Abstract
SUMMARY Background and purpose: d‐Serine, the endogenous co‐agonist of N‐methyl‐d‐aspartate (NMdA) receptors, has been recognized as an important gliotransmitter in the mammalian brain. d‐serine has been shown to prevent psychostimulant‐induced decrease in hippocampal neurogenesis. However, the mechanism whereby d‐serine regulates neurogenesis has not been fully characterized. Therefore, this study was designed to investigate the impacts of d‐serine on the proliferation, migration, and differentiation of primary cultured neural stem cells (NSCs). Methods and results: Immunohistochemistry analysis revealed NSCs expressed d‐serine as well as serine racemase (SR). Degradation of endogenous d‐serine with d‐amino acid oxidase (dAAO) significantly inhibited the proliferation and neuronal differentiation of NSCs, but failed to affect their radial migration. Reversely, addition of exogenous d‐serine did not affect the proliferation and migration of NSCs, but promoted NSC differentiation into neurons. Furthermore, dAAO could suppress the amplitude of glutamate‐induced Ca2+ transient, and thereby, inhibited the phosphorylation of glycogen synthase kinase3β (GSK3β), extracellular signal‐regulated kinases1/2 (ERK1/2), and cAMP‐responsive element‐binding protein (CREB). Conclusions: Our findings demonstrate for the first time that NSCs can synthesize d‐serine and, thereby, promote themselves proliferation and neuronal differentiation, which may afford a novel therapeutic strategy for the neurological disorders that require nerve cell replenishment, such as neurodegenerative diseases and stroke.
Keywords: Differentiation, d‐Serine, Neural stem cell, N‐methyl‐d‐aspartate, Proliferation
Introduction
d‐Amino acids were initially thought to exist only in lower organisms, such as bacterium and invertebrate [1]. In recent years, they have been detected at higher levels in mammalian tissues, especially in the brain [2, 3]. d‐Aspartate was the first d‐amino acid to be found in mammalian brain, and then Hashimoto et al. confirmed that another d‐amino acid called d‐serine was present in a significant amount in the brain [4]. d‐Serine is present at very high levels in the rodent and human brain (0.27 μmol/g), accounting for one‐third of the l‐serine [3]. Endogenous d‐serine is synthesized from l‐serine by serine racemase (SR) and is metabolized by the peroxisomal flavoprotein d‐amino acid oxidase (dAAO), converting it into pyruvate [5]. In adult mammalian brain, the amounts of d‐serine in cortex, hippocampus, striatum, and amygdaloid nucleus are larger than those in the other regions [6]. Recent data indicate that d‐serine, serving as an endogenous ligand for the glycine site of N‐methyl‐d‐aspartate (NMdA) receptors, is a major gliotransmitter in the central nervous system [6, 7, 8]. Increasing evidence has been focused on the roles of d‐serine in NMdA receptor‐mediated pathophysiological processes. The NMdA receptor is a major pharmacological target to prevent or decrease stroke damage, as its overactivation is a main culprit in the postischemic cell death that occurs after stroke [9]. Therefore, the prominent role of d‐serine in NMdA receptor‐mediated neurotoxicity has important pathological implications [10]. d‐Serine has also been shown to be involved in pain sensation in spinal cord [11] and NMdA hypofunction‐mediated schizophrenia [12]. In recent years, additional roles have been proposed for d‐serine, such as regulation of chondrogenesis [13]. Furthermore, d‐serine was reported to prevent a decrease in neurogenesis induced by psychostimulant phencyclidine (PCP) in the dentate gyrus (DG) by recuperating NMdA receptor function from chronic inhibition [14]. However, the precise mechanism of d‐serine modulation of neurogenesis remains unknown.
In the adult mammalian brain, neurogenesis occurs mainly in the subventricular zone (SVZ) and the subgranular zone (SGZ) of DG in the hippocampus [15]. The new interneurons in the SVZ can migrate with each other in chains through the rostral migratory stream (RMS) to the olfactory bulb, and then differentiate into either granule neurons or periglomerular neurons. In the adult DG, new neurons are born in the SGZ and migrate to granular cell layer where they integrate into the preexisting neuronal circuits [16]. Adult neurogenesis includes proliferation, migration, survival, differentiation, and functional integration of neuronal progeny into neuronal circuits, all these processes are regulated precisely by cell microenvironment [16]. Thus, the factors that control proliferation, self‐renewal, and differentiation of neural stem cells (NSCs) have received increasing interest. A plethora of developmental cues and physiological humoral factors have been shown to promote proliferation and maintenance of progenitor cells, such as Wnt, Sonic Hedgehog (Shh), membrane‐associated Notch signaling, and cytokines [17]. The balance of self‐renewal and differentiation of NSCs depends on the signals provided by the surrounding cells. Among those cells, astrocytes play a significant role in the adult neurogenesis by regulating the levels of multiple ions, neurotransmitters, hormones, and growth factors in the microenvironment for NSCs [16]. So far the NSCs have been regarded as a valuable source of cells used for transplantation to recover impairments in the nervous system [18]. The local environment may dictate the fate choice of transplanted NSCs. Nevertheless, our knowledge about the molecular mediators, especially the endogenous mediators, to regulate NSC proliferation, survival, migration, and differentiation is rather limited. So, a deep understanding of the molecular mechanism underlying the regulation of adult neurogenesis will help to find the novel target for drug development.
In this study, the effects of endogenous d‐serine on the proliferation, migration, and differentiation of NSCs derived from the newborn mouse SVZ were investigated. We demonstrate for the first time that d‐serine can regulate in vitro neurogenesis by modulating NMdA receptor‐dependent calcium influx and related signaling pathways. These results suggest that d‐serine is physiologically significant for NSC proliferation and for neuronal differentiation.
Materials and Methods
Animals
Male CD1 mice aged 2–3 months housed with free access to food and water in a room with an ambient temperature of 22 ± 2°C and a 12:12 h light/dark cycle. All experiments were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell Culture
Primary NSC cultures were generated as previously described [19]. Briefly, brains of newborn 1–3‐day‐old CD1 mice were microdissected to obtain the rostral periventricular region upon coronal sectioning. Tissues were finely minced and dissociated by trypsinization (0.125% (w/v) trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA) in Ca2+‐ and Mg2+‐free Hanks’ balanced salt solution [BSS]) at 37°C for 10 min. After centrifugation, cells were carefully dissociated by passaging in fire‐polished Pasteur pipettes and resuspended in serum‐free medium consisting of Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1) medium (Gibco, Grand Island, NY, USA), supplemented with 2% B27 (Gibco, Grand Island, NY, USA), epidermal growth factor (EGF; 20 ng/mL; Peprotech, Rocky Hill, NJ, USA; http://www.peprotech.com), and fibroblast growth factor (FGF)‐2 (20 ng/mL; Peprotech). Cells were seeded onto 25‐cm2 T‐flask and incubated at 37°C in 5% CO2 atmosphere. The NSCs formed neurospheres after 7 days in vitro. Then the neurospheres were collected and gently mechanically dissociated. Dissociated cells were resuspended in serum‐free medium and formed the secondary spheres after 7 days for the subsequent tests. The secondary sphere‐forming cells processed the potential to grow infinitely, and the proportion of the cells positive for nestin, a marker for immature neural progenitors, remained stable throughout the course of cell culture [19].
Immunohistochemistry
After perfusion, brains were postfixed overnight in paraformaldehyde at 4°C. Paraffin‐embedded brains were cut to 5 μm sections on a standard microtome. These sections were then deparaffinized before staining for d‐serine or SR. Dissociated NSCs were seeded on 24‐well culture plates coated with poly‐l‐ornithine (100 μg/mL; Sigma‐Aldrich, St. Louis, MO, USA) and laminin (5 μg/mL; Sigma‐Aldrich, St. Louis, MO, USA). After adherence, NSCs were rinsed carefully with 0.1 M phosphate buffered saline (PBS) (pH 7.20) and fixed with 4% paraformaldehyde, followed by blocking with PBS containing 5% bovine serum albumin for 1 h. After blocking, cells or sections were incubated at 4°C overnight, with the primary rabbit anti‐d‐serine antibody (1:1500; Gemacbio, Cenon, France; http://www.gemacbio.com) or rabbit anti‐SR antibody (1:500; Santa Cruz, Santa Cruz, CA, USA; http://www.scbt.com), and mouse anti‐Nestin antibody (1:200; Chemicon, MAB353; http://www.millipore.com). After being washed, cells or sections were incubated by goat anti‐rabbit F588 (1:800; Invitrogen, Carlsbad, CA, USA) and goat anti‐mouse F488 (1:1000; Invitrogen) for 1 h at room temperature. At last, the cells and sections were stained with Hoechst 33342 (5 μg/mL; Sigma‐Aldrich). Specimens were observed under a confocal microscope (Nikon, Japan) for visualization and photography.
High‐Performance Liquid Chromatography Analysis of d‐Serine Levels in Culture Medium
Dissociated NSCs from neurospheres were seeded on coated 24‐well culture plates at a density of 100,000 cells/mL in DMEM/F12 supplemented with B27, EGF, and FGF‐2. After confluence, the culture medium was replaced by 400 μL/well BSS buffer (NaCl 140 mM, KCl 5 mM, CaCl2 2 mM, HEPES 20 mM, glycine 0.03 mM, glucose 3 mM). BSS buffer excellular medium (20 μL) was taken after different incubation times. The levels of d‐serine and glutamate were measured by high‐performance liquid chromatography (HPLC) with fluorescent detection after precolumn derivatization with o‐phtaldialdehyde (OPA) using the system of Shimadzu scientific instruments (Kyoto, Japan). The OPA reagent (10 μL) was added to the sample (20 μL), mixed, incubated for 1.5 min, and then injected into the column. The amino acid derivatives were separated using a reverse phase C18 column (Waters), a HPLC RF‐10A fluorescence dector (Shimadzu Corp., Kyoto, Japan), and a liquid chromatography LC‐10AD (Shimadzu Corp., Kyoto, Japan). The emission wavelength was set at 443 nm and the excitation wavelength at 334 nm. The mobile phase was 100% methanol (B) and 40 mM Na2HPO4, 0.1 mM EDTA2Na, and 0.2% Triethylamine in water (A); pH was adjusted to 6.2 with H3PO4. The flow rate was set at 0.9 mL/min.
Cell Proliferation Assays
Proliferation was determined using 5‐bromodeoxyuridine (BrdU) incorporation [19]. The secondary spheres were collected and gently mechanically dissociated. Cells were plated on coated 24‐well culture plates at a density of 10,000 cells/mL (1 mL per well). After culture for 24 h, the medium was replaced by fresh culture medium, and then treated with d‐serine (1, 10, or 100 μM), MK‐801 (3, 10, or 30 μM), sodium benzoate (NaBZ 20 or 40 μM), or 0.1 U/mL dAAO, respectively, for 48 h. Cells were incubated for exactly 1 h with 10 μM BrdU, and then fixed and washed. DNA was denatured by treating the cells with 2 N HCl at 4°C for 30 min, washed with 0.1 M sodium borate (pH 8.5) for 10 min, and rinsed three times with PBS. After being blocked for 1 h in PBST containing 5% BSA at room temperature, cells were incubated with primary mouse anti‐BrdU antibody (1:15,000, Chemicon, MAB3510; http://www.millipore.com) for 24 h at 4°C followed by goat anti‐mouse F488 antibody (1:1000, Invitrogen). Then, cell nuclei were stained with Hoechst 33342. The number of BrdU+ cells and total number of cell nuclei were counted in nine randomly selected fields at 400× with an Nikon Optical TE2000‐S inverted microscope (Nikon, Japan).
NSC Migration Assay
The secondary neurospheres of similar diameter (about 200 μm) were selected and plated on coated 24‐well culture plates containing DMEM‐F12, supplemented with 2% B27, but without FGF‐2 and EGF. Then, they were incubated with d‐serine, dAAO, or MK‐801 for 48 h. Migration was quantified as the mean difference between the leading edge of radially migrating cells and the original neurosphere diameter by applying the measurement function in Image Pro Plus 5.1 (Media Cybernetics, Silver Spring, MD, USA).
NSC Differentiation
Dissociated NSCs from neurospheres were seeded on coated 24‐well culture plates at a density of 10,000 cells per well in DMEM/F12, containing 2% B27 and 1% fetal cattle serum (FCS, Gibco). dAAO, d‐serine, MK‐801, and NaBZ were added in different groups. After 7 days of differentiation, cells were probed with antibodies against neuron‐specific beta‐3 tubulin (TUJ1; 1:1000; Chemicon), GFAP (1:400; ABCam, Cambridge, MA, USA; http://www.abcam.com). The percentages of TUJ1+ and GFAP+ were quantified by normalizing total TUJ1+ or GFAP+ cells to the total number of cell nuclei labeled with Hoechst 33342.
Measurement of [Ca2+]
Neurospheres were plated on coated coverslips in DMEM/F12 with mitogens for 24 h. Then, cells were loaded for 30 min at 37°C with fluo‐3 AM (5 μM, Invitrogen). At the same time, cells were treated with 0.1 U/mL dAAO to exhaust the d‐serine. Intracellular glutamate‐induced Ca2+ transient were performed on cells bathed in imaging buffer comprising 140 mM NaCl, 3 mM KCl, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4). Through confocal series‐scan imaging with a CCD camera (Orca‐ER; Hamamatsu, Japan), fluo‐3 fluorescence images were acquired continuously every 5 seconds for 5 min. At least three independent cultures of neurospheres from three different litters were used for analysis.
Western Blotting
Cells were collected and washed by ice‐cold phosphate‐buffered saline and lysed in homogenization buffer (composed of 50 mM Tris‐HCl, 150 mM NaCl, 2 mM EDTA, 1 mM DTT, 1% Triton X‐100, 2.5 mM sodium pyrophophate, 1 mM β‐glycerol phosphate, 1 mM sodium vanadate, and 1 μg/mL leupeptin; pH 7.5) supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany). Protein lysates were quantified by Bradford assays (Bio‐Rad). Western blot analysis was performed by standard protocols using anti‐CREB, anti‐phospho‐CREB (pCREB)‐ser133 (Cell Signaling Technology, MA, USA), anti‐p42/p44 MAPKs, antiactivated p42/p44 MAPKs (Cell Signaling Technology), anti‐phospho‐Ser9‐GSK3β, and anti‐GSK3β (Cell Signaling Technology). Immunoreactive proteins were detected using HRP‐conjugated secondary antibodies and an ECL kit (Amersham Biosciences, NJ, USA), according to the manufacturer's instructions. The membranes were scanned and analyzed using an Omega 16ic Chemiluminescence Imaging System (Ultra‐Lum, CA, USA). The amount of phosphorylated proteins was normalized on respective total protein to assess the actual change in the fraction of total protein that was phosphorylated (and thus activated).
Statistics
All values were expressed as means ± SEM. The significance of the difference between control and samples treated with various drugs was determined by one‐way ANOVA, followed by the post hoc least significant difference test. Differences were considered significant at P < 0.05.
Results
d‐Serine Synthesis and Release from NSCs
To detect the localization of d‐serine and SR in the brain, we carried out immunofluorescence double staining of d‐serine or SR with NSC marker. As shown in Figure 1(A), nearly all of the NSCs labeled with Nestin were d‐serine and SR positive. Furthermore, all primary cultured cells expressed nestin, the intermediate filament protein of undifferentiated neural cells (Figure 1B), indicating that these cells were NSCs. If SR physiologically forms d‐serine, then this enzyme and its product should have similar localizations. Immunofluorescence analysis showed that the cultured NSCs expressed d‐serine and its biosynthetic enzyme SR, suggesting that the cultured NSCs could synthesize d‐serine. After 24‐h incubation to permit attachment of cells to the plate, the culture medium was removed and carefully replaced with BSS buffer. Then 20 μL of the supernatant was taken at different time points (0.5, 1, 2, 4, and 8 h) and was measured by HPLC. As shown in Figure 1(C), d‐serine level increased time dependently while the extracellular glutamate level hadn't changed significantly. These results indicate that NSCs can synthesize and release d‐serine. Cells cultured in BSS buffer for 8 h did not show any apoptotic changes in morphological features (data not shown).
Figure 1.
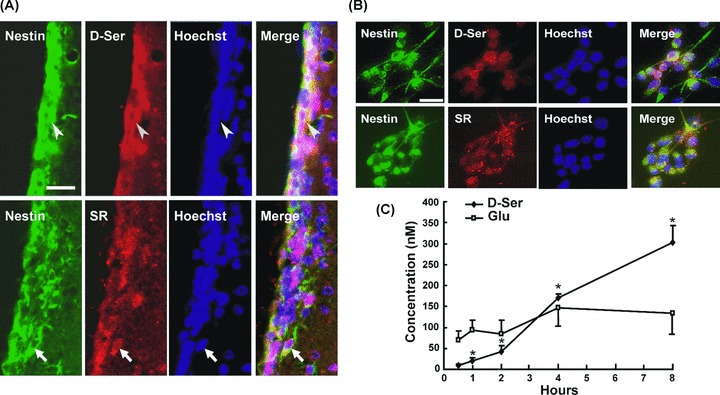
NSCs synthesize and release d‐serine by themselves. (A) Colocalization of d‐serine (arrowheads) and SR (arrows) with Nestin, a NSC marker, in adult mice SVZ. (B) Colocalization of d‐serine and SR with Nestin in cultured NSCs. Scale bar = 50 μM. (C) The extracellular concentrations of d‐serine and glutamate measured by HPLC. The amount of d‐serine in NSC medium was increased significantly during the incubation time from 0.5 to 8 h, while the content of glutamate had no significant change. n = 4. *P < 0.05 compared to the concentration at 0.5 h.
d‐Serine Maintains the Proliferation of NSCs
The effects of exogenous d‐serine on NSC proliferation were firstly examined. Addition of d‐serine (1, 10, or 100 μM) failed to affect the proliferation of NSCs, whereas the incubation with dAAO (0.1 U/mL) exhausting d‐serine could significantly suppress the multiplication rate of NSCs (26%) compared with control group (46%; Figure 2B). Moreover, the suppression of NSCs proliferation by dAAO could be attenuated by the pretreatment of the dAAO enzyme inhibitor, NaBZ (20 or 40 μM) or supplement with d‐serine (1, 10, or 100 μM; Figure 2D and F). To ascertain whether a NMdA receptor‐dependent mechanism is involved in d‐serine action, we further examined the effect of MK‐801, a noncompetitive NMdA receptor antagonist, on NSC proliferation. Similar to dAAO, MK‐801 could inhibit the proliferation of NSCs (Figure 2B). Preincubation with d‐serine (100 μM) alleviated MK‐801‐induced inhibition of NSC proliferation (Figure 2H). Furthermore, pretreatment with dAAO for 30 min followed by incubation with 1, 10, and 100 μM d‐serine for additional 48 h failed to change the proliferation of NSCs (Figure 2F).
Figure 2.
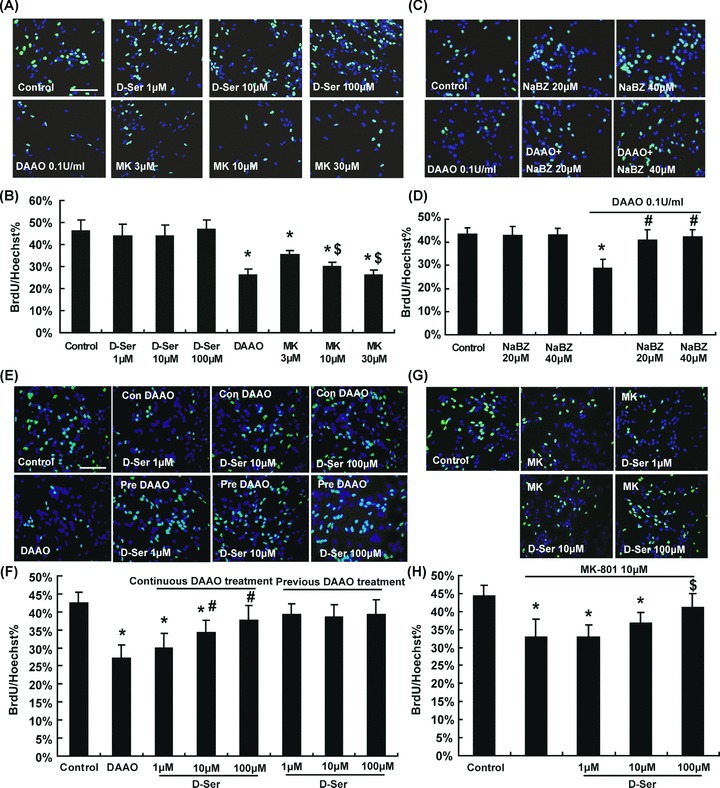
d‐Serine (1, 10, or 100 μM) could concentration dependently attenuate proliferation inhibition induced by continuous dAAO treatment. Exogenous d‐serine had no significant influence on NSCs proliferation; nevertheless, dAAO could significantly restrain their proliferation. Blocking of the NMdA receptor by MK‐801 could inhibit the proliferation of NSCs in a concentration dependent manner (A and B). The proliferation of NSCs couldn't be changed by the dAAO activity inhibitor sodium benzoate (NaBZ), while NaBZ alleviated dAAO‐induced NSCs proliferation inhibition (C and D). n = 5. *P < 0.05 as compared with the control group, $P < 0.05 as compared with the 3 μM MK‐801‐treated group and # with dAAO‐treated group. Scale bar = 100 μM. Pretreatment with dAAO, followed by incubation with 1, 10, or 100 μM d‐serine didn't influence the NSCs proliferation (E and F). Preincubation with d‐serine (100 μM) alleviated the MK‐801‐induced inhibition of NSCs proliferation (G and H). n = 5. *P < 0.05 as compared with the control group, #P < 0.05 as compared with the dAAO‐treated group and $ with 10 μM MK‐801‐treated group. Scale bar = 100 μM. MK: MK‐801. Con: continuous. Pre: previous.
d‐Serine Fails to Influence NSC Migration
Neurospheres cultured on plates coated with poly‐l‐ornithine and laminin cell culture substrate can migrate outward radially after adhesion, which is used to determine the NSCs migration ability. As shown in Figure 3, d‐serine, MK‐801, or dAAO didn't alter the distance of NSCs migration significantly.
Figure 3.
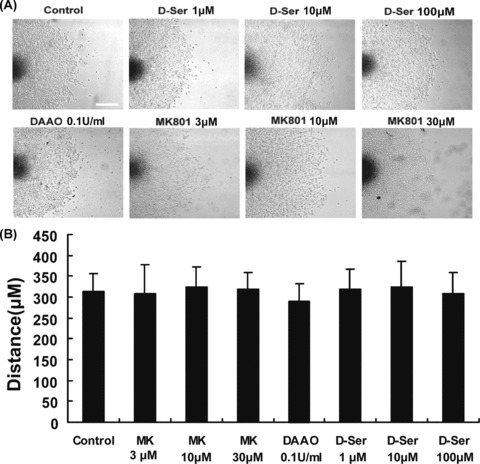
In vitro neurosphere migration assay. (A) SVZ neurospheres cultured on poly‐l‐ornithine and laminain‐coated coverslips migrate outwards radially. Scale bar = 200 μM. (B) d‐Serine, dAAO, and MK‐801 didn't influence the distance of radial migration of NSCs in vitro. n = 4.
d‐Serine Potentiates Neuronal Differentiation of NSCs
To investigate the role of d‐serine in neuronal and astroglial differentiation of NSCs, the percentage of TUJ1+ and GFAP+ cells was quantified by normalizing total TUJ1+ and GFAP+ cells to the total number of cell nuclei labeled with Hoechst 33342. After incubation with d‐serine (1, 10, or 100 μM) for 7 days, only 100 μM d‐serine significantly increased the neuronal differentiation of NSCs (16.5%) compared with control group (11.3%; Figure 4B). After incubation with dAAO (0.1 U/mL) for 7 days, the neuronal differentiation of NSCs remarkably decreased (6.8%). This effect could be alleviated by NaBZ (20 μM; Figure 4E). Likewise, the NMdA receptor antagonist MK‐801 inhibited the neuronal differentiation of NSCs. Furthermore, treatment of d‐serine (100 μM) could reverse the inhibition of neuronal differentiation triggered by dAAO and MK‐801 (Figure 4E). However, the proportion of GFAP+ cells was not significantly different among these groups (Figure 4C and F), suggesting that d‐serine, dAAO as well as MK‐801 failed to affect astroglial differentiation of NSCs.
Figure 4.
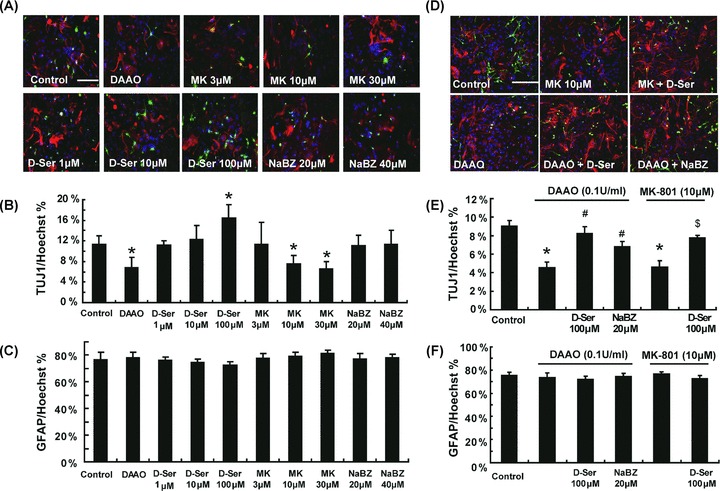
d‐Serine potentiates neuronal differentiation of NSCs. (A and B) d‐Serine (100 μM) significantly promoted the neuronal differentiation of NSCs. Either dAAO (0.1 U/mL) or MK‐801 (10 or 30 μM) prevented the neuronal differentiation remarkably, whereas compared with control group, no significant effect on the differentiation was detected in the presence of NaBZ. n = 5. *P < 0.05 as compared with the control group. Scale bar = 100 μM. (D and E) d‐Serine (100 μM) or NaBZ (20 μM) could alleviate dAAO‐induced prohibition of neuronal differentiation of NSCs. d‐Serine (100 μM) could also alleviate MK‐801‐induced inhibition of neuronal differentiation. n = 5. *P < 0.05 as compared with the control group, #P < 0.05 as compared with the dAAO‐treated group and $ with 10 μM MK‐801‐treated group. Scale bar = 100 μM. (C and F) On the other hand, dAAO, d‐serine, MK‐801, and NaBZ had no influence on the proportion of GFAP+ cells.
dAAO Suppresses Glutamate‐Induced Ca2+ Transient in NSCs
Extracellular Ca2+ can enter NSCs via ligand‐ and voltage‐gated Ca2+ channels [20]. NMdA receptors are ligand‐gated channels that upon activation are permeable for Ca2+. Glutamate, an excited amino acid, binds to the NMdA receptors on NSCs, then permits the influx of Na+ and Ca2+ and initiates the subsequent biochemical cascade events. The experiment results showed that 100 μM glutamate could result in Ca2+ transient increase in NSCs and the peak amplitude (ΔF/F0) of glutamate ‐induced Ca2+ transient was 3.22 ± 0.35 (n = 36 cells). After preincubation with dAAO for 30 min, the peak amplitude of glutamate‐induced Ca2+ transient was significantly suppressed (0.97 ± 0.13, n = 37 cells; Figure 5).
Figure 5.
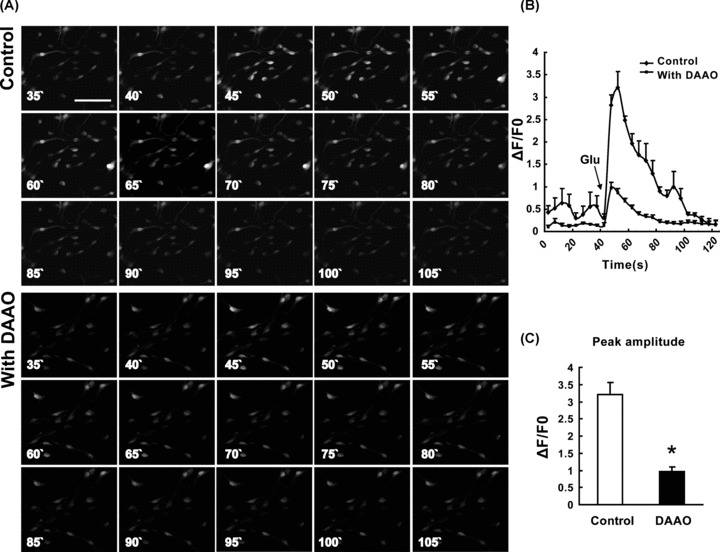
Effect of dAAO on the Ca2+ transient induced by glutamate. Sequence of images recorded over 90 seconds from control and dAAO group NSCs loaded with fluo‐3 AM following glutamate‐induced depolarization (A). Fluo‐3 fluorescence expressed as ΔF/F0; increased fluorescence indicates elevated [Ca2+]i. There was a significant inhibition of peak amplitudes in dAAO group compared with control group (B). Peak amplitudes of the glutamate‐induced Ca2+ transient show a significant decrease by preincubation with dAAO (C). n = 3. *P < 0.01 as compared with the control group. Scale bar = 50 μM.
dAAO Inhibits Phosphorylation of ERK1/2, CREB, and GSK‐3β in NSCs
Extracellular signal‐regulated kinases (ERK1/2) and cyclic AMP‐responsive element binding protein (CREB) have been implicated in signaling pathways related to proliferation and differentiation of NSCs [21, 22]. Thus, further study was to investigate the effect of dAAO on ERK1/2, CREB, and GSK‐3β phosphorylation. As shown in Figure 6, incubation with dAAO for 3 h significantly inhibited ERK1/2 phosphorylation in NSCs. After addition of dAAO up to 48 h, the phosphorylation of CREB and GSK‐3β was suppressed continuously and remarkably.
Figure 6.
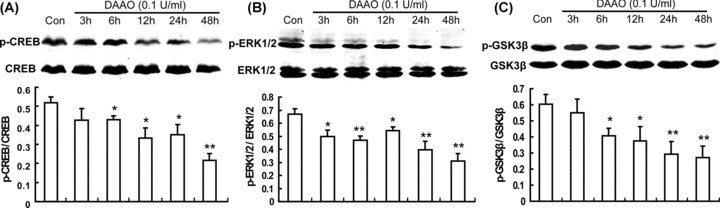
Effect of dAAO on CREB, ERK1/2, and GSK‐3β phosphorylation. After incubation with dAAO for 3 h, ERK1/2 phosphorylation significantly restrained (B). Six h after dAAO treatment, CREB phosphorylation (A) and GSK‐3β phosphorylation (C) remarkably decreased, and the suppression continued until 48 h. n = 4. *P < 0.05; **P < 0.01 as compared with the control group.
Discussion
d‐Serine, the endogenous ligand for the glycine site of the NMdA receptor [23], is a major gliotransmitter in the central nervous system [5]. Deletion of SR alters NMdA receptor neurotransmission and long‐term potentiation [7, 24]. Repeated d‐serine administration could decrease the PCP‐induced impairment of NSC proliferation [14]. Furthermore, during early neuronal development, d‐serine released from Bergmann glia seems to be essential in the migration of granule cells [25]. However, whether d‐serine plays a critical role in regulating the fundamental properties of NSCs remains unknown. In this study, we showed that the primary‐cultured forebrain NSCs could synthesize and release d‐serine. Moreover, d‐serine supported the proliferation and neuronal differentiation of NSCs, and could alleviate MK‐801‐induced inhibition of NSC proliferation. Our findings suggest that d‐serine promotes neurogenesis through acting on NMdA receptor and subsequently regulating the Ca2+‐related signaling pathways, including the phosphorylation of ERK1/2‐CREB and GSK‐3β. This may allow us to translate this proproliferation and proneural differentiation effect into therapeutic interventions for neurodegenerative diseases.
NSCs are the progenitor cells in the brain, which are capable of continuous self‐renewal and differentiation into neurons, astrocytes, and oligodendrocytes [17]. They are present not only during the embryonic development but also in the adult brain of all mammalian species. These cells hold great promise for neural repair after injury or disease [26]. Transplantation of stem cells or their derivatives, and mobilization of endogenous stem cells within the adult brain, have been proposed as future therapies for neurodegenerative diseases [27]. We confirmed that the NSCs expressed SR and d‐serine. Furthermore, the amount of d‐serine, instead of glutamate, in the medium of NSCs exhibited a time‐dependent increase during the incubation. These results indicate that NSCs not only synthesize d‐serine, but also release it into the extracellular environment to participate in the regulation of NSC function.
Our study showed that addition of exogenous d‐serine failed to influence the proliferation of NSCs. However, proliferation of NSCs was significantly suppressed after dAAO was used to exhaust extracellular d‐serine. dAAO is a FAD‐containing enzyme that catalyzes oxidative deamination of d‐amino acids yielding hydrogen peroxide and an imino acid. It almost inactive towards the corresponding l‐isomer [28]. Thus, d‐serine is essential to maintain basal proliferation of NSCs. Furthermore, d‐serine could reverse the attenuation of NSC proliferation by the NMdA receptor antagonist MK‐801, suggesting that d‐serine may regulate the proliferation of NSCs through acting on NMdA receptor. In the brain, the affinity of NMdA receptors for d‐serine is at least as high as glycine [9]. Moreover, the glycine site at the NMdA receptor is saturated by d‐serine [29]. NMdA‐receptor levels on NSCs are relatively low [30, 31]. Endogenous d‐serine originated from NSCs sufficiently saturates glycine site at the NMdA receptor due to both autocrine and paracrine effects. Therefore, adding d‐serine in culture media failed to affect the NSC proliferation, whereas exhausting d‐serine could significantly suppress the proliferation. Our findings reveal that d‐serine is responsible for NSC proliferation and regulates neurogenesis via acting on NMdA receptor.
NSCs are multipotent stem cells, which can differentiate to neurons, astrocytes, and oligodendrocytes [32]. The differentiation of NSCs is influenced by multiple extracellular molecular regulators, such as ions, neurotransmitters, hormones, and extracellular matrix [33]. In this study, exhausting d‐serine with dAAO remarkably inhibited the neuronal differentiation, while 100 μM d‐serine could promote the neuronal differentiation and alleviate MK‐801‐induced inhibition of neuronal differentiation. Therefore, we suppose that d‐serine might promote neuronal differentiation by strengthening NMdA receptor‐mediated pathways. NMdA receptor has been demonstrated to play an important role in the differentiation of NSCs [34]. NMdA receptor activation induces an immediate influx of Ca2+ into NSCs, then increases expression of NeuroD (a positive regulator of neuronal differentiation) by Ca2+/calmodulin‐dependent protein kinase pathway, and ultimately promotes the neuronal differentiation of NSCs [20, 35]. Furthermore, the NMdA receptors expressed on newborn neurons gradually increase as the differentiating neurons maturate during the differentiation of the NSCs [36]. We suppose that d‐serine acts on the emerging NMdA receptors on neurons and makes them more mature, thereby promoting the neuronal differentiation of NSCs further. Simultaneously, our study showed that d‐serine failed to alter glial differentiation and radial migration of NSCs.
The spatial and temporal pattern of Ca2+ influx is crucial for the regulation of several cellular physiological processes, such as cell growth and metabolism. Ca2+ signaling affects every aspect of a cell's life and death, including regulating self‐renewal and fate specification of NSCs [37, 38]. Alterations in the intracellular Ca2+ activities could induce embryonic stem cells to differentiate into neuronal phenotypes [39]. Therefore, the influence of d‐serine on NSC differentiation as well as proliferation might be associated with the regulation of intracellular Ca2+ ([Ca2+]i). Our study showed that 100 μM glutamate induced an immediate influx of Ca2+ into NSCs, in accordance with a significant increase of the intracellular fluorescence intensity of the fluo‐3 for Ca2+. And the peak amplitude of glutamate‐induced Ca2+ transient was decreased by dAAO. These findings indicate that d‐serine contributes to the neural differentiation via regulating Ca2+‐related pathways.
Increased Ca2+ may activate ERK1/2, as in the astrocytes [40] and striatal neurons [41], which may initiate a cascade of events resulting in proliferation of NSCs. Therefore, we investigated whether ERK1/2 is a mediator in the signaling cascades initiated by d‐serine. CREB lies at the center of a diverse array of intracellular signaling pathways and is a major transcriptional regulator of numerous functions. It has been demonstrated that activated CREB promotes proliferation of NSCs [42]. CREB phosphorylation is affected by many upstream protein kinases, such as ERK1/2, protein kinase A (PKA), and calmodulin‐dependent protein kinase II/IV (CaMKII/IV) [43]. We found that the phosphorylation of CREB and its upstream protein kinase ERK1/2 was downregulated significantly after incubation with dAAO, indicating that ERK1/2‐CREB participates in the regulation of NSC proliferation by d‐serine. GSK‐3β is another important kinase to participate in the regulation of NSC proliferation [44]. Phosphorylation of GSK‐3β could stabilize β‐catenin, which activates downstream transcription factors of lymphoid enhancer factor/T cell factor (LEF/TCF) family and cyclin D upregulation, promoting proliferation of NSCs eventually [44]. In this study, we found that exhausting d‐serine resulted in a significant suppression of GSK‐3β phosphorylation, suggesting that maintenance of GSK‐3β phosphorylation is responsible for d‐serine‐mediated action on NSCs.
In conclusion, this study demonstrates for the first time that NSCs can biosynthesize and release d‐serine that sustains the proliferation and promotes neuronal differentiation of NSCs by acting on NMdA receptor and regulating [Ca2+]i, as well as the ERK1/2‐CREB and GSK‐3β signaling pathways. Therefore, our findings not only reveal a novel physiological profile of d‐serine as an endogenous regulator of neurogenesis, but also help to develop a novel therapeutic strategy for the neurological disorders that require nerve cell replenishment, such as neurodegenerative diseases and stroke.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This study was supported by grants from the National Key Basic Research Program of China (No.2009CB521906 and No.2011CB504100) and the National Natural Science Foundation of China (No.81030060 and No.30973517).
References
- 1. Corrigan JJ. D‐amino acids in animals. Science 1969;164:142–149. [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto A, Nishikawa T, Hayashi T, et al The presence of free D‐serine in rat brain. FEBS Lett 1992;296:33–36. [DOI] [PubMed] [Google Scholar]
- 3. Hashimoto A, Oka T, Nishikawa T. Anatomical distribution and postnatal changes in endogenous free D‐aspartate and D‐serine in rat brain and periphery. Eur J Neurosci 1995;7:1657–1663. [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto A, Kumashiro S, Nishikawa T, et al Embryonic development and postnatal changes in free D‐aspartate and D‐serine in the human prefrontal cortex. J Neurochem 1993;61:348–351. [DOI] [PubMed] [Google Scholar]
- 5. Scolari MJ, Acosta GB. D‐serine: A new word in the glutamatergic neuro‐glial language. Amino Acids 2007;33:563–574. [DOI] [PubMed] [Google Scholar]
- 6. Wolosker H, Dumin E, Balan L, Foltyn VN. D‐amino acids in the brain: D‐serine in neurotransmission and neurodegeneration. FEBS J 2008;275:3514–3526. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, Ge W, Chen Y, et al Contribution of astrocytes to hippocampal long‐term potentiation through release of D‐serine. Proc Natl Acad Sci U S A 2003;100:15194–15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens ER, Esguerra M, Kim PM, et al D‐serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A 2003;100:6789–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolosker H. NMDA receptor regulation by D‐serine: New findings and perspectives. Mol Neurobiol 2007;36:152–164. [DOI] [PubMed] [Google Scholar]
- 10. Oliet SH, Mothet JP. Regulation of N‐methyl‐D‐aspartate receptors by astrocytic D‐serine. Neuroscience 2009;158:275–283. [DOI] [PubMed] [Google Scholar]
- 11. Wake K, Yamazaki H, Hanzawa S, et al Exaggerated responses to chronic nociceptive stimuli and enhancement of N‐methyl‐D‐aspartate receptor‐mediated synaptic transmission in mutant mice lacking D‐amino‐acid oxidase. Neurosci Lett 2001;297:25–28. [DOI] [PubMed] [Google Scholar]
- 12. Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci 2003;1003:318–327. [DOI] [PubMed] [Google Scholar]
- 13. Takarada T, Takahata Y, Iemata M, et al Interference with cellular differentiation by D‐serine through antagonism at N‐methyl‐D‐aspartate receptors composed of NR1 and NR3A subunits in chondrocytes. J Cell Physiol 2009;220:756–764. [DOI] [PubMed] [Google Scholar]
- 14. Maeda K, Sugino H, Hirose T, et al Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci 2007;103:299–308. [DOI] [PubMed] [Google Scholar]
- 15. Aimone JB, Deng W, Gage FH. Adult neurogenesis: Integrating theories and separating functions. Trends Cogn Sci 2010;14:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132:645–660. [DOI] [PubMed] [Google Scholar]
- 17. Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res 2009;19:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pluchino S, Zanotti L, Deleidi M, Martino G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Brain Res Rev 2005;48:211–219. [DOI] [PubMed] [Google Scholar]
- 19. Ferron SR, Andreu‐Agullo C, Mira H, Sanchez P, Marques‐Torrejon MA, Farinas I. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat Protoc 2007;2:849–859. [DOI] [PubMed] [Google Scholar]
- 20. Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation‐neurogenesis coupling in adult neural stem/progenitor cells. Neuron 2004;42:535–552. [DOI] [PubMed] [Google Scholar]
- 21. Hao Y, Creson T, Zhang L, et al Mood stabilizer valproate promotes ERK pathway‐dependent cortical neuronal growth and neurogenesis. J Neurosci 2004;24:6590–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 2007;67:1348–1361. [DOI] [PubMed] [Google Scholar]
- 23. Mothet JP, Parent AT, Wolosker H, et al D‐serine is an endogenous ligand for the glycine site of the N‐methyl‐D‐aspartate receptor. Proc Natl Acad Sci U S A 2000;97:4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panatier A, Theodosis DT, Mothet JP, et al Glia‐derived D‐serine controls NMDA receptor activity and synaptic memory. Cell 2006;125:775–784. [DOI] [PubMed] [Google Scholar]
- 25. Kim PM, Aizawa H, Kim PS, et al Serine racemase: Activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A 2005;102:2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci 2006;7:395–406. [DOI] [PubMed] [Google Scholar]
- 27. Lindvall O, Kokaia Z, Martinez‐Serrano A. Stem cell therapy for human neurodegenerative disorders‐how to make it work. Nat Med 2004;10(Suppl):S42–S50. [DOI] [PubMed] [Google Scholar]
- 28. Khoronenkova SV, Tishkov VI. D‐amino acid oxidase: Physiological role and applications. Biochemistry (Mosc) 2008;73:1511–1518. [DOI] [PubMed] [Google Scholar]
- 29. Czepita D, Daw NW, Reid SN. Glycine at the NMDA receptor in cat visual cortex: Saturation and changes with age. J Neurophysiol 1996;75:311–317. [DOI] [PubMed] [Google Scholar]
- 30. Varju P, Schlett K, Eisel U, Madarasz E. Schedule of NMDA receptor subunit expression and functional channel formation in the course of in vitro‐induced neurogenesis. J Neurochem 2001;77:1444–1456. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci 2006;24:645–653. [DOI] [PubMed] [Google Scholar]
- 32. Kriegstein A, Alvarez‐Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009;32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol 2009;217:169–180. [DOI] [PubMed] [Google Scholar]
- 34. Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 2009;25:253–275. [DOI] [PubMed] [Google Scholar]
- 35. Joo JY, Kim BW, Lee JS, et al Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci 2007;120:1358–1370. [DOI] [PubMed] [Google Scholar]
- 36. Jelitai M, Schlett K, Varju P, Eisel U, Madarasz E. Regulated appearance of NMDA receptor subunits and channel functions during in vitro neuronal differentiation. J Neurobiol 2002;51:54–65. [DOI] [PubMed] [Google Scholar]
- 37. Schneider JW, Gao Z, Li S, et al Small‐molecule activation of neuronal cell fate. Nat Chem Biol 2008;4:408–410. [DOI] [PubMed] [Google Scholar]
- 38. Clapham DE. Calcium signaling. Cell 2007;131:1047–1058. [DOI] [PubMed] [Google Scholar]
- 39. Ulrich H, Majumder P. Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: From basic research towards clinical applications. Cell Prolif 2006;39:281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bezzi P, Domercq M, Brambilla L, et al CXCR4‐activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat Neurosci 2001;4:702–710. [DOI] [PubMed] [Google Scholar]
- 41. Zanassi P, Paolillo M, Feliciello A, Avvedimento EV, Gallo V, Schinelli S. cAMP‐dependent protein kinase induces cAMP‐response element‐binding protein phosphorylation via an intracellular calcium release/ERK‐dependent pathway in striatal neurons. J Biol Chem 2001;276:11487–11495. [DOI] [PubMed] [Google Scholar]
- 42. Dworkin S, Malaterre J, Hollande F, Darcy PK, Ramsay RG, Mantamadiotis T. cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 2009;27:1347–1357. [DOI] [PubMed] [Google Scholar]
- 43. Tiraboschi E, Tardito D, Kasahara J, et al Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology 2004;29:1831–1840. [DOI] [PubMed] [Google Scholar]
- 44. Ming GL, Song H. DISC1 partners with GSK3beta in neurogenesis. Cell 2009;136:990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]


