Abstract
HUH endonucleases are numerous and widespread in all three domains of life. The major function of these enzymes is processing a range of mobile genetic elements by catalysing cleavage and rejoining of single-stranded DNA using an active-site Tyr residue to make a transient 5′-phosphotyrosine bond with the DNA substrate. These enzymes have a key role in rolling-circle replication of plasmids and bacteriophages, in plasmid transfer, in the replication of several eukaryotic viruses and in various types of transposition. They have also been appropriated for cellular processes such as intron homing and the processing of bacterial repeated extragenic palindromes. Here, we provide an overview of these fascinating enzymes and their functions, using well-characterized examples of Rep proteins, relaxases and transposases, and we explore the molecular mechanisms used in their diverse activities.
Although the double helix is the most iconic feature of DNA, the molecule must assume a transient single-stranded form during replication, transcription, bacterial conjugation and a variety of repair and recombination processes. Single-stranded DNA (ssDNA) also serves as the packaged genetic material in some viruses and bacteriophages. It is therefore no surprise that there is a dedicated endonuclease superfamily, the HUH endonucleases (in which U is a hydrophobic residue), the members of which exclusively process ssDNA using particular recognition and reaction mechanisms for site-specific ssDNA cleavage and ligation.
HUH endonucleases are numerous and widespread in all three domains of life. Two major classes within this superfamily are the Rep (replication) and relaxase, or Mob (mobilization), proteins involved in DNA processing during plasmid replication and conjugation, respectively. However, HUH endonucleases have also been identified in other processes involving DNA, such as replication of certain phages and eukaryotic viruses, and different types of transposition. These proteins have also been appropriated for cellular processes such as intron homing and processing of bacterial repetitive extragenic palindromic sequences (REP sequences). Here, we provide an overview of these fascinating enzymes, their numerous roles and functions, and the mechanisms used in their diverse activities. We explore their functional diversity using well-characterized examples of Rep proteins, relaxases and transposases.
Mechanism and overall protein architecture
The first member of the HUH superfamily, protein A (gpA) of phage φX174, was identified in early studies of phage replication1. Subsequent bioinformatic approaches2,3 revealed many related proteins forming a vast superfamily, which includes members involved in the catalysis of viral and plasmid rolling-circle replication (RCR) (Rep proteins), in conjugative plasmid transfer (relaxases) and in DNA transposition (transposases)4–9. This familial relationship is based on several conserved protein motifs, including the HUH motif, composed of two His residues separated by a bulky hydrophobic residue, and the Y motif, containing either one or two Tyr residues separated by several amino acids. The initial classification was based largely on Rep proteins and included only a few relaxases, leading to some misclassfications in the latter group2,3. Indeed, the initial Y motif identified for the R388 and F plasmid relaxases was incorrect, presumably owing to the limited number of examples available at the time9. For convenience, here we define enzymes with only a single conserved catalytic Tyr as Y1 and those with more than one catalytic Tyr as Y2, even though some Y2 HUH endonucleases require only one of their Y motif Tyr residues for catalysis, whereas others require both.
Y1 HUH endonucleases include Rep proteins of some plasmids that replicate using ssDNA intermediates (such as pUB110 (REF 10)), Rep proteins of a wide range of eukaryotic viruses11, most relaxases, transposases of insertion sequences with a common region (ISCRs; which are insertion sequences related to the IS91 family)8 and transposases of the IS200-IS605 family6,7. Y2 enzymes12 include φΧ174 gpA and the Rep proteins of other isometric-headed ssDNA and double-stranded DNA (dsDNA) phages (such as P2 (REF 13)), those of some cyanobacterial and archaeal plasmids, and those of parvoviruses (for example, adeno-associated virus (AAV)), as well as transposases ofthe IS91 and Helitron families4, and relaxases of the MobF family.
HUH endonucleases catalyse ssDNA breakage and joining with a unique mechanism. They use a Y motif Tyr to create a 5′-phosphotyrosine intermediate and a free 3′-OH at the cleavage site (FIG. 1a). Subsequently, the 3′-OH can be used for different tasks. The most obvious task is to prime replication, as observed for the HUH domains in Rep proteins of single-stranded phages, RCR plasmids and conjugative relaxases. The 3′-OH group can also act as the nucleophile for strand transfer to resolve the phosphotyrosine intermediate in the termination step of RCR replication, conjugative transfer and transposition (see below).
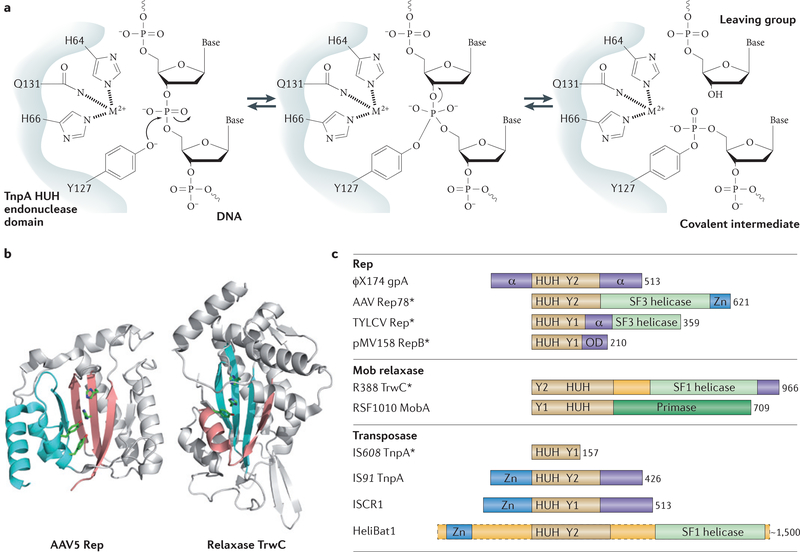
Figure 1 |. Reaction mechanism and organization of selected HUH proteins.
a | The HUH endonuclease domain (in which U is a hydrophobic residue), together with the divalent metal ion (M2+) ligand, nicks single-stranded DNA (ssDNA) to form a covalent intermediate and release the cleaved leaving group. b | Both the Rep (replication) domain of adeno-associated virus 5 (AAV5) Rep (Protein Data Bank (PDB) accession 1M55) and the relaxase domain of plasmid R388 TrwC (PDB accession 1OMH) have a five-stranded β-sheet with a βαββ core that harbours the HUH motif on the central strand. Owing to the different domain organization in Rep proteins and relaxases, the position of this core differs (βαββαβαβ for Rep proteins and βαβαβαββ for relaxases). The more amino-terminal residues in the primary sequence are shown in pink; those more carboxy-terminal are in blue. c | The organization of representative HUH domain-containing proteins is shown; they contain HUH, helicase, oligomerization (OD) and proposed Zn-binding (not necessarily structurally related) domains. The length of each protein is indicated in numbers of amino acids, and those proteins for which HUH domain structures are available are indicated with an asterisk; the HUH motif data are taken from REF. 3 (motif 2) and REF. 9 (motif III); the Y motif data are taken from REF. 3 (motif 3) and REF. 9 (motif I). The assigned domain organizations are taken from phage φXl74 protein A (gpA)17, AAV Rep78 (REF. 102), tomato yellow leaf curl virus (TYLCV) Rep57, plasmid pMV158 RepB17, plasmid R388 TrwC22, plasmid RSF1010 MobA (mobilization protein A)75, transposases from the insertion sequences IS608 (REF. 7), IS91 (REF. 7) and insertion sequence with a common region 1 (ISCR1) (S. Messing, A.B.H. and F.D., unpublished observations), and HeliBat1 (a consensus sequence from a bioinformatic prediction)113. Image in part a is modified, with permission, from REF. 7 © (2005) Cell Press.
The cleavage polarity of HUH endonucleases is inverse to that of Tyr recombinases, which make 3′-phosphoty-rosine intermediates and generate free 5′-OH groups that cannot be used as replication primers14. A further contrast to the cofactor-independent Tyr recombinases is that HUH enzyme activities require a divalent metal ion to facilitate cleavage by localizing and polarizing the scissile phosphodiester bond. Depending on the enzyme, Mg2+, Mn2+ or other divalent metal ions can be used in vitro15–20, although it is often presumed that Mg2+ or Mn2+ are the physiological cofactors. The His pair of the HUH motif provides two of the three ligands necessary for metal ion coordination (FIG. 1a). The location and identity of the third, invariably polar residue, which can be Glu, Asp, His or (as shown in FIG. 1a) Gln, varies across the superfamily.
Three-dimensional structures of several Rep and relaxase HUH domains have been determined with and without bound DNA (Supplementary information S1 (table) for Protein Data Bank accessions) and have been crucial to understanding the mechanisms of these enzymes. The common element of the HUH fold (FIG. 1b) is a central four- or five-stranded antiparallel β-sheet generally sandwiched between α-helices. The HUH motif is invariably located on a single β-strand, whereas the catalytic Tyr residue (or residues) is located on a nearby α-helix (FIG. 1b). The order of HUH and Y motifs varies in the primary sequence: in the relaxase group, the Y motif is upstream of the HUH motif, whereas in the Rep group it is downstream (FIG. 1b,c). This circular permutation3,21,22 of the motifs in the primary sequence (FIG. 1c) changes the topology of the domains (FIG. 1b). Nevertheless, the three-dimensional constellation of active-site residues is virtually identical across the superfamily.
Given the diverse functions of HUH proteins, it is not surprising that other domains are often appended to the HUH domain. In many cases, these additional domains are of unknown function. However, ATP-dependent helicase, Zn-binding, primase and multimerization domains are recurring themes4,23–28 (FIG. 1c). For example, the ssDNA substrates needed by HUH endonucleases can be generated by a dedicated DNA helicase domain carboxy-terminal to the HUH domairi4,24,29,30 or, alternatively, by recruitment of a host helicase25–28. RCR uses 3′−5′ helicase activity acting on the template strand to facilitate DNA unwinding at the replication fork, whereas during conjugation, helicases (as part of the relaxase) are transported into the recipient cell and track 5′−3′ on the transported ssDNA.
DNA recognition
A feature of many HUH endonucleases is that they can recognize and bind DNA hairpin structures, and the DNA cleavage sites are located either in the hairpin or in the ssDNA located on the 5′ or 3′ side of the stem. The crucial role of hairpins has been firmly established for many processes, including plasmid conjugation, replication of plasmids and of eukaryotic viruses, and transposition6,7,16,31–34. In other systems, palindromic sequences that can form DNA hairpins are present near the probable HUH endonuclease cleavage sites35. Such hairpins can be formed in vivo under a number of physiological conditions (see REF. 36).
Structural studies of HUH endonucleases revealed that small DNA hairpins can be recognized in several different ways (FIG. 2): sequence-specific recognition of the dsDNA stem, structure-specific recognition of irregularities in the stem, or sequence-specific recognition of the hairpin loop7,15,20,22,23,33.
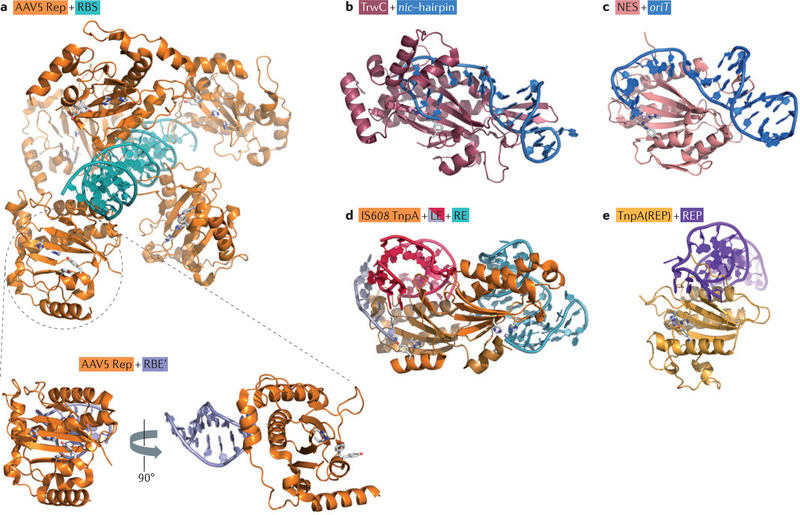
Figure 2 |. Structures of various HUH enzymes with their substrate DNAs.
a | The interaction of adeno-associated virus (AAV) Rep (replication) protein with the inverted terminal repeat hairpin in the DNA; the top panel shows the interaction with the 20 bp Rep-binding site (RBS) (Protein Data Bank (PDB) accession 1RZ9), and the lower panel shows the interaction with the Rep-binding element (RBE’) (PDB accession 1UUT). b | The interaction between the plasmid R388 relaxase, TrwC, and a 25-base oligonucleotide containing both the plasmid nic site and the recognition hairpin (PDB accession 2CDM). c | The interaction of nicking enzyme in Staphyloeoeeus (NES) with the origin of transfer (oriT), represented by an oligonucleotide of 30 bases (PDB accession 4HT4). d | A model of the interaction between insertion sequence IS608 transposase (TnpA) and both the transposon left end (LE) (PDB accession 2VJV) and right end (RE) (PDB accession 2VHG). e | The interaction of TnpA(REP) with a repetitive extragenic palindromic (REP) sequence (PDB accession 4ER8).
The hairpin-flanking DNA — in many cases, in single-stranded form — is also often important for recognition. Relaxases, for example, make extensive contacts with the bases extending between the hairpin and the cleavage site20,22,37 (FIG. 2), and for TnpA(REP), a protein related to the IS608 transposase (TnpA), it has been shown that nucleotides on the 5′ side of the hairpin are crucial for binding and for sequence-specific recognition33. Other family members23,38 have more complex binding modes (see below).
Below, we explore the functional diversity of HUH endonucleases using well-characterized examples of various Rep proteins, relaxases and transposases.
Rep proteins and RCR
RCR (FIG. 3) was first proposed more than 40 years ago39 as a mechanism for the replication of single-stranded phages. This process is now also well-established as a mechanism adopted by certain eukaryotic viruses and bacterial plasmids (reviewed in REFS 1,35,40).
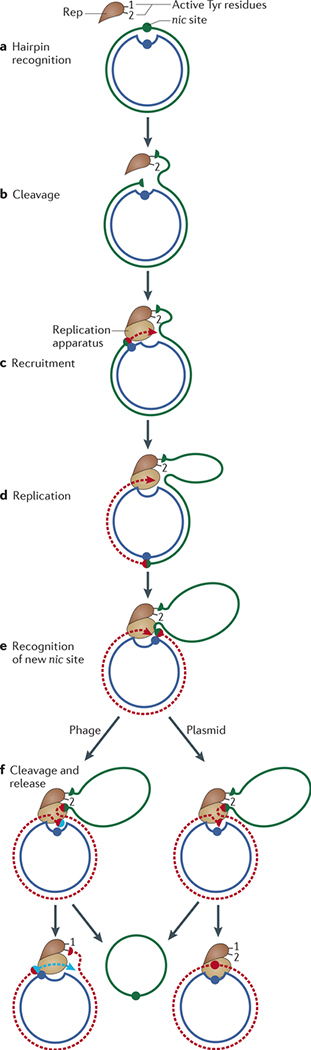
Figure 3 |. Rolling-circle replication.
a | The Rep (replication) protein contains two active-site Tyr residues, 1 and 2, and recognizes circular double-stranded DNA (positive-sense strand in green, negative-sense strand in blue) at the the 3′ recognition sequence. b | Rep cleaves the DNA at thenicsite and forms a phosphotyrosine bond with Tyr residue 1. c | The replication apparatus is recruited. d | Replication is initiated and reconstitutes thenicsite. e | When the entire circle of DNA has been replicated, Rep and the replication apparatus reach the reconstitutednicsite. f | In the case of phage replication, thenicsite is cleaved by the Rep active-site Tyr residue 2 to form a new phosphotyrosine. The resulting 3′-OH attacks the original phosphotyrosine bond (with Tyr residue 1) to release a single-stranded circular phage copy. Replication of the Rep-bound circular DNA then continues round the circle again. In the case of copy number-regulated plasmid replication, no second phosphotyrosine bond is formed, and the plasmid regains its initial circular dsDNA form possibly through the use of a second nucleophile (such as water) instead of a Tyr47. Failure to terminate at this stage would lead to multimeric single-stranded products.
At the origin: phage φΧ174.
Studies on φΧ174 gpA revealed several functional details that are recapitulated in other HUH domain-containing systems; thus, φΧ174 provides a general framework for understanding DNA processing involving HUH endonucleases.
When φΧ174 infects its Escherichia coli host, its circular positive-sense ssDNA ((+)ssDNA) genome is injected into the cell and converted by host enzymes into a duplex form resembling a supercoiled dsDNA plasmid. RCR is initiated when the Rep protein gpA recognizes the 3′ region of a short (approximately 30 bp) sequence at the origin of replication (FIG. 3a), unwinds DNA upstream and nicks the (+)strand at a specific site (called the nic site) upstream of the recognition sequence to form a covalent 5′-phosphotyrosine intermediate and a free 3′-OH41–43 (FIG. 3b). Although the DNA recognition sequence and cleavage site are known, there is surprisingly no published detailed analysis of how gpA recognizes and binds the target DNA. Replication is initiated using the 3′-OH end, and the (+)strand is ‘peeled off’ (FIG. 3c,d). As replication proceeds, the recognition and nic sites are regenerated. After one complete cycle of replication round the circular DNA template, the replication apparatus and gpA reach the reconstituted recognition and nic site, and gpA recognizes this regenerated sequence (FIG. 3e) and nicks the (+)strand using the second Tyr in the catalytic Y motif44, which becomes attached to the newly replicated DNA (FIG. 3f). The newly generated 3′-OH (at the end of the original, displaced ssDNA copy) then attacks the first phosphotyrosine linkage, generating a free ssDNA circle (FIG. 3f). Alternating the use of the active-site Tyr residues allows gpA to spin off many ssDNA circles45.
This process led to a model in which the Tyr residues, located on the same face of an α-helix, can be positioned alternately to attack the scissile phosphate that is localized and polarized by the HUH-bound metal ion43,45. Alternating the use of Tyr residues probably necessitates conformational changes, which might also be a crucial feature of the catalytic cycle of other HUH endonucleases (for example, see REFS 13,46).
RCR plasmids.
Plasmid RCR closely mirrors that of phages. However, the lifestyle of viruses benefits from the synthesis of multiple genomic copies, whereas that of bacterial plasmids requires plasmid replication to be closely coordinated with host cell growth. Plasmids must therefore possess mechanisms to prevent unregulated premature reinitiation of replication.
Several models have been proposed to explain how this is achieved. For the monomeric Y1 Rep protein of pC194, the final step in replication can be accomplished by an essential active-site Glu that replaces the second Tyr. This Glu allows cleavage of the phosphotyrosine bond by a bound water molecule (FIG. 3f) and release of the enzyme. Importantly, a mutant with a second Tyr instead of a Glu, emulating the phage Rep protein sequence, generates multimeric ssDNA plasmid copies47. An alternative so-called suicide mechanism was proposed for other plasmid Y1 RCR systems that use a dimeric Rep, in which covalent modification of one Rep monomer generates an inactive Rep (containing one active monomer and one inactive monomer) on termination48.
The only published example of a crystallographic structure for a Rep protein from an RCR plasmid is that of RepB from pMV158 (REF. 17). This protein has two closely spaced Y motif Tyr residues and might be considered a Y2 enzyme, although only one of the Tyr residues (Tyr99) is required for catalysis49 and the other is not correctly placed for catalysis17. In addition to an amino-terminal HUH domain, RepB has a small C-terminal oligomerization domain that assembles six endonuclease domains into a ring with a small central channel, potentially providing multiple second Tyr residues for catalysis. RepB DNA recognition is complex, with two binding sites, the distal and proximal direct repeats, downstream of the hairpin that contains the cleavage site38.
Viral Rep proteins.
Replication of eukaryotic parvoviruses provides an intriguing variation of the RCR cycle50. Parvoviruses such as AAV have linear ssDNA genomes and replicate by a ‘rolling hairpin’ mechanism (FIG. 4). Crucial for this are the final ~160 bases on each end of the viral ssDNA genome, which can fold into fully complementary three-way DNA junctions known as inverted terminal repeats (ITRs) (represented two-dimensionally as a T-shaped folded structure; FIG. 4a insert). As a result, replication of the 5′ end is straightforward: the 3′ ITR provides a free 3′-OH group from which leading-strand synthesis can be initiated using host cell enzymes (FIG. 4a-c). However, replication of this 3′ ITR requires a site-specific nick to generate a second 3′-OH at the terminal resolution site (trs) in the 3′ ITR. Cleavage is catalysed by the viral Rep (FIG. 4d,e), which possesses an N-terminal HUH domain and a C-terminal superfamily 3 (SF3) hexameric 3′−5′ helicase domain separated by a functionally important linker region51–54 (FIG. 1c). When the nick is introduced at the 3′ ITR, the cellular replication apparatus completes viral genome replication (FIG. 4f).
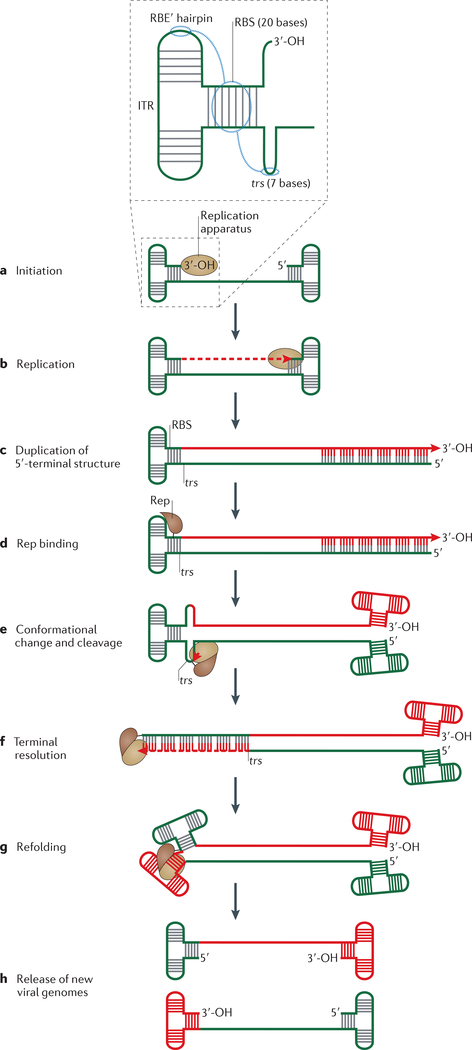
Figure 4 |. Rolling hairpin replication of adeno-associated virus.
For simplicity, secondary structures at the ends of the single-stranded adeno-associated virus (AAV) genome (called inverted terminal repeats (ITRs)) are shown as T junctions with complementary base pairing. Parental DNA is green and newly synthesized DNA is red. a | The insert shows a schematic organization of the ITR, and blue circles indicate ITR regions that interact with the protein Rep (replication). Replication is initiated using the host replication machinery and the 3′-OH of the 3′ ITR as a primer. b,c | Replication continues to the end of the genome, duplicating the 5′-terminal ITR structure. d | For AAV5, five Rep molecules bind to the 20-base (GCTC)5 Rep-binding site (RBS) in the 3′ ITR and contact the Repbinding element (RBE′) hairpin tip. e | This binding is proposed to provoke a conformational change in the DNA at the palindromic terminal resolution site (trs), and to induce Rep-mediated cleavage at trs to generate a phosphotyrosine bond between Rep and the DNA. f | Recruitment of the host replication machinery then allows replication of the 3′-terminal structure (a step called terminal resolution). g | Refolding of the ends generates structures resembling those that are present before replication initiation. h | The end result is a fully replicated viral genome.
Structural characterization of the N-terminal HUH domain in AAV5 Rep revealed that several AAV5 Rep monomers recognize and bind tandem tetranucleotide repeats in the Rep-binding site (RBS) located on one branch of the 3′ ITR (FIG. 4 insert), to form a spiral curling around the RBS site23,55. In this case, the HUH endonuclease domain recognizes dsDNA via major- and minor-groove interactions and contacts two adjacent tetranucleotide RBS repeats. The tip of one hairpin branch (called the Rep-binding element (RBE′)) is also recognized by AAV Rep, although via a different surface of the HUH domain23 (FIG. 2a), and this interaction is important for AAV replication31. Rep helicase activity is thought to generate ssDNA for trs hairpin formation29 (FIG. 4e). After trs cleavage, AAV Rep remains covalently attached to the viral DNA through its active-site Tyr. In addition, the helicase domain directs packaging of the newly replicated genomes into capsids56. Although AAV Rep is a Y2 endonuclease, there is no evidence that the second Y motif is required for replication.
The HUH endonuclease domains have been structurally characterized for several other Rep proteins of eukaryotic viruses with circular ssDNA genomes. Replication of these genomes presumably proceeds using an RCR mechanism similar to that used by phages and plasmids. These Rep proteins include those of tomato yellow leaf curl virus (a geminivirus)57, the nanovirus faba bean necrotic yellows virus and porcine circovirus58,59. The structure of the dimeric Rep protein from the archaeal Rudivirus Sulfolobus islandicus rod-shaped virus 1 has also been determined, and the biochemical activity of the enzyme has been characterized. The genome of this virus is linear and does not replicate by RCR, but replication could share some mechanistic aspects with parvovirus replication60. All of these viral Rep proteins have only one conserved Tyr at their active sites, raising the possibility that a second is provided by another protomer or that an alternative second nucleophile (such as water) is used, as has been proposed for copy number control in RCR plasmids (see above)47,61–63.
Plasmid relaxases
Plasmid conjugation (FIG. 5), which was discovered more than 60 years ago64, involves transfer of an ssDNA plasmid copy from one cell to another through a pore formed by a specialized type IV secretion system (T4SS)65. Conjugation is the process primarily responsible for horizontal gene transfer, for example of antibiotic resistance genes between bacterial species. Recently, a large family of non-autonomous elements called conjugative transposons or integrative conjugative elements (ICEs) has been identified. These elements are extremely wide- spread66. They are integrated into the host genome, excised as circular intermediates and transferred by conjugation. Depending on the ICE family, integration and excision can be catalysed either by a phage-like Tyr recombinase67 or by a DDE family transposase68,69. Like conjugative plasmids, ICEs encode relaxases with both a cluster that potentially binds divalent metal ions (but not necessarily HUH ligands) and a conserved Tyr, identified first in Tn916 (REF 70) and subsequently in many examples from clinically important Grampositive bacteria (see REF 9). Moreover, certain ICEs have also been shown to undergo replication as part of their life cycle68,71.
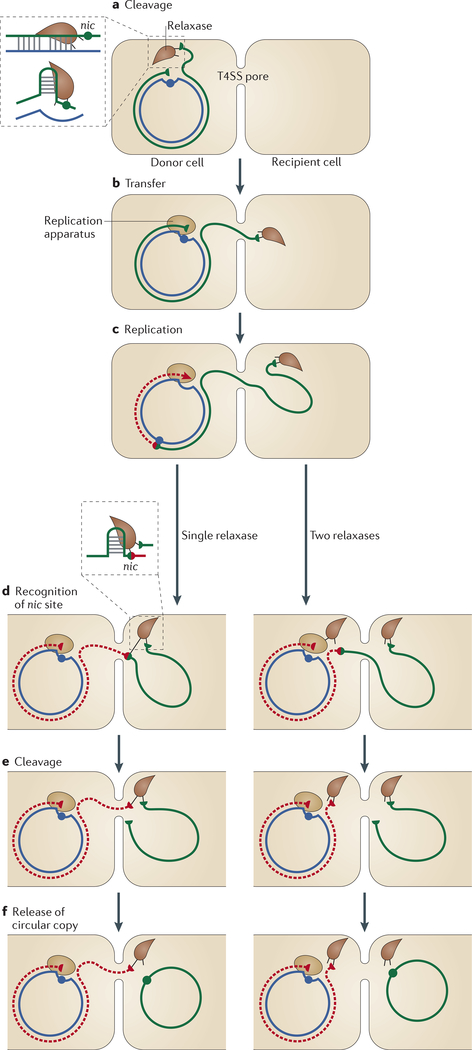
Figure 5 |. Rolling-circle replication-mediated conjugation.
a | Conjugative transfer occurs through a type IV secretion system (T4SS) pore between the donor and recipient cells. Conjugation is initiated by the relaxase recognizing one side of a double-stranded inverted repeat structure (insert) proximal to a 3′ nic site. Nucleophilic attack of the nic site generates a phosphotyrosine bond between the cleaved strand of the nic site and a Tyr residue in the relaxase. b | The linked single-stranded DNA (ssDNA) is transferred with the relaxase into the recipient cell. c | Replication is initiated in the donor cell using the host replication apparatus and regenerates the nic site. d-f | Replication continues until the entire circle of DNA has been replicated. The nic site that was reconstituted at the onset of replication is then cleaved by a relaxase (insert) to generate a circular ssDNA copy of the genome in the recipient cell. This cleavage can be mediated by the relaxase bound to the original nicked end, in the recipient cell, or by a second relaxase, in the donor cell. Replication in the donor cell is generally concomitant with transfer but is not essential. Replication of the transferred single strand is carried out by the replication apparatus of the recipient cell.
General reaction mechanism.
The biochemical signal for initiating conjugative DNA processing is unknown but is thought to be triggered by donor cell-recipient cell interaction. Conjugation begins with nicking of the circular dsDNA plasmid at the nic site within the plasmid origin of transfer (oriT); this nicking is mediated by the HUH endonuclease domain of the plasmid-encoded relaxase (FIG. 5a). This initiates ‘conjugative RCR’, which peels off a specific strand of the dsDNA plasmid and transfers this ssDNA to the recipient cell (FIG. 5b,c). Relaxase remains covalently attached to this ssDNA and is transported into the recipient cell, where it tracks 5′−3′ along the ssDNA while still attached to the end72. Relaxase catalyses recircularization of the transferred ssDNA plasmid in the recipient cell (FIG. 5d-f), where complementary-strand synthesis by a host cell polymerase converts the plasmid into its dsDNA form. Donor plasmid replication (using the non-transferred, circular strand as a template) can occur in the donor cell concomitantly with transfer, but is not essential for the transfer process73. Replication termination and the generation of a circular plasmid copy can occur in several ways, depending on whether the second Tyr is carried in the same relaxase molecule or in a different one, and whether the termination occurs in the donor or recipient cell (see below).
Catalytic centre.
Relaxases (FIG. 1c) have been classified into six families on the basis of their N-terminal HUH domains9. Four of these families, MobF, MobQ, MobP and MobV, generally include the HUH motif and a third residue for metal coordination (usually another His) along with the conserved Tyr residue (or residues), whereas the two other families, MobH and MobC, have — a different architecture. Thus, as with plasmid RCR (in which not all RCR plasmids use HUH-containing Rep proteins), conjugative plasmid transfer can have variations in the way that strand cleavage and strand transfer occur. Of the four HUH relaxase families, MobF comprises Y2 enzymes, whereas MobQ, MobP and MobV are all Y1 relaxase families. To date, crystallographic structures of the endonuclease domains of three MobF enzymes18,22,74 and two MobQ proteins20,75 have been determined. In addition to the HUH and Y motifs, a third motif with a conserved Asp or Glu might enhance the interaction of the His triad with the divalent cation55,76. There seem to be mechanistic differences even between members of one relaxase family. For example, in the MobF family member TrwC (encoded by plasmid R388), Tyr18 is necessary for the cleavage of supercoiled plasmid DNA in vitro and is absolutely required, together with Tyr26, for complete relaxase activity in vivo. This suggests that Tyr18 is involved in the initial cleavage reaction and that Tyr26 is responsible for the final strand transfer reaction77. By contrast, of the four candidate catalytic Tyr residues in Tral (encoded by plasmid F), only Tyr16 is essential for relaxase activity, suggesting that two Tral molecules are necessary for plasmid F transfer78.
DNA binding.
HUH relaxases are thought to initially recognize the dsDNA form of an inverted repeat close to the nic site in the supercoiled plasmid79,80 and, with the help of accessory proteins65, locally melt the DNA around the nic site (FIG. 5a insert). In the recipient cell, both arms of the inverted repeat in the transferred ssDNA will form a hairpin that is again recognized by the relaxase (FIG. 5d insert).
In the TrwC-DNA co-crystal structure22 (FIG. 2b), the hairpin is firmly embraced by the protein through a β-ribbon conserved in all three available MobF structures, whereas in the MobQ-DNA co-crystal of DNA bound by the Staphylococcus aureus MobQ protein NES (FIG. 2c), the hairpin is bound by two loops20. Three C-terminal α-helices form the MobF relaxase ‘fingers’, which position the ssDNA region containing the nic site (immediately next to the hairpin) in the relaxase active site. These fingers are absent in MobQ proteins, and the nic site is instead positioned by burying an adjacent nucleotide in the protein. Conserved Arg and Ser residues have also been implicated in the stabilization of DNA-relaxase interactions and, in the case of Ser, positioning the ssDNA substrate in the active site22,81.
For MobF relaxases, which are all Y2 proteins, one Tyr is involved in RCR initiation in the donor cell (FIG. 5a), whereas another Tyr in the same relaxase molecule catalyses termination in the recipient cell (left panels in FIG. 5d,e)77. The regenerated nic site in the transferred ssDNA is recognized by the initiating relaxase, which remains covalently linked to the 5′ ssDNA end. In all MobF relaxases, the C-terminal half contains a RecD-like translocase domain, which could track along the incoming ssDNA and reload the regenerated nic site onto the relaxase catalytic centre in the recipient cell (FIG. 2b). Then, a second nic site cleavage circularizes the transferred ssDNA but also joins the relaxase to the newly synthesized nic site (left panels in FIG. 5d-f). This, in principle, might lead to transfer of a second plasmid copy. It is uncertain how relaxase is removed from the ssDNA to terminate the reaction. A second model proposes that the second cleavage occurs in the donor cell and is mediated by a second relaxase, so the termination reaction will simply be the reversal of the cleavage reaction78 (right panels in FIG. 5d-f).
Relaxases from the MobQ, MobP and MobV families, which all contain Y1 and HUH domains, have variable C-terminal domains82,83, such as primase, helicase or DNA-binding domains. For plasmid RSF1010, the primase domain of the relaxase MobA (FIG. 1c) is required for initiation of replication in the recipient cell, but the same primase can also be produced separately without the relaxase domain to form the protein RepB′, which is also essential for replication of the plasmid84.
HUH endonucleases as transposases
HUH endonucleases also act as transposases for members of the bacterial and archaeal IS200-IS605 (REF. 6), IS91 (REF. 85) and ISCR8 insertion sequence families and for the eukaryotic Helitrons4 (BOX 1). The best understood HUH transposases are those of the IS200-IS605 family.
Box 1 | The Helitron superfamily.
All the HUH transposases (in which U represents a hydrophobic residue) described in the main text are specific to bacteria and archaea and thus catalyse the movement of DNA elements in bacterial and archaeal genomes. However, Helitrons are a family of mobile genetic elements that are present in eukaryotic genomes. Helitrons are found in high numbers in Arabidopsis thaliana, Oryza sativa,Zea maize, Caenorhabditis elegans, Drosophila melanogaster112 and Myotis lucifugus113. Bioinformatic analysis suggests that these elements are mobilized by rolling-circle replication-based transposition, similarly to certain bacterial and archaeal elements112. It has been proposed that Helitrons gave rise to geminiviruses114, although the helicase domains of Helitrons differ significantly from the superfamily 3 (SF3) helicases that are generally associated with geminiviruses.
Helitrons contain a particularly long ORF, RepHel, composed of a number of exons (sometimes more than ten)4,112 encoding a protein with both HUH and Y2 motifs, together with putative amino-terminal Zn-finger and carboxy-terminal SF1-like helicase domains (FIG. 1c). Moreover, in many Helitrons, RepHel exhibits additional apurinic endonuclease and/or cysteine protease domains112. These elements can also include a separate protein that binds single-stranded DNA. Helitrons have fairly well conserved ends (generally 5′-TC and often CTAG-3′) and short 16–20 -bp subterminal hairpins at the right end, but no inverted repeats, and are also often flanked by 5′-T and A-3′.
IS200-IS605 transposons.
The IS200-IS605 transposon family includes two subgroups: IS200 and IS605. IS200 group members carry only the transposase gene (tnpA). Those from the IS605 group carry both tnpA and a second ORF, tnpB (FIG. 6a), the function of which is unclear but is not required for transposition. The first family member, IS200, was identified about 30 years ago86 in Salmonella spp. However, the family paradigms are the more recently identified IS608 (in Helicobacter pylori87) and ISDra2 (in Deinococcus radiodurans88). These insertion sequences use obligatory ssDNA intermediates for mobility. The transposons excise as an ssDNA circle with abutted left and right ends, and insert 3′ to a conserved, element-specific penta- or tetra- nucleotide target. Although this target sequence is not part of the IS, it is essential for further transposition. The transposons do not carry terminal inverted repeats but include hairpins in the left and right ends that are recognized and bound by TnpA (FIG. 6a).
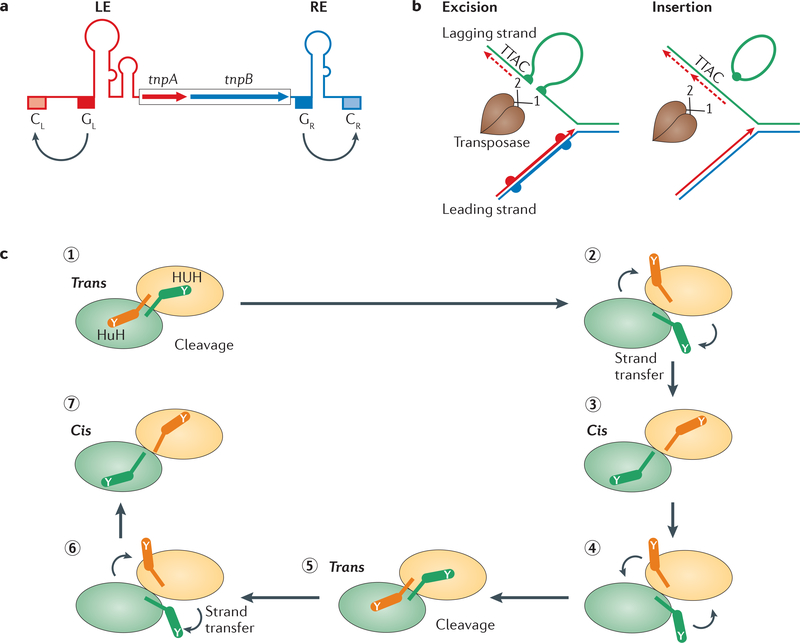
Figure 6 |. Single-strand transposons.
a | The genetic organization of the insertion sequence IS608, a member of the IS200-IS605 family. The ORFs tnpA and tnpB encode transposase (TnpA; a Y1 transposase) and a protein of unknown function, TnpB. The left end (LE) and right end (RE) regions have potential secondary structures, as indicated schematically. The left and right guide sequences (GL and GR, respectively) recognize and interact with the left and right cleavage sites (Cl and CR, respectively). b | IS200-IS605 transposons preferentially excise from and integrate into the lagging-strand template of the replication fork. The dashed red line represents an Okazaki fragment, and the solid red line represents the newly synthesized leading strand. The dimeric TnpA (black sticks indicating catalytic Tyr residues) catalyses excision of the single-stranded insertion sequence, resulting in a deletion in the lagging-stand template. The single-stranded insertion sequence then attacks the lagging-strand template in a target molecule and inserts 3′ to the TTAC target tetranucleotide. c | Each monomer of the IS608 TnpA dimer undergoes a series of cis-trans conformational changes during the transposition cycle, displacing the HUH motif (in which U is a hydrophobic residue) in the main body of the protein and the catalytic Tyr residue (Y) situated on an α-helix (αD). For binding and cleavage of the left and right ends of the insertion sequence, the active sites adopt a trans configuration, in which the HUH motif of an active site is contributed by one TnpA monomer and the Tyr residue is contributed by the other (step 1). Following DNA cleavage, the two αD-helices rotate to the cis configuration (step 2). In this configuration, the αD-helices then catalyse the formation of a circular single-stranded transposon and join the cleaved ends of the donor sequence (step 3). The configuration is then reset to the trans form to end excision (step 4). Integration starts with the active sites, in a trans configuration, cleaving the single-stranded transposon and the target DNA (step 5). The two αD-helices rotate for strand transfer (step 6), and the active sites return to a cis configuration to integrate the transposon (step 7).
The transposons excise from, and insert preferentially into, the lagging-strand template at chromosome and plasmid replication forks (FIG. 6b). This preference for the lagging strand generates an insertion bias that reflects the mode of replication (uni- or bidirectional) of the target replicon89. Transposition of ISDra2 is strongly induced following recovery of the highly radiation-resistant host from irradiation90, and this induction is certainly due to the large amount of ssDNA that is generated during reassembly of the shattered D. radiodurans genome91.
IS200-IS605 family transposases are single-domain proteins containing only the essential HUH motif and a single catalytic Tyr (they are Y1 transposases) (FIG. 1c). Both IS608 TnpA and ISDra2 TnpA are obligatory dimers, and the active sites of these enzymes are believed to adopt two functionally important conformations: the trans configuration, in which each active site is composed of the HUH motif from one monomer and the Tyr residue on an α-helix (αD) from the other monomer, and the cis configuration, in which both motifs in an active site are contributed by the same monomer. Only the trans configuration has been observed crystallographically.
Like relaxases, TnpA also binds hairpins and cleaves ssDNA at some distance. The left-end and right-end cleavage sites (which differ in sequence) are recognized not directly by the protein, but instead by particular base interactions with short ‘guide’ sequences 5′ to each hairpin. Recognition of the cleavage site requires a network of base interactions15,46,92 that also stabilizes the nucleo- protein complex — the transpososome — within which the DNA strand cleavages and transfers are carried out92. Changes in the guide sequences generate predictable changes in insertion site specificity93.
Excision and integration probably occur by alternating the catalytic site conformation between the trans and cis configurations (FIG. 6c). On cleavage of both cleavage sites by the trans dimer, the left end of the transposon and the chromosomal sequence immediately downstream of the right end are each attached by their 5′-phosphate to the attacking Tyr residue, leaving a 3′-OH on the break upstream of the left end and on the right end. Strand joining probably occurs by reciprocal rotation of the two Tyr-carrying a-helices, to move into the cis configuration, and subsequent attack of the phosphotyrosine bonds by the 3′-OH groups. This joins the left and right ends together, and also joins the two transposon-flanking sequences. The αD helices must be reset to the trans configuration to integrate the circular transposon elsewhere in the chromosome92.
RCR transposons: the IS91, ISCR and Helitron families.
The earliest identified transposable elements with HUH domain transposases were members of the IS91 family5. The IS91 transposases are significantly larger than Y1 transposases (FIG. 1c), carry a Y2 motif and include an N-terminal Zn-binding motif and additional domains of as-yet-unidentified function.
Recently, a group of related elements, the ISCRs, has been described (see REF 8). ISCRs have a common region consisting of an ORF resembling the IS91 family transposase gene but with only a single catalytic Tyr residue (FIG. 1c). ISCRs are often associated with a range of antibiotic resistance genes. In addition, eukaryotic relatives of these elements, the Helitrons, have been identified by bioinformatic approaches (BOX 1).
There is little information concerning either ISCR or Helitron transposition, but IS91 is thought to transpose by an RCR mechanism related to RCR plasmid replication. Both Tyr residues are essential for IS91 transposition in vivo. Although IS91 ends contain two short flanking inverted repeats, it is not clear whether this is a general feature of the family. Like IS200-IS605 family members, IS91 inserts with the right inverted repeat (IRR; also called ori-IS) 3′ to a specific tetra- nucleotide that is also required for further transposition (see REFS 94,95). Transposition is postulated to initiate at IRr as a result of transposase-mediated cleavage, and the 3′-OH generated in the donor molecule would act as a primer for DNA replication. Transfer of the donor sequence to a target is driven by replication in the donor, during which displacement of the active transposon strand would be driven by leading-strand replication. Transfer of an ssDNA IS91 copy into the target DNA is accomplished when IRL (also called ter-IS) is reached and cleaved (reviewed in REF 5). Termination fails at a high frequency (1% of all insertions), and the transfer process in these cases is known as one-ended transposition, as it results in the insertion of additional flanking donor-plasmid genes 3′ to the IRL sequence85. Although IRr is essential for insertion sequence activity, IRL is not: removal of this sequence simply increases the relative frequency of one-ended transposition. Acquisition of flanking antibiotic resistance genes by ISCRs could be a manifestation of this property.
Despite this attractive model, in vivo studies have identified both ssDNA and dsDNA transposon circles containing covalently joined termini without the IRR- flanking tetranucleotide. Circle formation depends on an intact transposase Y2 motif95, and these circles might therefore be transposition intermediates analogous to the IS608 ssDNA circular transposition intermediates. The dsDNA circles could result from replication restart or from extension of a trapped Okazaki fragment on the excised ssDNA circle.
The relationship between transposition of IS91 and replication of the donor and target replicons is not known.
Domestication, diversity and versatility
Domestication.
A number of studies, particularly of eukaryotes, have provided many examples of transposable elements evolving to assume alternative roles within their host96. There are few bacterial examples of such ‘domestication’, but two of these examples involve HUH endonucleases closely related to IS200-IS605 family transposases (BOX 2). These examples are TnpA(REP) proteins, which are TnpA-like transposases that can cleave and rejoin REP sequences in vitro and are thought to be responsible for the dissemination of these sequences within bacterial genomes (BOX 2); and the TnpA-like endonucleases that are encoded in some group I introns (called IStrons), which presumably play the part of homing endonucleases (BOX 2).
Box 2 | Domestication of IS200-IS605 family transposases.
In bacteria, two groups of mobile genetic elements, the repetitive extragenic palindromic sequences (REP sequences; not to be confused with the Rep (replication) HUH proteins) and the IStrons, use transposases that are closely related to the transposase (TnpA) proteins of the insertion sequence IS200-IS605 family.
REP sequences
REP sequences, which were identified 30 years ago115, are inverted DNA repeats that can form hairpin structures and are almost exclusively located in intergenic regions of bacterial genomes115. REP sequences are often present in high copy numbers (there are ~590 copies in Escherichia coli K12 and >1,600 copies in Stenotrophomonas maltophilia) and grouped into pairs in structures called bacterial interspersed mosaic elements (BIMEs). BIMEs are composed of a REP sequence and an inverted REP (iREP) sequence116, and might have several key roles in aspects of host physiology, including organization of the genome structure, regulation of gene expression, and genome plasticity (see REF 32).
Recently, a group of REP-associated proteins called TnpA(REP) proteins32 (also known as RAYTS117) was identified and found to be closely related to IS200-IS605 family transposases. tnpA(REP) is generally present in a single copy located between flanking REP sequences. TnpA(REP) catalyses REP sequence cleavage and joining in vitro32, but is less specific than IS200-IS605 family transposases.
TnpA(REP) is structurally closely related to the TnpA proteins of IS608 and ISDra2 but, unlike these dimeric TnpA proteins, is a monomer33 (FIG. 2). The structure has been determined for TnpA(REP) bound to a REP hairpin extended on its 5′ side by 4 bases (analogous to the guide sequence found in IS608), and in this structure the single-stranded DNA ‘guide sequence’ is extensively contacted by the protein. As is the case for ISDra2 TnpA, hairpin recognition by TnpA(REP) requires a mismatch in the stem, and the 5′ guide sequence is important for cleavage33. In spite of the evident catalytic properties of TnpA(REP) and the high prevalence of REP sequences, only scant information is available about the origin and behaviour of REP sequences or the role of TnpA(REP) in vivo. Recently, bioinformatic approaches detected a BIME excision event in the Pseudomonas fluorescence GW25 genome, suggesting that BIMEs are in fact mobile elements118, but there is no direct evidence for the involvement of TnpA(REP) in this process. As TnpA(REP)-mediated cleavage and rejoining requires only a single REP sequence (the iREP sequence is refractory to TnpA(REP) activity32), it is intriguing that REP sequences are grouped in BIMEs. This paired configuration might be linked to genome replication because the iREP sequence on the lagging strand would become a REP sequence on the leading strand. The mechanism by which REP sequences invade and propagate within genomes remains to be addressed.
Group I introns
Other known TnpA-like proteins occur in certain group I bacterial introns called IStrons. These elements are rich in secondary structure and encode a TnpA-like potential homing endonuclease119 similar to TnpA of ISDra2. All of the IStron copies analysed so far have been found to be inserted 3′ to the pentanucleotide TTGAT, which is also the target sequence used by ISDra2. TTGAT is complementary to the intron internal guide sequence and, at the RNA level, is presumably required in the splicing reaction. Little is known about IStron behaviour.
Diversity in the catalytic centre.
As stated above, HUH endonucleases require divalent metal ions and bind these using a triad of amino acids consisting of the HUH His pair and a third residue, which can be Glu, Asp, His or Gln. In addition to variation in the third metal ion ligand, certain viral Rep proteins with almost identical Rep folds, such as those of circoviruses and nanoviruses58,59,97, include variations in the HUH motif itself, as do certain MobP relaxases98. In circoviruses and nanoviruses, the motif becomes HUQ, so bioinformatic searches for HUH endonucleases would not identify these enzymes. Indeed, there are even proteins that catalyse plasmid RCR or conjugation but do not contain an HUH motif at all3,9,40. For example, MobH relaxases contain an HHH motif and a hydrolase motif (both of which are potential divalent metal ion-binding clusters) and an upstream conserved Y motif, whereas MobC relaxases contain a conserved potential metal ion-binding D..E..E triad together with the Y motif9. The relationship of these viral proteins to HUH endonucleases cannot be defined until three-dimensional structural information becomes available. Thus, the superfamily of endonucleases that use the HUH mechanism of strand cleavage and rejoining is likely to extend significantly further than the currently defined HUH group.
Similarities with other enzymes.
HUH endonucleases are structurally similar to the replication origin-binding domains encoded by some dsDNA viruses55,57 and also have strong similarity to the viral RNA recognition motif (RRM)55,57,99, suggesting that there are intricate and ancient evolutionary relationships between these proteins.
Helicases and the sources of ssDNA.
A central question in the activity of HUH endonucleases is how their substrates become available in the ssDNA form. This role could be fulfilled by the associated helicase domains of many HUH endonucleases (FIG. 1c) or by the interaction of HUH proteins with cellular helicases that can unwind dsDNA. For AAV Rep, the associated SF3 helicase activity is needed for DNA cleavage, suggesting that this activity contributes to the formation of the trs-containing hairpin (FIG. 4a insert).
In bacterial systems, in which plasmids are super-coiled, a certain level of supercoiling can lead to hairpin extrusion without the need for helicase activity. By contrast, phage φX174 replication is absolutely dependent on the cellular Rep helicase (an SF1 3′−5′ helicase)100, but the role of this helicase is in unwinding the phage dsDNA form during RCR, after gpA-mediated nicking.
The 5′−3′ helicase activity of relaxase is thought to promote tracking of the enzyme along the single transferred strand to position it correctly for the termination step (FIG. 5). Although relaxase is capable of binding to one arm of the dsDNA site at oriT, additional accessory proteins are involved in melting this region to enable hairpin formation and nicking (FIG. 5a insert). Bacterial ssDNA transposases of the IS200-IS605 family rely on sources of ssDNA89 such as the lagging-strand template at the replication fork, the ssDNA intermediates generated during DNA repair and possibly R-loops generated during intensive transcription, and these enzymes therefore do not require a dedicated helicase. However, the situation is not clear for IS91 or, by extension, for the related ISCRs and the eukaryotic Helitrons, both of which are thought to require transposon-specific replication5. Helitrons encode a 5′−3′ SF1 helicase; neither IS91 nor ISCR transposases contain helicase domains, but both have a C-terminal domain of unknown function, and it is tempting to speculate that this domain interacts with a cellular helicase.
Interchangeable functions?
As an illustration of the versatility of HUH endonucleases, members of the Rep and relaxase families can also promote intermolecular strand transfer, leading to integration. AAV2 can integrate site specifically into human chromosome 19 at a locus called AAVS1, which carries an RBS-like tandem repeat sequence and a nearby trs site101. Site-specific integration is Rep dependent, but the mechanism is unclear. Synapsis between the viral ITR and AAVS1 might be facilitated, for instance, by Rep protomers assembled on AAVS1 as hexameric or octameric rings102,103. In sharp contrast to Y1 transposases, integration mediated by the AAV2 transposase is not precise104. Most events occur hundreds of bases away from AAVS1 in an apparently asymmetrical manner, implying that insertion is not a simple event.
Some relaxases can also site-specifically integrate the transferred strand into the genome of a recipient bacterial cell72, provided that a second oriT is present in the target genome. Both the relaxase and a second TrwC domain in the 600 N-terminal residues are reauired for this activity. The reaction involves a complex sequence of events to resolve the presumed intermediates105–107. Moreover, relaxases can be specifically targeted to the nucleus108, although relaxase-mediated site-specific integration of the transferred DNA in the recipient chromosome has yet to be demonstrated.
This type of site-specific integration reaction might have wide potential applications in biotechnology and biomedicine to directly transfer DNA from bacteria to eukaryotic cells, although practical implementation of this approach is clearly premature at present109–111.
Conclusions
HUH endonucleases use the same basic catalytic mechanism to carry out a diverse set of biological functions. These enzymes all use Tyr residues as nucleophiles and form covalent 5′-phosphotyrosine bonds with the cleaved DNA strand. The phosphotyrosine bond stores energy that can be harnessed by a 3′-OH located at the other end of the Tyr-linked strand or on the end of a different ssDNA strand, allowing for either intra- or intermolecular strand transfer.
Although HUH endonucleases are involved in a wide variety of biological processes in nature, this is not a consequence of these enzymes carrying out different catalytic reactions. Rather, it is principally a consequence of the different functional modules appended to the HUH domain or recruited as separate entities. The choice between RCR, conjugation or transposition is dictated by the topological and temporal coordination between these various alternative functional modules. Indeed there are examples of one HUH protein assuming more than one role: for example, the relaxase NicK carries out both transfer and replication functions for the transposon ICEBs1 (REF 71).
There remain many unanswered questions concerning this widespread class of enzymes. It is still unknown whether and how these proteins interact with the replication apparatus at the fork, and no structural data are available concerning these higher-order interactions, even in the case of φΧ174, the paradigm of RCR studies. In addition, ISCRs represent an important vector of multiple antibiotic resistance genes, which clearly affect public health8, and understanding the mechanisms of acquisition and transmission of these genes is undoubtedly a principal concern. Finally, the impact of Helitrons, particularly in shaping plant genomes, makes study of their detailed mechanism an area of priority.
Supplementary Material
Repetitive extragenic palindromic sequences.
Abundant non-coding repeats that are found in bacterial genomes and form DNA hairpin structures that can have regulatory functions.
Rolling-circle replication.
Unidirectional replication of circular DNA molecules (such as plasmids and phage genomes) in which a single-stranded product is ‘peeled’ from a circular DNA template.
Insertion sequences with a common region.
Insertion sequences that contain a common ORF which has similarity to the transposase ORF encoded in insertion sequence IS91. These elements seem to be able to sequester additional neighbouring genes during their transposition.
Insertion sequences.
Short transposable DNA segments that include one or more genes involved in their own mobility.
Helitron.
A type of eukaryotic transposon that is thought to transpose using a rolling-circle replication mechanism similar to that of insertion sequence IS91.
Helicase.
An enzyme that unwinds and separates double-stranded DNA. Helicases are classified into six major superfamilies (SF1-SF6) on the basis of the motifs and consensus sequences shared by the molecules and their activities (for example, 5′−3′ or 3′−5′ directionality).
Type IV secretion system.
A multiprotein apparatus that is used by bacteria to transport both DNA and proteins across the bacterial cell envelope.
Integrative and conjugate element.
A mobile genetic element that has the transfer properties of a conjugative plasmid but that is generally unable to replicate autonomously and possesses dedicated integration system to allow the element to be maintained by integration into the host chromosome.
DDE family transposase.
A transposase that contains a characteristic DDE amino acid motif.
R-loops.
Regions of DNA in which the two strands are separated by a short RNA segment that forms a complementary RNA-DNA hybrid with one of the DNA strands.
Acknowledgements
The authors thank C. Guynet, S. Messing and I. Molineau for discussions. This work was supported by intramural funding from the French Centre National de la Recherche Scientifique (to M.C. and B.T.H.) and the Intramural Program of the US National Institute of Diabetes and Digestive and Kidney Diseases (to A.B.H. and F.D.), and by grants from the French Agence National de Recherche (ANR-12-BSV8-0009-01; to B.T.H.), the Spanish Ministry of Science and Innovation (BIO2010-14809; to G.M.), the Spanish Ministry of Education (BFU2011-26608; to F.d.l.C.) and the European Seventh Framework Program (248919/FP7-ICT-2009-4 and 282004/ FP7-HEALTH.2011.2.3.1-2; to F.d.l.C.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
ISfinder: https://www-is.biotoul.fr
References
- 1.Kornberg A & Baker TA DNA Replication 2nd edn (Freeman, 1992). [Google Scholar]
- 2.Ilyina TV & Koonin EV Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 20, 3279–3285 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koonin EV & Ilyina TV Computer-assisted dissection of rolling circle DNA replication. Biosystems 30, 241–268 (1993).References 2 and 3 are the first bioinformatic analyses of HUH endonucleases.
- 4.Kapitonov VV & Jurka J Rolling-circle transposons in eukaryotes. Proc. Natl Acad. Sci. USA 98, 8714–8719 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcillan-Barcia MP, Bernales I, Mendiola MV & De la Cruz F in Mobile DNA Vol. II (eds Craig NL, Craigie R, Gellert M, & Lambowitz A) 891–904 (ASM Press, 2002). [Google Scholar]
- 6.Ton-Hoang B et al. Transposition of ISHp608, member of an unusual family of bacterial insertion sequences. EMBO J. 24, 3325–3338 (2005).A description of IS608 and its behaviour.
- 7.Ronning DR et al. Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. Mol. Cell 20, 143–154 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Toleman MA, Bennett PM & Walsh TR ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev 70, 296–316 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcillan-Barcia MP, Francia MV & de la Cruz F The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev 33, 657–687 (2009).A discussion of the diversity of relaxases and their classification.
- 10.Gruss A & Ehrlich SD The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol. Rev 53, 231–241 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosario K, Duffy S & Breitbart M A field guide to eukaryotic circular single-stranded DNA viruses: insights gained from metagenomics. Arch. Virol 157, 1851–1871 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Curcio MJ & Derbyshire KM The outs and ins of transposition: from Mu to Kangaroo. Nature Rev. Mol. Cell Biol 4, 865–877 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Odegrip R & Haggard-Ljungquist E The two active- site tyrosine residues of the A protein play nonequivalent roles during initiation of rolling circle replication of bacteriophage p2. J. Mol. Biol 308, 147–163 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Grindley ND, Whiteson KL & Rice PA Mechanisms of site-specific recombination. Annu. Rev. Biochem 75, 567–605 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Hickman AB et al. DNA recognition and the precleavage state during single-stranded DNA transposition in D. radiodurans. EMBO J. 29, 3840–3852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boer R et al. Unveiling the molecular mechanism of a conjugative relaxase: the structure of TrwC complexed with a 27-mer DNA comprising the recognition hairpin and the cleavage site. J. Mol. Biol 358, 857–869 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Boer DR et al. Plasmid replication initiator RepB forms a hexamer reminiscent of ring helicases and has mobile nuclease domains. EMBO J. 28, 1666–1678 (2009)The multimeric structure of the Rep protein from plasmid pMV158.
- 18.Datta S, Larkin C & Schildbach J F Structural insights into single-stranded DNA binding and cleavage by F factor Tral. Structure 11, 1369–1379 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Larkin C et al. Inter- and intramolecular determinants of the specificity of single-stranded DNA binding and cleavage by the F factor relaxase. Structure 13, 1533–1544 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Edwards JS et al. Molecular basis of antibiotic multiresistance transfer in Staphylococcus aureus. Proc. Natl Acad. Sci. USA 10.1073/pnas.1219701110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyda F & Hickman AB A Mob of Reps. Structure 11, 1310–1311 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Guasch A et al. Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. Nature Struct. Biol 10, 1002–1010 (2003).The structure of the TrwC relaxase complexed with its DNA substrate.
- 23.Hickman AB, Ronning DR, Perez ZN, Kotin RM & Dyda F The nuclease domain of adeno- associated virus Rep coordinates replication initiation using two distinct DNA recognition interfaces. Mol. Cell 13, 403–414 (2004).A description of the different binding modes of AAV Rep from a structural point of view.
- 24.Clerot D & Bernardi F DNA helicase activity is associated with the replication initiator protein Rep of tomato yellow leaf curl geminivirus. J. Virol 80, 11322–11330 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odegrip R, Schoen S, Haggard-Ljungquist E, Park K & Chattoraj DK The interaction of bacteriophage P2 B protein with Escherichia coli DnaB helicase. J. Virol 74, 4057–4063 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit MA et al. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol. Microbiol 29, 261–273 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Bruand C & Ehrlich SD UvrD-dependent replication of rolling-circle plasmids in Escherichia coli. Mol. Microbiol 35, 204–210 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Chang TL et al. Biochemical characterization of the Staphylococcus aureus PcrA. helicase and its role in plasmid rolling circle replication. J. Biol. Chem 277, 45880–45886 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Brister JR & Muzyczka N Rep-mediated nicking of the adeno-associated virus origin requires two biochemical activities, DNA helicase activity and transesterification. J. Virol 73, 9325–9336 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im DS & Muzyczka N The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell 61, 447–457 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Brister JR & Muzyczka N Mechanism of Rep- mediated adeno-associated virus origin nicking. J. Virol 74, 7762–7771 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ton-Hoang B et al. Structuring the bacterial genome: Y1-transposases associated with REP-BIME sequences. Nucleic Acids Res. 40, 3596–3609 (2012).The identification and structure of TnpA(REP), and an analysis of the activity of this enzyme.
- 33.Messing SA et al. The processing of repetitive extragenic palindromes: the structure of a repetitive extragenic palindrome bound to its associated nuclease. Nucleic Acids Res. 40, 9964–9979 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orozco BM & Hanley-Bowdoin LA DNA structure is required for geminivirus replication origin function. J. Virol 70, 148–158 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Solar G, Giraldo R, Ruiz-Echevarria MJ, Espinosa M & Diaz-Orejas R Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev 62, 434–464 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikard D, Loot C, Baharoglu Z & Mazel D Folded DNA in action: hairpin formation and biological functions in prokaryotes. Microbiol. Mol. Biol. Rev 74, 570–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin C, Datta S, Nezami A, Dohm JA & Schildbach J F Crystallization and preliminary X-ray characterization of the relaxase domain of F factor Tral. Acta Crystallogr. D Biol. Crystallogr 59, 1514–1516 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Maso JA, Lurz R, Espinosa M & del Solar G Interactions between the RepB initiator protein of plasmid pMV158 and two distant DNA regions within the origin of replication. Nucleic Acids Res. 35, 1230–1244 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert W & Dressler D DNA replication: the rolling circle model. Cold Spring Harb. Symp. Quant. Biol 33, 473–484 (1968). [DOI] [PubMed] [Google Scholar]
- 40.Khan SA Plasmid rolling-circle replication: recent developments. Mol. Microbiol 37, 477–484 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Brown DR et al. DNA structures required for ϕΧ174 A-protein-directed initiation and termination of DNA replication. Cold Spring Harb. Symp. Quant. Biol 47, 701–715 (1983). [DOI] [PubMed] [Google Scholar]
- 42.Brown DR, Schmidt-Glenewinkel T, Reinberg D & Hurwitz J DNA sequences which support activities of the bacteriophage φΧ174 gene A protein. J. Biol. Chem 258, 8402–8412 (1983). [PubMed] [Google Scholar]
- 43.Roth MJ, Brown DR & Hurwitz J Analysis of bacteriophage ϕΧ174 gene A protein-mediated termination and reinitiation of ϕΧ DNA synthesis. II. Structural characterization of the covalent ϕΧ A protein-DNA complex. J. Biol. Chem 259, 10556–10568 (1984). [PubMed] [Google Scholar]
- 44.Hanai R & Wang JC The mechanism of sequence-specific DNA cleavage and strand transfer by ϕΧ174 gene A* protein. J. Biol. Chem 268, 23830–23836 (1993). [PubMed] [Google Scholar]
- 45.van Mansfeld AD, van Teeffelen HA, Baas PD & Jansz HS Two juxtaposed tyrosyl-OH groups participate in φΧ174 gene A protein catalysed cleavage and ligation of DNA. Nucleic Acids Res. 14, 4229–4238 (1986).A demonstration of the importance of two Tyr residues in the φX174 Rep protein for catalysis.
- 46.Barabas O et al. Mechanism of IS200/IS605 family DNA transposases: activation and transposon-directed target site selection. Cell 132, 208–220 (2008).The structural model for single-strand transposition of IS608 and other IS200-IS605 family members.
- 47.Noirot-Gros MF & Ehrlich SD Change of a catalytic reaction carried out by a DNA replication protein. Science 274, 777–780 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Novick RP Contrasting lifestyles of rolling-circle phages and plasmids. Trends Biochem. Sci 23, 434–438 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Moscoso M, Eritja R & Espinosa M Initiation of replication of plasmid pMV158: mechanisms of DNA strand-transfer reactions mediated by the initiator RepB protein. J. Mol. Biol 268, 840–856 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Tattersall P & Ward DC Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature 263, 106–109 (1976). [DOI] [PubMed] [Google Scholar]
- 51.James JA et al. Crystal structure of the SF3 helicase from adeno-associated virus type 2. Structure 11, 1025–1035 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Smith RH & Kotin RM The Rep52 gene product of adeno-associated virus is a DNA helicase with 3′-to-5′ polarity. J. Virol 72, 4874–4881 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maggin JE, James JA, Chappie JS, Dyda F & Hickman AB The amino acid linker between the endonuclease and helicase domains of adeno- associated virus type 5 Rep plays a critical role in DNA-dependent oligomerization. J. Virol 86, 3337–3346 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarate-Perez F et al. The interdomain linker of AAV-2 Rep68 is an integral part of its oligomerization domain: role of a conserved SF3 helicase residue in oligomerization. PLoS Pathog. 8, e1002764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hickman AB, Ronning DR, Kotin RM & Dyda F Structural unity among viral origin binding proteins: crystal structure of the nuclease domain of adeno-associated virus Rep. Mol. Cell 10, 327–337 (2002). [DOI] [PubMed] [Google Scholar]
- 56.King JA, Dubielzig R, Grimm D & Kleinschmidt JA DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 20, 3282–3291 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos-Olivas R, Louis JM, Clerot D, Gronenborn B & Gronenborn AM The structure of a replication initiator unites diverse aspects of nucleic acid metabolism. Proc. Natl Acad. Sci. USA 99, 10310–10315 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vega-Rocha S, Gronenborn B, Gronenborn AM & Campos-Olivas R Solution structure of the endonuclease domain from the master replication initiator protein of the nanovirus faba bean necrotic yellows virus and comparison with the corresponding geminivirus and circovirus structures. Biochemistry 46, 6201–6212 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega-Rocha S, Byeon IJ, Gronenborn B, Gronenborn AM & Campos-Olivas R Solution structure, divalent metal and DNA binding of the endonuclease domain from the replication initiation protein from porcine circovirus 2. J. Mol. Biol 367, 473–487 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Oke M et al. A dimeric Rep protein initiates replication of a linear archaeal virus genome: implications for the Rep mechanism and viral replication. J. Virol 85, 925–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noirot-Gros MF, Bidnenko V & Ehrlich SD Active site of the replication protein of the rolling circle plasmid pC194. EMBO J. 13, 4412–4420 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasooly A & Rasooly RS How rolling circle plasmids control their copy number. Trends Microbiol. 5, 440–446 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Laufs J et al. Geminivirus replication: genetic and biochemical characterization of Rep protein function, a review. Biochimie 77, 765–773 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Lederberg J & Tatum EL Sex in bacteria; genetic studies, 1945–1952. Science 118, 169–175 (1953). [DOI] [PubMed] [Google Scholar]
- 65.de la Cruz F, Frost LS, Meyer RJ & Zechner EL Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol. Rev 34, 18–40 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Guglielmini J, Quintais L, Garcillan-Barcia MP, de la Cruz F & Rocha EP The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 7, e1002222 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burrus V, Pavlovic G, Decaris B & Guedon G Conjugative transposons: the tip of the iceberg. Mol. Microbiol 46, 601–610 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Guerillot R, Da Cunha V, Sauvage E, Bouchier C & Glaser P Modular evolution of TnGBSs, a new family of integrative and conjugative elements associating insertion sequence transposition, plasmid replication, and conjugation for their spreading. J. Bacteriol 195, 1979–1990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smyth DS & Robinson DA Integrative and sequence characteristics of a novel genetic element, ICE6013, in Staphylococcus aureus. J. Bacteriol 191, 5964–5975 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rocco JM & Churchward G The integrase of the conjugative transposon Tn916 directs strand- and sequence-specific cleavage of the origin of conjugal transfer, oriT, by the endonuclease Orf20. J. Bacteriol 188, 2207–2213 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CA, Babic A & Grossman AD Autonomous plasmid-like replication of a conjugative transposon. Mol. Microbiol 75, 268–279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Draper O, Cesar CE, Machon C, de la Cruz F & Llosa M Site-specific recombinase and integrase activities of a conjugative relaxase in recipient cells. Proc. Natl Acad. Sci. USA 102, 16385–16390 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kingsman A & Willetts N The requirements for conjugal DNA synthesis in the donor strain during F lac transfer. J. Mol. Biol 122, 287–300 (1978). [DOI] [PubMed] [Google Scholar]
- 74.Nash RP, Habibi S, Cheng Y, Lujan SA & Redinbo MR The mechanism and control of DNA transfer by the conjugative relaxase of resistance plasmid pCU1. Nucleic Acids Res. 38, 5929–5943 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monzingo AF, Ozburn A, Ma S, Meyer RJ & Robertus JD The structure of the minimal relaxase domain of MobA at 2.1 A resolution. J. Mol. Biol 366, 165–178 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larkin C, Haft RJF, Harley MJ, Traxler B & Schildbach JF Roles of active site residues and the HUH motif of the F plasmid TraI relaxase. J. Biol. Chem 282, 33707–33713 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Perez B et al. Analysis of DNA processing reactions in bacterial conjugation by using suicide oligonucleotides. EMBO J. 26, 3847–3857 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dostal L, Shao S & Schildbach JF Tracking F plasmid TraI relaxase processing reactions provides insight into F plasmid transfer. Nucleic Acids Res. 39, 2658–2670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lucas M et al. Relaxase DNA binding and cleavage are two distinguishable steps in conjugative DNA processing that involve different sequence elements of the nic site. J. Biol. Chem 285, 8918–8926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim YJ, Lin LS & Meyer RJ Two domains at the origin are required for replication and maintenance of broad-host-range plasmid R1162. J. Bacteriol 169, 5870–5872 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pansegrau W, Schroder W & Lanka E Concerted action of three distinct domains in the DNA cleavingjoining reaction catalyzed by relaxase (TraI) of conjugative plasmid RP4. J. Biol. Chem 269, 2782–2789 (1994). [PubMed] [Google Scholar]
- 82.Meyer R Replication and conjugative mobilization of broad host-range IncQ plasmids. Plasmid 62, 57–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geibel S, Banchenko S, Engel M, Lanka E & Saenger W Structure and function of primase RepB′ encoded by broad-host-range plasmid RSF1010 that replicates exclusively in leading-strand mode. Proc. Natl Acad. Sci. USA 106, 7810–7815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Honda Y, Sakai H, Komano T & Bagdasarian M RepB′ is required in trans for the two single-strand DNA initiation signals in oriV of plasmid RSF1010. Gene 80, 155–159 (1989). [DOI] [PubMed] [Google Scholar]
- 85.Mendiola MV, Bernales I & de la Cruz F Differential roles of the transposon termini in IS91 transposition. Proc. Natl Acad. Sci. USA 91, 1922–1926 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lam S & Roth JR IS200: a Salmonella-specific insertion sequence. Cell 34, 951–960 (1983). [DOI] [PubMed] [Google Scholar]
- 87.Kersulyte D et al. Transposable element ISHp608 of Helicobacter pylori: nonrandom geographic distribution, functional organization, and insertion specificity. J. Bacteriol 184, 992–1002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Islam SM et al. Characterization and distribution of IS8301 in the radioresistant bacterium Deinococcus radiodurans. Genes Genet. Syst 78, 319–327 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Ton-Hoang B et al. Single-stranded DNA transposition is coupled to host replication. Cell 142, 398–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pasternak C et al. Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. PLoS Genet. 6, e1000799 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zahradka K et al. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 443, 569–573 (2006). [DOI] [PubMed] [Google Scholar]
- 92.He S et al. Reconstitution of a functional IS608 single-strand transpososome: role of non-canonical base pairing. Nucleic Acids Res. 39, 8503–8512 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guynet C et al. Resetting the site: redirecting integration of an insertion sequence in a predictable way. Mol. Cell 34, 612–619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcillan-Barcia MP & de la Cruz F Distribution of IS91 family insertion sequences in bacterial genomes: evolutionary implications. FEMS Microbiol. Ecol 42, 303–313 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Pilar Garcillan-Barcia M, Bernales I, Mendiola MV & de la Cruz CF Single-stranded DNA intermediates in IS91 rolling-circle transposition. Mol. Microbiol 39, 494–502 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Sinzelle L, Izsvak Z & Ivics Z Molecular domestication of transposable elements: from detrimental parasites to useful host genes. Cell. Mol. Life Sci 66, 1073–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vega-Rocha S, Gronenborn AM, Gronenborn B & Campos-Olivas R 1H, 13C, and 15N NMR assignment of the master Rep protein nuclease domain from the nanovirus FBNYV. J. Biomol. NMR 38, 169 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Varsaki A, Lucas M, Afendra AS, Drainas C & de la Cruz F Genetic and biochemical characterization of MbeA, the relaxase involved in plasmid ColE1 conjugative mobilization. Mol. Microbiol 48, 481–493 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Burd CG & Dreyfuss G Conserved structures and diversity of functions of RNA-binding proteins. Science 265, 615–621 (1994). [DOI] [PubMed] [Google Scholar]
- 100.Scott JF, Eisenberg S, Bertsch LL & Kornberg A A mechanism of duplex DNA replication revealed by enzymatic studies of phage ϕΧ174: catalytic strand separation in advance of replication. Proc. Natl Acad. Sci. USA 74, 193–197 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotin RM et al. Site-specific integration by adeno-associated virus. Proc. Natl Acad. Sci. USA 87, 2211–2215 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith RH, Spano AJ & Kotin RM The Rep78 gene product of adeno-associated virus (AAV) self-associates to form a hexameric complex in the presence of AAV ori sequences. J. Virol 71, 4461–4471 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mansilla-Soto J et al. DNA structure modulates the oligomerization properties of the AAV initiator protein Rep68. PLoS Pathog. 5, e1000513 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCarty DM, Young SM Jr & Samulski RJ Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet 38, 819–845 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Cesar CE, Machon C, de la Cruz F & Llosa M A new domain of conjugative relaxase TrwC responsible for efficient oriT-specific recombination on minimal target sequences. Mol. Microbiol 62, 984–996 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Cesar CE & Llosa M TrwC-mediated site-specific recombination is controlled by host factors altering local DNA topology. J. Bacteriol 189, 9037–9043 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agundez L, Gonzalez-Prieto C, Machon C & Llosa M Site-specific integration of foreign DNA into minimal bacterial and human target sequences mediated by a conjugative relaxase. PLoS ONE 7, e31047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agundez L et al. Nuclear targeting of a bacterial integrase that mediates site-specific recombination between bacterial and human target sequences. Appl. Environ. Microbiol 77, 201–210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schröder G, Schuelein R, Quebatte M & Dehio C Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. Proc. Natl Acad. Sci. USA 108, 14643–14648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez-Gonzalez E et al. Transfer of R388 derivatives by a pathogenesis-associated type IV secretion system into both bacteria and human cells. J. Bacteriol 193, 6257–6265 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.González-Prieto C, Agúndez L, Linden RM & Llosa M HUH site-specific recombinases for targeted modification of the human genome. Trends Biotechnol. 31, 305–312 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Pritham EJ & Feschotte C Massive amplification of rolling-circle transposons in the lineage of the bat Myotis lucifugus. Proc. Natl Acad. Sci. USA 104, 1895–1900 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kapitonov VV & Jurka J Helitrons on a roll: eukaryotic rolling-circle transposons. Trends Genet. 23, 521–529 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Feschotte C & Wessler SR Treasures in the attic: rolling circle transposons discovered in eukaryotic genomes. Proc. Natl Acad. Sci. USA 98, 8923–8924 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Higgins CF, Ames GF, Barnes WM, Clement JM & Hofnung M A novel intercistronic regulatory element of prokaryotic operons. Nature 298, 760–762 (1982). [DOI] [PubMed] [Google Scholar]
- 116.Bachellier S, Clément J-M & Hofnung M Short palindromic repetitive DNA elements in enterobacteria: a survey. Res. Microbiol 150, 627–639 (1999). [DOI] [PubMed] [Google Scholar]
- 117.Nunvar J, Huckova T & Licha I Identification and characterization of repetitive extragenic palindromes (REP)-associated tyrosine transposases: implications for REP evolution and dynamics in bacterial genomes. BMC Genomics 11,44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bertels F & Rainey P B. Within-genome evolution of REPINs: a new family of miniature mobile DNA in bacteria. PLoS Genet. 7, e1002132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Braun V et al. A chimeric ribozyme in Clostridium difficile combines features of group I introns and insertion elements. Mol. Microbiol 36, 1447–1459 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.