, 20(3), 234–240. [DOI] [PubMed] [Google Scholar]
- Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, … Schmitt K (2008). Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. Journal of Acquired Immune Deficiency Syndromes (1999), 47(5), 597–606. 10.1097/QAI.0b013e3181684c51 [DOI] [PubMed] [Google Scholar]
- Deng L-P, Gui X-E, Zhang Y-X, Gao S-C, & Yang R-R (2009). Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. World Journal of Gastroenterology, 15(8), 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade-Nwulia O, Sutcliffe C, Moon J, Chander G, Wansom T, Keruly J, … Sulkowski M (2017). High hepatitis C cure rates among black and nonblack human immunodeficiency virus-infected adults in an urban center. Hepatology (Baltimore, Md.), 66(5), 1402–1412. 10.1002/hep.29308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JE, Starbird LE, Anderson J, Perrin NA, Lowensen K, Ross T, & Carroll KC (2017). Methodologic considerations of household-level methicillin-resistant Staphylococcus aureus decolonization among persons living with HIV. American Journal of Infection Control, 45(10), 1074–1080. 10.1016/j.ajic.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer T, Brook G, McSorley J, Murphy S, & Mohamed A (2014). Using short message service text reminders to reduce “did not attend” rates in sexual health and HIV appointment clinics. International Journal of STD & AIDS, 25(4), 289–293. 10.1177/0956462413502325 [DOI] [PubMed] [Google Scholar]
- Finitsis DJ, Pellowski JA, & Johnson BT (2014). Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PloS One, 9(2), e88166 10.1371/journal.pone.0088166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusfeld L, Aggarwal J, Dougher C, Vera-Llonch M, Bubb S, Donepudi M, & Goss TF (2013). Assessment of motivating factors associated with the initiation and completion of treatment for chronic hepatitis C virus (HCV) infection. BMC Infectious Diseases, 13, 234 10.1186/1471-2334-13-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Giordano TP, Marks G, Wilson TE, Craw JA, Drainoni M-L, … Retention in Care Study Group. (2014). Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 59(5), 725–734. 10.1093/cid/ciu357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, … Antiretroviral Treatment and Access Study Study Group. (2005). Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS (London, England), 19(4), 423–431. [DOI] [PubMed] [Google Scholar]
- Gottlieb LN (2014). Strengths-based nursing. The American Journal of Nursing, 114(8), 24–32; quiz 33,46. 10.1097/01.NAJ.0000453039.70629.e2 [DOI] [PubMed] [Google Scholar]
- Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, & Koziel MJ (2001). Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 33(4), 562–569. 10.1086/321909 [DOI] [PubMed] [Google Scholar]
- Grebely J, Bryant J, Hull P, Hopwood M, Lavis Y, Dore GJ, & Treloar C (2011). Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. Journal of Viral Hepatitis, 18(4), e104–116. 10.1111/j.1365-2893.2010.01370.x [DOI] [PubMed] [Google Scholar]
- Gupta K, Romney D, Briggs M, & Benker K (2007). Effects of a brief educational program on knowledge and willingness to accept treatment among patients with hepatitis C at inner-city hospitals. Journal of Community Health, 32(4), 221–230. [DOI] [PubMed] [Google Scholar]
- Harris M, & Rhodes T (2013). Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduction Journal, 10, 7 10.1186/1477-7517-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Merz JF, & Nicholas R (1995). Response burden, reliability, and validity of the CAGE, Short MAST, and AUDIT alcohol screening measures. Behavior Research Methods, Instruments, & Computers, 27(2), 277–280. 10.3758/BF03204745 [DOI] [Google Scholar]
- Henry SR, Goetz MB, & Asch SM (2012). The effect of automated telephone appointment reminders on HIV primary care no-shows by veterans. The Journal of the Association of Nurses in AIDS Care: JANAC, 23(5), 409–418. 10.1016/j.jana.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Holtzman CW, Shea JA, Glanz K, Jacobs LM, Gross R, Hines J, … Yehia BR (2015). Mapping patient-identified barriers and facilitators to retention in HIV care and antiretroviral therapy adherence to Andersen’s Behavioral Model. AIDS Care, 27(7), 817–828. 10.1080/09540121.2015.1009362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, & May S (1999). Applied Survival Analysis (2nd ed.). New York: John Wiley & Sons. [Google Scholar]
- Jayaweera D, Althoff K, Eron J, Huhn G, Milligan S, Mills A, … Elion R (2018). Untreated HCV in HIV/HCV co-infection: Data from the TRIO network (Vol. 68, p. S261). Presented at the The International Liver Congress, Paris, France: Journal of Hepatology. [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko CR, Lau B, Chander G, & Moore RD (2018). Time Spent with HIV Viral Load > 1500 Copies/mL Among Persons Engaged in Continuity HIV Care in an Urban Clinic in the United States, 2010–2015. AIDS and Behavior, 1–8. 10.1007/s10461-018-2085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, … Schackman BR (2014). The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PloS One, 9(5), e97317 10.1371/journal.pone.0097317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Re V, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, … Justice AC (2014). Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Annals of Internal Medicine, 160(6), 369–379. 10.7326/M13-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, & Holmberg SD (2012). The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of Internal Medicine, 156(4), 271–278. 10.7326/0003-4819-156-4-201202210-00004 [DOI] [PubMed] [Google Scholar]
- Maryland Department of Health. (2018, February 21). Clinical Criteria for Hepatitis C (HCV) Therapy. Department of Health and Mental Hygiene; Retrieved from https://mmcp.health.maryland.gov/pap/docs/HCV%20%20Clinical%20Criteria%20Feb%2021%202018%20final.pdf [Google Scholar]
- Maryland Department of Health and Mental Hygiene. (2015, January 8). Clinical Criteria for Hepatitis C (HCV) Therapy. Department of Health and Mental Hygiene; Retrieved from https://mmcp.health.maryland.gov/pap/docs/Hep%20C%20clinical%20criteria%20.pdf [Google Scholar]
- Masson CL, Delucchi KL, McKnight C, Hettema J, Khalili M, Min A, … Perlman DC (2013). A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. American Journal of Public Health, 103(10), e81–88. 10.2105/AJPH.2013.301458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, … Thomas DL (2008). Limited uptake of hepatitis C treatment among injection drug users. Journal of Community Health, 33(3), 126–133. 10.1007/s10900-007-9083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, & Strathdee SA (2005). A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS (London, England), 19 Suppl 3, S179–189. [DOI] [PubMed] [Google Scholar]
- Moore RD (1998). Understanding the Clinical and Economic Outcomes of HIV Therapy: The Johns Hopkins HIV Clinical Practice Cohort. Journal of Acquired Immune Deficiency Syndromes. [DOI] [PubMed] [Google Scholar]
- Munoz-Plaza CE, Strauss S, Astone-Twerell J, Jarlais DD, Gwadz M, Hagan H, … Rosenblum A (2008). Exploring drug users’ attitudes and decisions regarding hepatitis C (HCV) treatment in the U.S. The International Journal on Drug Policy, 19(1), 71–78. 10.1016/j.drugpo.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, … ION-4 Investigators. (2015). Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. The New England Journal of Medicine, 373(8), 705–713. 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CS, Devereaux R, Pollio DE, Hong BA, & Jain MK (2014). Patient perspectives on hepatitis C and its treatment. European Journal of Gastroenterology & Hepatology, 26(1), 74–81. 10.1097/MEG.0b013e32836382b5 [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2017). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf [Google Scholar]
- Patel N, Nasiri M, Koroglu A, Amin R, McGuey L, McNutt L-A, … Miller C (2015). Prevalence of drug-drug interactions upon addition of simeprevir- or sofosbuvir-containing treatment to medication profiles of patients with HIV and hepatitis C coinfection. AIDS Research and Human Retroviruses, 31(2), 189–197. 10.1089/AID.2014.0215 [DOI] [PubMed] [Google Scholar]
- Rapp RC, Otto AL, Lane DT, Redko C, McGatha S, & Carlson RG (2008). Improving linkage with substance abuse treatment using brief case management and motivational interviewing. Drug and Alcohol Dependence, 94(1–3), 172–182. 10.1016/j.drugalcdep.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiberger T, Obermeier M, Payer BA, Baumgarten A, Weitner L, Moll A, … Peck-Radosavljevic M (2011). Considerable under-treatment of chronic HCV infection in HIV patients despite acceptable sustained virological response rates in a real-life setting. Antiviral Therapy, 16(6), 815–824. 10.3851/IMP1831 [DOI] [PubMed] [Google Scholar]
- Sherr L, Lampe F, Norwood S, Leake-Date H, Fisher M, Edwards S, … Harding R (2007). Successive switching of antiretroviral therapy is associated with high psychological and physical burden. International Journal of STD & AIDS, 18(10), 700–704. 10.1258/095646207782193821 [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, … Podsadecki T (2015). Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA, 313(12), 1223–1231. 10.1001/jama.2015.1328 [DOI] [PubMed] [Google Scholar]
- Surjadi M, Torruellas C, Ayala C, Yee HF, & Khalili M (2011). Formal patient education improves patient knowledge of hepatitis C in vulnerable populations. Digestive Diseases and Sciences, 56(1), 213–219. 10.1007/s10620-010-1455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL (2014). Cure of hepatitis C virus infection without interferon alfa: scientific basis and current clinical evidence. Topics in Antiviral Medicine, 21(5), 152–156. [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Newland J, Rance J, & Hopwood M (2010). Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. Journal of Viral Hepatitis, 17(12), 839–844. 10.1111/j.1365-2893.2009.01250.x [DOI] [PubMed] [Google Scholar]
- Treloar Carla, Hull P, Dore GJ, & Grebely J (2012). Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug and Alcohol Review, 31(7), 918–924. 10.1111/j.1465-3362.2012.00468.x [DOI] [PubMed] [Google Scholar]
- Tyler D, Nyamathi A, Stein JA, Koniak-Griffin D, Hodge F, & Gelberg L (2014). Increasing hepatitis C knowledge among homeless adults: results of a community-based, interdisciplinary intervention. The Journal of Behavioral Health Services & Research, 41(1), 37–49. 10.1007/s11414-013-9333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven MHMMT, Brusamento S, Majeed A, & Car J (2013). Scope and effectiveness of mobile phone messaging for HIV/AIDS care: a systematic review. Psychology, Health & Medicine, 18(2), 182–202. 10.1080/13548506.2012.701310 [DOI] [PubMed] [Google Scholar]
- Wagner G, Ryan G, Osilla KC, Bhatti L, Goetz M, & Witt M (2009). Treat early or wait and monitor? A qualitative analysis of provider hepatitis C virus treatment decision-making in the context of HIV coinfection. AIDS Patient Care and STDs, 23(9), 715–725. 10.1089/apc.2009.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B, Conigrave KM, Wallace C, Whitfield JB, Wurst F, & Haber PS (2007). Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug and Alcohol Review, 26(3), 231–239. 10.1080/09595230701247681 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2016). Combating hepatitis B and C to reach elimination by 2030 (Advocacy Brief). Geneva.
- Wyles D, Bräu N, Kottilil S, Daar ES, Ruane P, Workowski K, … ASTRAL-5 Investigators. (2017). Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 65(1), 6–12. 10.1093/cid/cix260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, … ALLY-2 Investigators. (2015). Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. The New England Journal of Medicine, 373(8), 714–725. 10.1056/NEJMoa1503153 [DOI] [PubMed] [Google Scholar]
- Yap L, Carruthers S, Thompson S, Cheng W, Jones J, Simpson P, … Butler T (2014). A descriptive model of patient readiness, motivators, and hepatitis C treatment uptake among Australian prisoners. PloS One, 9(2), e87564 10.1371/journal.pone.0087564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Schranz AJ, Umscheid CA, & Lo Re V (2014). The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PloS One, 9(7), e101554 10.1371/journal.pone.0101554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickmund SL, Campbell SA, Tirado CF, Zook CL, & Weinrieb RM (2012). Perceived barriers to hepatitis C therapy for patients receiving opioid agonist treatment. Journal of Addiction Medicine, 6(3), 233–239. 10.1097/ADM.0b013e31825f491b [DOI] [PubMed] [Google Scholar]