Summary
Aims
1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) is a neurotoxin widely used to produce experimental models of Parkinson's disease in laboratory animals. It is believed to cause a selective destruction of substantia nigra dopamine neurons, mainly based on a large reduction of tyrosine hydroxylase (TH), the catecholamine's synthesizing enzyme. Unlike Parkinson's disease in humans, however, all animal models are able to recover more or less rapidly from the MPTP induced Parkinsonian syndrome. This raises the question as whether MPTP causes a cell death with a decrease in dopamine transporter or a simple impairment of TH.
Methods
To respond to this question, we quantified in a cat model of Parkinson's disease (MPTP 5 mg/kg i.p. during 5 days) the dopamine transporter using positron emission tomography (PET) imaging and autoradiography of [11C]PE2I and compared the data with the TH‐immunoreactivity.
Results
We found no changes in [11C]PE2I PET binding either 5 or 26 days after MPTP treatment when compared to baseline levels. Similarly, there were no significant changes in [11C]PE2I autoradiographic binding in the cat brain one week after MPTP treatment. In sharp contrast, MPTP treated cats exhibited severe Parkinson‐like motor syndrome during the acute period with a marked decrease in TH‐immunoreactivity in the striatum.
Conclusion
These data suggest that MPTP toxicity impairs efficiently TH and that such an effect is not necessarily accompanied by significant reduction of dopamine transporter seen with in vitro or in vivo [11C]PE2I binding.
Keywords: Autoradiography; Cat; 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine; Neurodegeneration; Parkinson's disease; PE2I; Positron emission tomography
Introduction
The underlying mechanisms of Parkinson's disease remain partly unknown, although several hypotheses have been proposed for linking disruption of dopaminergic pathways to oxidative stress, inflammation, or exposure to environmental agents 1, 2, 3. In this context, the use of toxin‐based animal models has given useful insights into the pathology of Parkinson's disease. The neurotoxin 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) is supposed to destroy dopamine neurons by a well‐known mechanism. After systematic administration, MPTP crosses the blood–brain barrier and is converted by monoamine oxidase B to 1‐methyl‐4‐phenyl‐2,3‐dihydropyridinium (MPDP), which is oxidized to 1‐methyl‐4‐phenylpyridinium (MPP+). MPP+ is then taken up by dopamine neurons through the dopamine transporter (DAT), and acts as an inhibitor of mitochondrial complex I of the respiratory chain causing cell death. MPTP functions as a potent neurotoxin in both mice and primates, although mice appear to be less sensitive than monkeys 4, 5, 6.
The feline model constitutes an alternative model of Parkinsonism. Schneider and colleagues have shown that the cat is sensitive to the neurotoxic effects of MPTP 7, 8, 9, as MPTP administration produces a behavioral Parkinson‐like syndrome, accompanied by a striatal dopamine depletion 10, 11. Unlike humans in which MPTP intoxication causes permanent Parkinson's syndrome, animal models, including the cat and monkey, are able to recover more or less rapidly from this syndrome. This raises the question as whether MPTP causes a cell death or a simple impairment of tyrosine hydroxylase (TH) in dopaminergic neurons. To respond to this question, the use of markers other than TH to follow‐up dopaminergic neurons, such as the DAT appears necessary.
Positron emission tomography (PET) enables the direct measurement of various components of the dopamine system in the animal and human brain and has served as a useful tool in monitoring neurotransmission in vivo and in diagnosis. However, the utility of imaging in animal models of Parkinson's disease has not been extensively evaluated, particularly in the feline model. Therefore, our aim was to evaluate the alterations in DAT during the initial phase of MPTP neurotoxicity and correlate these changes with behavioral and TH‐immunohistochemical data. For this purpose, we used [11C]PE2I (N‐(3‐iodopro‐2E‐enyl)‐2beta‐carbomethoxy‐3beta‐(4′‐methylphenyl) nortropane), a high affinity DAT inhibitor, as tracer to evaluate the striatal DAT levels both in vivo using PET and in vitro using autoradiography in the cat.
Methods
Animal model
All experiments followed European Ethics Committee (86/6091EEC) and French National Committee (decree 87/848) directives. The experimental protocol was approved by the Ethic Committee of the University of Lyon. Every effort was made to minimize the number of animals used and any pain and discomfort.
Experiments were carried out in cats of both sexes weighing 3.2–4.1 kg, born and bred in our own animal facilities. They were implanted electrodes for behavioral EEG and sleep‐wake monitoring to study the possible correlation between the MPTP‐induced behavioral and sleep‐wake effects and the loss of dopaminergic neurons. After baseline recordings, they were given by intraperitoneal route 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP, Sigma, St. Louis, MO, USA) at dose of 5 mg/kg i.p. for five consecutive days 12. Cortical EEG and sleep‐wake parameters were observed during 3–5 weeks after the first MPTP injection. Among these animals, six cats were used in addition to the sleep‐wake study for PET imaging, autoradiographic, and immunohistochemical studies, and the data are reported in this study. EEG and sleep‐wake data will be reported separately. [11C]PE2I PET imaging was examined before (control), 5 or 26 days after the first MPTP injection.
Magnetic Resonance Imaging (MRI) Procedure
Before the beginning of MPTP administrations, three cats were used to build an MRI template. They were anesthetized with 2.5% isoflurane, and their head was immobilized in a stereotaxic plexiglas frame with ear bars, orbital, and hard palate pieces. MRI acquisitions (1.5‐T Siemens Magnetom scanner; Siemens AG, Erglangen, Germany) consisted of a three‐dimensional anatomical T1‐weighted sequence, which lasted 40 min. The anatomical volume covered the whole brain with 0.7 mm3 voxels.
PET Procedure
The PE2I precursor was synthesized and radiolabelled by [11C]CH3I O‐methylation as previously described 13. The specific activity of the injected [11C]PE2I ranged from 74 to 148 × 103 MBq/μmol (2–4 Ci/μmol, at the time of injection). PET acquisitions were performed on a Siemens CTI‐ECAT HR+ (Knoxville, TN, USA). The three cats were scanned twice to determine baseline values, and once after 1 week of daily injections of MPTP (5 mg/kg, i.p. ×5). One of the cats was also scanned after a second series of daily MPTP injections (5 mg/kg, i.p. ×5) and 3 weeks later.
After isoflurane anesthesia, a catheter was inserted into the forearm branch of the brachiocephalic vein. Animals were secured with a stereotaxic plexiglas frame defining the horizontal plane. A 10‐min transmission scan was performed, followed by a bolus injection of 74 MBq (2 mCi) of 11C‐PE2I. Radioactivity was measured in series of sequential time frames of increasing duration from 30 s to 10 min for a total time of 60 min. Sinograms were normalized, attenuated, corrected for scatter and reconstructed with a filtered backprojection yielding a dynamic study of 15 volumes of 128 × 128 × 63 with a voxel size of 0.86 × 0.86 × 2.42 mm3 14.
Dynamic PET volumes were integrated and manually co‐registered with MRI data (MNI‐BIC Software; Montreal, QC, Canada) using a rigid body transformation with 6 degrees of freedom. A 330 mm3 ellipse was drawn in the center of cerebellum on the MRI. The cerebellum was chosen as the region of reference as it is almost devoid of dopamine transporter binding sites 15.
Parametric images of BP (the ratio of available receptor density to receptor affinity), k 2 (the tracer's efflux in the vascular system), and R 1 (the ratio of plasma to brain transport constant) were calculated from individual voxel time–activity curves using Receptor Parametric Mapping software 16. BP volumes were then automatically co‐registered, using PET‐to‐PET cross‐correlation with 7 degrees of freedom. The same transformation matrices were applied to parametric images of k 2 and R 1.
All PET volumes of BP were averaged into a single volume, which was then fused with the MRI to draw the regions containing BP (Figure 1), regrouped into anatomical volumes of interest (VOIs). Snyder and Niemer's stereotaxic atlas of the cat brain 17 was used as an anatomical reference to draw the right and left striatum (2 × 270 mm3), and midbrain region (100 mm3). Regional radioactivity concentration (nCi/cc) was also measured in the dynamic PET volumes for each VOI and plotted versus time.
Figure 1.
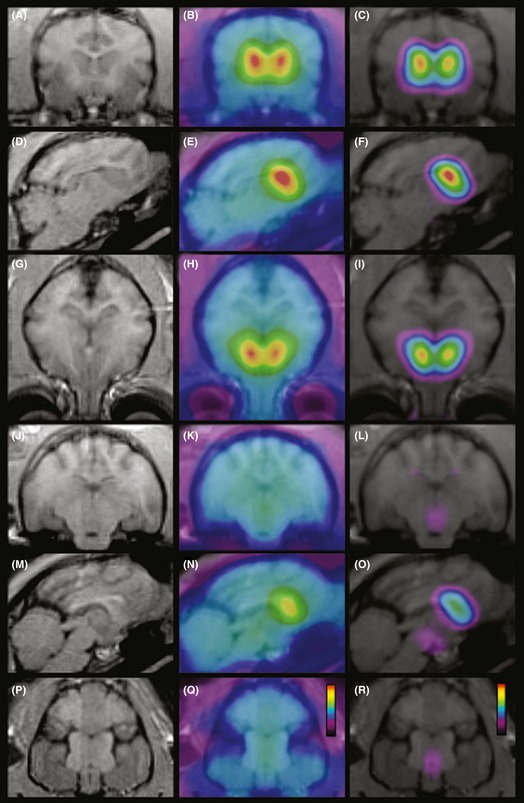
MRI (A, D, G, J, M, P), PET distribution of 11C‐PE2I binding (B, E, H, K, N, Q), and co‐registered MRI/PET BP (C, F, I, L, O, R) images in the cat brain at different anatomical levels in the coronal (A–C, J–L), sagittal (D–F, M–O), and horizontal (G–I, P–R) planes. PET data were obtained from the sum of eleven scans performed in three cats (74 MBq of 11C‐PE2I, anesthetized with isoflurane). Pseudocolor scales range from 0 to 2000 nCi/cc and 0.5–7 in Q and R, respectively.
Differences in BP between control and MPTP treated cats were assessed by non‐parametric repeated measures ANOVA (Friedman's test) followed by Mann–Whitney's test for P < 0.05 (InStat 3.06, GraphPad Software, San Diego, CA, USA).
Autoradiography Procedure
In vitro autoradiographic studies were performed similarly for one control cat and for one MPTP pretreated cat. After euthanasia by an i.p. pentobarbital overdose, the cat was intracardiacally perfused with a Ringer solution. The brain was carefully removed and immediately frozen in 2‐methylbutane cooled with dry ice at −29°C. Thirty‐μm‐thick coronal sections were cut using a −20°C‐cryostat (Microm‐Microtech, Paris, France), thaw‐mounted on glass slides and stored at −80°C. The day of [11C]PE2I synthesis, the slides were allowed to reach ambient temperature and were then incubated 20 min in Tris phosphate‐buffered saline buffer (138 mm NaCl, 2.7 mm KCl, pH 7.6) containing 37 kBq/mL (1 μCi/mL) of [11C]PE2I. After incubation, the slides were dipped in cold buffer and distilled water (4°C), then dried and juxtaposed to a phosphor imaging plate for 60 min (BAS‐1800 II; Fujifilm, Tokyo, Japan). Regions of interest were drawn manually using Multigauge software (Fujifilm). Measurements obtained in the caudate nucleus and putamen were normalized using the cerebellum and expressed as Standard Uptake Value.
Immunohistochemistry of Tyrosine Hydroxylase
After PET procedure, the MPTP‐treated cats were processed for TH immunohistochemistry as described in a previous study 18. Data from non‐treated animals, already obtained in our laboratory, were used as control. Briefly, under deep anesthesia, cats were perfused with paraformaldheyde and brain blocks were cut using a cryostat (−22°C). Freely floating coronal sections (25 μm thick) were incubated successively with (i) a rabbit polyclonal anti‐TH antibody (1:20000, for 72h at 4°C; Jacques Boy Institute, Reims, France); (ii) an anti‐rabbit IgG antibody (BA‐1000; Vector Laboratory, Burlingam, CA, USA); (iii) an avidin–biotin complex (Vectastain ABC Kit ref PK6100; Vector laboratory). The two latter incubations were at 4°C overnight. Finally, the immunohistochemical product was revealed using 3‐3′ diaminobenzidine tetrahydrochloride (DAB; Sigma)‐nickel technique.
Results
Behavior
As previously reported, MPTP‐treatment (5 mg/kg. i.p. ×5 days) caused, during the acute period (up to 2 weeks), a severe Parkinson‐like motor syndrome characterized by a sharp decrease in animal activity and locomotion (akinesia), and a marked hesitation to initiate movement 7, 12, 19. Moreover, these cats presented a severe hypersomnia in slow wave sleep (SWS), accompanied by wake deficiency and pronounced behavioral somnolence reminiscent of excessive daytime sleepiness. During the chronic period (3rd–4thweek post‐treatment), whereas the amount of waking and SWS returned to control level, paradoxical sleep showed transient increase (+30–50% of control over 2–4 days), accompanied by prolonged episode duration and narcolepsy‐like episodes similar to that seen in Parkinsonian patients. Data will be presented in a separate study in preparation.
PET
After the intravenous injection of [11C]PE2I, radioactivity rapidly accumulated in the brain with a high uptake of radioactivity in the striatum (Figure 1B,E,H,N) and to a lesser extent in the midbrain (Figure 1K,N,Q), which remained high during the remainder of the scan (Figure 2A–B). In contrast, after initial washout, few radioactivity remained in the cerebellum region (Figure 2A–B). This regional pattern was even more clear cut when observing the BP distribution, with high values in the striatum (Figure 1C,F,I,O), medium in the midbrain (Figure 1L,O,R), and almost nil in other brain regions.
Figure 2.
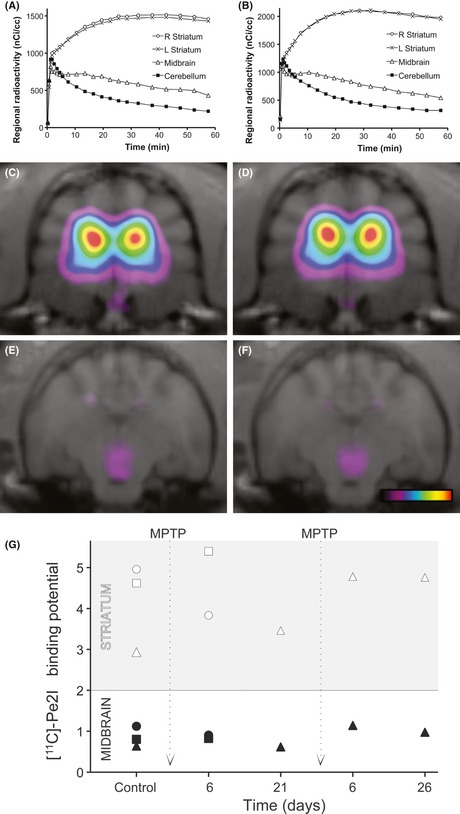
The time–activity curves (in A and B) are the sum of 11C‐PE2I binding in the striatum and cerebellum before (A) and after 1 week of daily injections of 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) (B) in the same three cats. Regional radioactivity is expressed in nCi/cc. C, E and D, F are color‐coded parametric images representing 11C‐PE2I BP in the striatum and midbrain, as described in materials and methods, before and after 1 week of MPTP treatment, respectively. Pseudocolor scale ranges from 0.5 to 6.
There were no apparent changes between [11C]PE2I radioactivity time curves before and after MPTP treatment (Figure 2A–B). Accordingly, the distribution and values of [11C]PE2I parameters (R1, k2, and BP) were also unchanged in the striatum and midbrain of the cat following one or 2 weeks of daily injections of MPTP, or even 3 weeks later (Figure 2G).
Autoradiography
Autoradiography revealed a high density of [11C]PE2I binding sites in the caudate nucleus and putamen (Figure 3A–B). In contrast, the cerebellum was almost devoid of any labeling (Figure 3C‐D). There were no apparent changes in [11C]PE2I binding in any region examined after 1 week of MPTP treatment (Figure 3).
Figure 3.
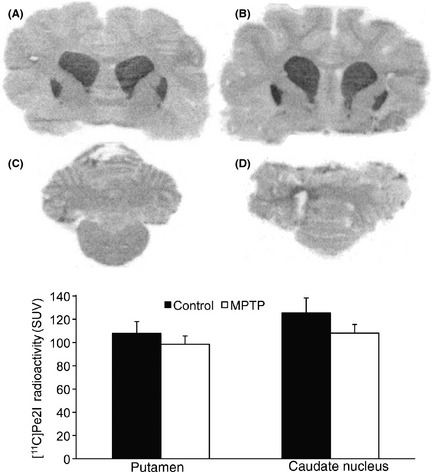
Autoradiographic distribution of 11C‐PE2I in the caudate nucleaus, putamen (A, B), and cerebellum (C, D) in a control cat (A, C) and after 1 week of daily injections of 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (B, D). Histogram representing 11C‐PE2I radioactivity measured in the aforementioned regions of interest.
Immunocytochemistry
In contrast and in consistence with previous studies 7, 8, 10, 12, immunohistochemistry of tyrosine hydroxylase (TH) revealed a marked decrease in TH‐immunoreactivity in the whole brain of the MPTP‐treated animals. There was a significant decrease in the number of TH‐immunoreactive cell bodies (estimated to 40–50%) and density of the labeling in the substantia nigra and adjacent midbrain dopaminergic structures either at day 6 or at days 21–26 of the MPTP treatment. The number and intensity of TH‐immunoreactive varicose fibers and terminal‐like dots were also sharply reduced in the striatum (see Figure 4, compare the right panel, MPTP treatment to the left one, control animal with saline injection).
Figure 4.
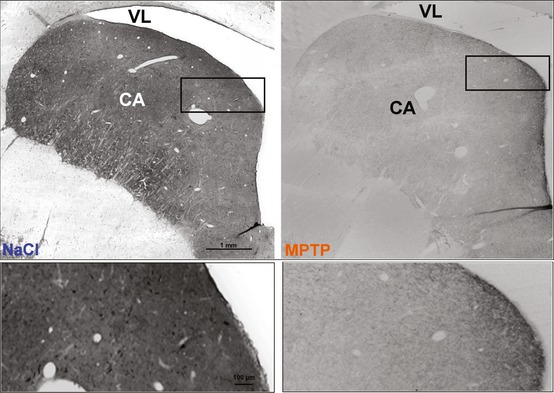
Photomicrographs depicting the effects of 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) (5 mg/kg, i.p. ×5 daily injection), on the ex‐vivo immunoreactivity of tyrosine hydroxylase (TH) in the striatum (Caudate nucleus, CA) 3 weeks after injections. Note that the MPTP treatment strikingly decreased the density of TH‐positive fibers and terminal‐like dots in the CA of the MPTP‐treated cats (right panel) as compared to control animals (treated with NaCl, left panel). Lower photomicrographs are higher power magnification of the boxed areas in upper photomicrographs. Other abbreviation: VL: lateral ventricle.
Discussion and Conclusion
The cat MPTP model of Parkinsonism is interesting not only because of its known sensitivity to MPTP 7, 8, 10 but also because of certain similarities in the organization of the striatum between cats and humans. Furthermore, the sheer size of the animal's brain allows them to be used in studies performed with clinical PET.
MPTP treatment in cats produces major signs of motor disorders similar to those seen in Parkinson's disease. The loss of TH‐positive cells and fibers in cats treated with MPTP was consistent with previous studies describing that MPTP administration during 5 days leads to a strong and long‐lasting decrease in tyrosine hydroxylase (TH) immunoreactivity in the striatum 7, 8, 10, 12. These studies have also shown that although the ventral striatum is only modestly reinnervated by TH‐positive fibers, cats spontaneously recover motor function after a few weeks 20. Interestingly, like in the monkey, repeated exposure to MPTP in recovered cats reinstates Parkinson‐like motor deficits without further decreasing the number of TH‐labeled cells in most brain regions 11.
This study is the first to examine the distribution of [11C]PE2I in the cat brain. Several [11C]PE2I studies have demonstrated the important loss of DAT‐binding sites after MPTP treatment in experimental models of Parkinsonism, like baboons, common marmoset, or cynologous monkeys 21, 22, 23, 24.
PET imaging and autoradiography both revealed a high density of [11C]PE2I binding sites, which were confined to the caudate nucleus and putamen. As the present study focuses on comparing [11C]PE2I binding in cats before and after acute or chronic MPTP treatment, we took a number of methodological precautions. In this way, co‐registration of PET volumes with the MRI template was crucial in ensuring the proper measurement of regions of interest. Considering the resolution of the PET camera and [11C] high energy, binding potential (BP) values were probably underestimated due to partial volume effects 25. Nevertheless, to minimize the error inherent to measuring radioactivity in a small brain region, we used a geometric template encompassing the midbrain to ensure a constant shape and size of volumes of interest between scans. Our [11C]PE2I BP values should thus be considered as an index for comparison between control and treated animals rather than absolute quantitative measures.
Neither autoradiography nor PET imaging showed any significant change in [11C]PE2I binding in any region examined after MPTP treatment despite large decreases in TH revealed by immunocytochemistry. Two factors could account for this result. Firstly, MPTP does not destroy a sufficient number of dopaminergic neurons in the cat brain but could transiently impair their ability to function, by down‐regulating the dopaminergic function. TH‐labeling could be decreased in major dopamine cells, whereas dopamine transporters could still be present 26. This would explain the modest recovery of TH labeling observed in dopamine cells 10. Secondly, the remaining dopaminergic neurons after MPTP treatment might compensate by rapidly upregulating dopamine biosynthesis and transporters. This hypothesis is consistent with the significant recovery in extracellular levels of DA in striatal regions 27, 28 and the 60 % increase in dopamine D2/D3 receptor number after MPTP administration 29, 30. However, it has been also reported that dopamine transporter mRNA expression and protein levels do not increase after MPTP treatment in cat 20. Further studies are warranted to elucidate these apparent discrepancies. Finally, as DAT‐binding sites were still present after MPTP treatment, it may be concluded that MPTP toxicity efficiently impairs TH without necessarily causing cell death of dopaminergic neurons on a large scale. Thus, these data could shed some light on the relatively rapid recovery of motor function in MPTP animal models of Parkinson's disease. Further studies are warranted to determine why certain neuronal populations of dopamine neurons do not seem to succumb to the neurotoxic effects of MPTP.
In summary, the feline MPTP model of Parkinsonism is unique as it may allow the identification of relationships between dynamic processes that may be associated with compensation from a large dopamine depleting lesion. These findings may have relevance for understanding the long‐term compensatory processes underlying the pre‐symptomatic period of Parkinson's disease. In terms of molecular imaging, these findings highlight the fact that PET results are not systematically transferable from one animal model to another.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by INSERM/UCBL‐U628 and by grants from Région Rhône‐Alpes (Research Program Cluster 11: Handicap, Aging and Neuroscience), FRC (Fédération de la Recherche sur le Cerveau) and France Parkinson to J.S. Lin. N. Aznavour was recipient of a postdoctoral fellowship from the Swiss National Science Foundation. The authors are grateful to F. Bonnefoi and V. Gualda for their technical support in the PET studies.
References
- 1. McCormack AL, Thiruchelvam M, Manning‐Bog AB, Thiffault C, Langston JW, Cory‐Slechta DA, Di Monte D. Environmental risk factors and Parkinson's disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002;10:119–127. [DOI] [PubMed] [Google Scholar]
- 2. Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM‐1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 2006;197:275–283. [DOI] [PubMed] [Google Scholar]
- 3. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008;4:600–609. [DOI] [PubMed] [Google Scholar]
- 4. Sonsalla PK, Heikkila RE. The influence of dose and dosing interval on MPTP‐induced dopaminergic neurotoxicity in mice. Eur J Pharmacol 1986;129:339–345. [DOI] [PubMed] [Google Scholar]
- 5. Petzinger GM, Langston JW. The MPTP‐lesioned nonhuman primate: a model for Parkinson's disease. Scottsdale: Prominent Press, 1998. [Google Scholar]
- 6. Przedborski S, Jackson‐Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 2001;76:1265–1274. [DOI] [PubMed] [Google Scholar]
- 7. Schneider JS, Markham CH. Neurotoxic Effects of N‐Methyl‐4‐Phenyl‐1,2,3,6‐Tetrahydropyridine (Mptp) in the Cat ‐ Tyrosine‐Hydroxylase Immunohistochemistry. Brain Res 1986;373:258–267. [DOI] [PubMed] [Google Scholar]
- 8. Schneider JS, Yuwiler A, Markham CH. Production of a Parkinson‐like syndrome in the cat with N‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP): Behavior, histology, and biochemistry. Exp Neurol 1986;91:293–307. [DOI] [PubMed] [Google Scholar]
- 9. Rothblat DS, Schneider JS. Spontaneous functional recovery from Parkinsonism is not due to reinnervation of the dorsal striatum by residual dopaminergic neurons. Brain Res Bull 1994;34:309–312. [DOI] [PubMed] [Google Scholar]
- 10. Schneider JS, Rothblat DS. Neurochemical evaluation of the striatum in symptomatic and recovered MPTP‐treated cats. Neuroscience 1991;44:421–429. [DOI] [PubMed] [Google Scholar]
- 11. Rothblat DS, Schneider JS. Repeated exposure to MPTP does not produce a permanent movement disorder in cats recovered from MPTP‐induced Parkinsonism. Neurodegeneration 1995;4:87–92. [DOI] [PubMed] [Google Scholar]
- 12. Lin JS, Hou Y, Kitahama K, Jouvet M. Selective suppression of type B monoamine oxidase immunoreactivity in the raphe nuclei following MPTP administration in the cat. NeuroReport 1995;6:321–324. [DOI] [PubMed] [Google Scholar]
- 13. Halldin C, Erixon‐Lindroth N, Pauli S, et al. [11C]PE2I ‐ a highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging 2003;30:1220–1230. [DOI] [PubMed] [Google Scholar]
- 14. Brix G, Zaers J, Adam LE, et al. Performance evaluation of a whole‐body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 1997;38:1614–1623. [PubMed] [Google Scholar]
- 15. Jucaite A, Odano I, Olsson H, Pauli S, Halldin C, Farde L. Quantitative analyses of regional [11C]PE2I binding to the dopamine transporter in the human brain: A PET study. Eur J Nucl Med Mol Imaging 2006;33:657–668. [DOI] [PubMed] [Google Scholar]
- 16. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. Neuroimage 1997;6:279–287. [DOI] [PubMed] [Google Scholar]
- 17. Snyder RSN. A stereotaxic Atlas of the Cat Brain. Chicago: University of Chicago Press, 1961. [Google Scholar]
- 18. Lin JS, Hou Y, Sakai K, Jouvet M. Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J Neurosci 1996;16:1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnulf I, Crochet S, Buda C, et al. Sleep‐wake changes in MPTP‐treated cats: An experimental model for studying sleep‐wake disorders in Parkinson's disease? Sleep 2005;28:52. [Google Scholar]
- 20. Rothblat DS, Schroeder JA, Schneider JS. Tyrosine hydroxylase and dopamine transporter expression in residual dopaminergic neurons: Potential contributors to spontaneous recovery from experimental Parkinsonism. J Neurosci Res 2001;65:254–266. [DOI] [PubMed] [Google Scholar]
- 21. Poyot T, Conde F, Gregoire MC, et al. Anatomic and biochemical correlates of the dopamine transporter ligand 11C‐PE2I in normal and Parkinsonian primates: Comparison with 6‐[18F]fluoro‐L‐dopa. J Cereb Blood Flow Metab 2001;21:782–792. [DOI] [PubMed] [Google Scholar]
- 22. Nagai Y, Obayashi S, Ando K, et al. Progressive changes of pre‐ and post‐synaptic dopaminergic biomarkers in conscious MPTP‐treated cynomolgus monkeys measured by positron emission tomography. Synapse 2007;61:809–819. [DOI] [PubMed] [Google Scholar]
- 23. Shetty HU, Zoghbi SS, Liow JS, et al. Identification and regional distribution in rat brain of radiometabolites of the dopamine transporter PET radioligand [11C]PE2I. Eur J Nucl Med Mol Imaging 2007;34:667–678. [DOI] [PubMed] [Google Scholar]
- 24. Ando K, Maeda J, Inaji M, et al. Neurobehavioral protection by single dose l‐deprenyl against MPTP‐induced Parkinsonism in common marmosets. Psychopharmacology 2008;195:509–516. [DOI] [PubMed] [Google Scholar]
- 25. DeLorenzo C, Kumar JS, Zanderigo F, Mann JJ, Parsey RV. Modeling considerations for in vivo quantification of the dopamine transporter using [(11)C]PE2I and positron emission tomography. J Cereb Blood Flow Metab 2009;29:1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurosaki R, Muramatsu Y, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T. Role of dopamine transporter against MPTP (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine) neurotoxicity in mice. Metab Brain Dis 2003;18:139–146. [DOI] [PubMed] [Google Scholar]
- 27. Rothblat DS, Schneider JS. Regional differences in striatal dopamine uptake and release associated with recovery from MPTP‐induced Parkinsonism: An in vivo electrochemical study. J Neurochem 1999;72:724–733. [DOI] [PubMed] [Google Scholar]
- 28. Elsworth JD, Taylor JR, Sladek JR, Collier TJ, Redmond DE, Roth RH. Striatal dopaminergic correlates of stable Parkinsonism and degree of recovery in Old‐World primates one year after MPTP treatment. Neuroscience 2000;95:399–408. [DOI] [PubMed] [Google Scholar]
- 29. Wade T, Rothblat DS, Schneider JS. Changes in striatal dopamine D(2) receptors in relation to expression of and recovery from experimental Parkinsonism. Brain Res 2000;871:281–287. [DOI] [PubMed] [Google Scholar]
- 30. Wade TV, Rothblat DS, Schneider JS. Changes in striatal dopamine D3 receptor regulation during expression of and recovery from MPTP‐induced Parkinsonism. Brain Res 2001;905:111–119. [DOI] [PubMed] [Google Scholar]


