Summary
To provide healthcare professionals with a comprehensive assessment of donepezil 23 mg and its role in treating Alzheimer's disease (AD), the Donepezil 23 mg Expert Working Group (EWG) convened in June 2011 to critically evaluate the clinical trial database for this higher dose formulation and the members' clinical experience with its use. Discussions were based on a large, 6‐month, phase 3 clinical trial in patients with moderate to severe AD that compared continuing donepezil 10 mg/day versus switching to 23 mg/day. In this trial, donepezil 23 mg/day demonstrated significantly greater cognitive benefits (mean change in Severe Impairment Battery score, 2.11 points; P < 0.001). Prespecified analyses showed that benefits were significant irrespective of concomitant memantine use. The EWG considered integrating these new data into clinical practice approaches. Dementia severity, tolerability of the 10 mg dose, and need for additional therapy were key selection criteria, as was monitoring of gastrointestinal side effects, as consideration of titration strategies is an important aspect of implementation. The EWG concluded that donepezil 23 mg is an efficacious therapy for moderate to severe AD, with or without concomitant memantine, extending the treatment opportunities available to manage moderate to severe AD dementia. EWG guidelines offer assistance to clinicians in choosing and implementing treatment options.
Keywords: Alzheimer's disease, Donepezil, Efficacy, Moderate to severe, Safety, Tolerability
Introduction
An estimated 5.4 million people in the United States currently have Alzheimer's disease (AD), a progressive neurodegenerative disorder that affects primarily the elderly population. AD is associated with significant burden on the person with the illness, his or her caregivers, and on society as a whole through the considerable cost of disease‐related care. Annual AD‐related care costs in the United States exceed US $180 billion 1. Based on the latest Census review, more than half of Americans with dementia due to AD are at the moderate or severe stages of the disease 2. These advanced stages are characterized by generalized progressive decline in cognitive function, increasing impairment in the ability to perform normal activities of daily living (ADL), and a high probability of behavioral disturbances.
The accumulating care burdens associated with the progression of AD place considerable stress on caregivers and families of the person with AD 3, 4, and often determine whether that person is placed in a nursing home 5. Furthermore, greater impairments in cognition, ADL, and behavior are associated with higher societal costs of AD‐related care 6. Successful symptomatic therapies, particularly at the moderate and severe stages of the disease, may provide substantial health and quality of life benefits, not only for the patient, but also for those in their caregiving network via reduced AD‐related burden and stress 7, 8, 9. Moreover, effective treatment of symptoms may also delay nursing home placement 10, 11 and help reduce costs related to patient care 12, 13, 14, 15.
Pharmacologic therapies approved for AD by the US Food and Drug Administration (FDA) are limited to the acetylcholinesterase inhibitors (AChEIs) donepezil, rivastigmine, and galantamine and the N‐methyl‐d‐aspartate receptor antagonist memantine. Rivastigmine and galantamine are currently approved by the FDA for the treatment of mild and moderate AD 16, 17, 18. Memantine is approved for moderate and severe AD 19. Donepezil is approved in the United States for mild, moderate, and severe stages of the disease 20.
A number of novel AD treatments are currently in clinical development, with the majority focused on delaying or arresting disease progression rather than alleviating disease‐related symptoms. Thus far, no “disease‐modifying” agent has been shown to be safe and effective in clinical trials 21, 22, 23, and it is unlikely that any new and effective FDA‐approved agent will be available for the treatment of AD for the next several years. As these disease‐modifying agents aim to impact the underlying pathophysiology of AD, they are expected to be most useful in the early stages of the disease. However, even a successful disease‐modifying agent would not be likely to prevent all patients from reaching a moderate or even severe stage of AD. In these patients, symptomatic therapy will still have a major role. Currently available symptomatic agents will therefore remain the best therapeutic options for the management of AD for the near future, particularly for persons with more advanced disease, and will likely remain an important part of the pharmacotherapy of AD for the next decade or more. Consequently, optimizing the use of symptomatic agents is of prime importance for improving AD management and care.
Donepezil has been approved in the United States at daily doses of 5 or 10 mg for more than a decade. In July 2010, a new high‐dose, once‐daily 23 mg tablet was approved by the FDA for the treatment of patients with moderate or severe AD. The rationale for the development of this higher dose formulation has been described in detail in a separate publication 24. In brief, the development of donepezil 23 mg was supported by the following observations: (1) modern imaging analyses showed that doses of 5 and 10 mg of donepezil inhibited cortical AChE activity in vivo by only 20–40%, suggesting that higher doses would increase AChE inhibition, possibly resulting in improved efficacy; (2) studies examining brain tissue samples indicated that cholinergic deficits are larger in AD patients with more advanced symptoms, suggesting that higher AChEI doses may be required in these patients; (3) clinical trials of patients with AD who were randomized to treatment with donepezil 5 mg/day, 10 mg/day, or placebo confirmed a dose–response relationship that favored the higher 10 mg dose, and the benefits of this higher dose were most apparent in studies of patients with more advanced AD; and (4) daily donepezil doses of 20 mg appeared safe and well tolerated in a small pilot, randomized, controlled trial of subjects with mild to moderate AD who were already stabilized on donepezil 10 mg/day 25.
Food and Drug Administration approval of donepezil 23 mg/day was based on results from a large, 24‐week, randomized, double‐blind study comparing the efficacy and safety of donepezil 23 mg/day with the established standard dose of donepezil (10 mg/day). A subsequent 12‐month, open‐label extension study, in which all participants received donepezil 23 mg/day, has also been conducted. Several articless and presentations have detailed either the primary outcomes from these trials or outcomes from post hoc analyses of trial data 26, 27, 28, 29, 30, 31, 32.
The donepezil 23 mg tablet is a new formulation, not a higher‐dose version of the 10‐mg immediate‐release tablet. Pharmacokinetic analyses comparing donepezil 23 mg with donepezil 10 mg have shown pharmacologically important differences: maximum plasma concentration (Cmax) and time to Cmax for the 23 mg dose are approximately twice as great, and higher blood levels are maintained over sustained periods. Advantages of this pharmacokinetic profile include the slower rise to Cmax, which may reduce adverse events (AEs); the higher Cmax, which may increase efficacy; and the more prolonged period at higher drug concentrations, which may extend efficacy.
Guidelines are expert‐based assistance documents that translate the results of clinical trials into models of clinical care. Clinical trials answer very focused questions within well‐defined populations, and information from trials must be interpreted and placed in context for “real‐world” application. The objective of this article is to provide primary care physicians, specialists, and other healthcare professionals with a comprehensive assessment of donepezil 23 mg, and its role in the treatment of AD based on the published literature, congress presentations, and consensus opinion of the authors, who comprise the Donepezil 23 mg Expert Working Group (EWG) (see Conflict of Interest).
Efficacy of Donepezil 23 mg/day Versus Donepezil 10 mg/day
The efficacy and safety of donepezil 23 mg/day were compared with those of donepezil 10 mg/day in a single, 24‐week, international, randomized, double‐blind trial of 1467 subjects with moderate or severe AD 29. This trial enrolled subjects with probable AD dementia, who had Mini‐Mental State Examination (MMSE) scores of 0–20 (moderate to severe impairment) and who had been receiving a stable dose of donepezil 10 mg/day for at least 3 months prior to their screening visit. Subjects who enrolled in the study were randomized 2:1 to either increase their daily dose to donepezil 23 mg/day or continue taking their current 10 mg/day dose. Concomitant memantine use of up to 20 mg/day was allowed in this study if the subject had been taking a stable dose of memantine for at least 3 months prior to screening. Memantine was not considered a study medication, and its compliance was not monitored during the study. Primary outcome measures were the Severe Impairment Battery (SIB; a cognitive assessment, with total scores ranging from 0 to 100) and the Clinician's Interview‐Based Impression of Change plus caregiver input (CIBIC‐plus; an assessment of global state, including cognition, function, and behavior). Secondary outcome measures were the Alzheimer's Disease Cooperative Study‐Activities of Daily Living–severe version (ADCS‐ADL‐sev; a measure of ADL) and the MMSE.
At the end of the 24‐week study, subjects in both donepezil treatment groups showed additional cognitive benefits over baseline, based on their SIB scores. The improvement in SIB score from baseline with donepezil 23 mg/day was 2.2 points, which was significantly greater than with donepezil 10 mg/day (Figure 1). Similar results were found in the observed‐cases (OC) population (2.4‐point difference between the two treatment groups [P < 0.001]). Although the clinical meaningfulness of a two‐point difference on the SIB has been debated, it is noteworthy that not only was this improvement over an active therapy rather than placebo but that, based on prior studies 33, 34, untreated patients with moderate to severe AD would be likely to show a decline on the SIB scale over a similar study period. For the second coprimary outcome measure, CIBIC‐plus, no incremental benefit over that achieved with donepezil 10 mg/day was demonstrated over 24 weeks in the intention‐to‐treat population (Table 1) or in the OC patient population analysis (P < 0.059). Likewise, donepezil 23 mg/day showed no benefits over donepezil 10 mg/day for either of the prespecified secondary endpoints (ADCS‐ADL‐sev and MMSE). Because this was a global study, differences in interpretation of assessment scales and geographic variations in population characteristics may have affected the accurate detection of treatment effects. For example, it is questionable whether the ADCS‐ADL is suitable for global studies due to cultural differences in caregiving and daily life 29.
Figure 1.
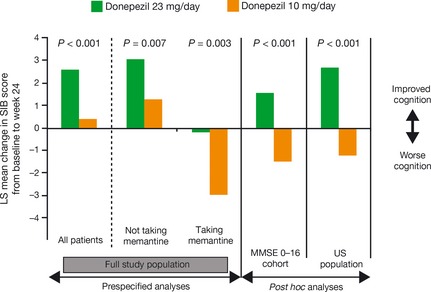
Effect of 24 weeks of treatment with donepezil 23 mg versus donepezil 10 mg on cognitive function in patients with moderate or severe Alzheimer's disease. Data presented are least square (LS) mean changes in Severe Impairment Battery scores derived from the intent‐to‐treat populations (all randomized patients who received at least one dose of study medication and who had both baseline and postbaseline efficacy data for at least one of the coprimary measures) using the last observation carried forward method to impute missing values 29. MMSE, Mini‐Mental State Examination.
Table 1.
Effect of 24 weeks' treatment with donepezil 23 mg versus donepezil 10 mg on global function in patients with moderate or severe AD a
| Prespecified analyses | Post hoc analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | Not taking memantine | Taking memantine | MMSE 0–16 cohort | US population | ||||||
| 23 mg | 10 mg | 23 mg | 10 mg | 23 mg | 10 mg | 23 mg | 10 mg | 23 mg | 10 mg | |
| CIBIC‐plus mean | 4.23 | 4.29 | 4.12 | 4.16 | 4.40 | 4.52 | 4.31b | 4.42 | 4.38b | 4.57 |
AD, Alzheimer's disease; MMSE, Mini‐Mental State Examination; CIBIC‐plus, Clinician's Interview‐Based Impression of Change plus caregiver input.
Data presented are mean CIBIC‐plus overall change scores at week 24 derived from the intent‐to‐treat populations (all randomized patients who received at least one dose of study medication and who had both baseline and postbaseline efficacy data for at least one of the coprimary measures) using the last observation carried forward method to impute missing values (lower CIBIC‐plus overall change scores represent less global impairment)
P < 0.05 for donepezil 23 mg/day versus donepezil 10 mg/day 29.
A further prespecified analysis of SIB and CIBIC‐plus scores compared subjects not taking concomitant memantine with those who were. The SIB outcomes were similar with regard to the magnitude of benefit conferred by donepezil 23 mg compared with those continuing with 10 mg, but were different with regard to the pattern of change. Subjects in the donepezil 23 mg group who were not taking memantine improved by almost two points on the SIB compared with no change for the 10‐mg group, whereas subjects in the 23 mg group on memantine showed no change, compared with a decline of almost two points in the 10‐mg group (Figure 1, Table 1) 27, 29. For the CIBC‐plus overall change scores, no incremental benefit of donepezil 23 mg/day over donepezil 10 mg/day was observed in patients receiving and not receiving concurrent memantine.
Post hoc analyses have been performed to evaluate further the efficacy of donepezil 23 mg/day using data from the double‐blind study. Analysis of the subgroup of subjects with more severe cognitive impairment at baseline (MMSE scores of 0–16, representing more than 70% of the overall study population) showed greater treatment effects with donepezil 23 mg/day versus 10 mg/day on both the cognitive and global function coprimary endpoints (Figure 1, Table 1) 29. A post hoc analysis performed in the subpopulation of subjects enrolled at sites within the United States (who were more cognitively impaired at the start of the study compared with the overall population) also showed significant differences in favor of donepezil 23 mg/day over donepezil 10 mg/day on both coprimary endpoints (Figure 1, Table 1) 29, 31. Of the 1467 subjects, 465 (32%) came from the United States, more than from any other country. As such, this post hoc analysis represented a meaningful assessment of a sizeable population of patients with more uniform standard of clinical care than the overall trial population, which comprised individuals from many different countries. Moreover, outcomes in this subpopulation would presumably more closely represent how the drug would perform in the US population with AD dementia. This supposition was an important factor in the FDA's consideration of the drug.
A further post hoc analysis was performed to evaluate the effects of donepezil 23 mg/day on language ability using a subscale of the SIB that specifically assesses language items (the SIB‐L scale) 30. The ability to communicate has an important effect on the AD patient's emotional and physical well‐being, helping to maintain a connection to their family, caregivers, and care team. In the full study population, language ability was improved with donepezil 23 mg/day by study end, but declined with donepezil 10 mg/day; the treatment difference was statistically significant in favor of the higher dose. Statistically significant language benefits of donepezil 23 mg/day over 10 mg/day were also seen in the subgroup of subjects with more severe cognitive impairment at baseline (MMSE scores of 0–16) and were maintained irrespective of concomitant memantine use.
Assessment of efficacy was not a primary objective of the open‐label extension study; however, SIB scores were obtained at each visit over the 12‐month treatment period. Using SIB data from subjects who completed the open‐label extension, a mixed‐model repeated measures, post hoc analysis that showed those subjects who received high‐dose donepezil continuously for 18 months obtained greater cognitive benefits over the entire 18‐month period than those who received donepezil 10 mg/day for 6 months followed by donepezil 23 mg/day for 12 months (least squares mean difference in SIB score of 2.1 points, P < 0.01) 26.
Safety and Tolerability of Donepezil 23 mg/day Versus Donepezil 10 mg/day
The safety and tolerability of the higher donepezil dose were assessed in comparison with donepezil 10 mg/day during the 24‐week double‐blind study 28, 29. Throughout the study, AEs were reported in more subjects receiving donepezil 23 mg/day (73.7%) than donepezil 10 mg/day (63.7%). Of subjects who experienced AEs, the majority (>85%) in both donepezil treatment groups had events of mild to moderate severity. The most common AEs considered likely to be related to treatment with donepezil 23 and 10 mg/day were cholinergic‐related gastrointestinal (GI) events, including nausea (6.1 vs. 1.9%), vomiting (5.0 vs. 0.8%), and diarrhea (3.2 vs. 1.5%). As would be expected based on prior experience of dose increases from 5 to 10 mg/day, the cholinergic‐related GI events occurred most frequently during the first month of treatment in those subjects randomized to increase their donepezil dose to 23 mg/day; the incidence of these events declined beyond this time point and was similar between the two groups for the remainder of the trial.
More subjects receiving donepezil 23 mg/day (18.6%) than donepezil 10 mg/day (7.9%) discontinued treatment due to an AE. The AEs that contributed most to early discontinuations were cholinergic related and included vomiting, nausea, diarrhea, and dizziness. The majority of subjects (68%) discontinuing treatment with donepezil 23 mg/day did so during the first month of therapy (Figure 2). Cardiac AEs were infrequent with both donepezil doses. The overall incidence of bradycardia was greater in the higher‐dose group, but this event occurred in fewer than 3% of subjects treated with donepezil 23 mg/day and was not commonly associated with other cardiovascular events. Weight loss as an AE was also more common in the donepezil 23 mg group; it occurred in fewer than 5% of subjects and only one subject experienced severe weight loss. There were 13 deaths during the study or within 30 days of study discontinuation. Of these, eight (0.8%) subjects were in the donepezil 23 mg group and five (1.1%) were in the donepezil 10‐mg group. None of these deaths was considered by the investigator to be possibly or probably related to study medication.
Figure 2.
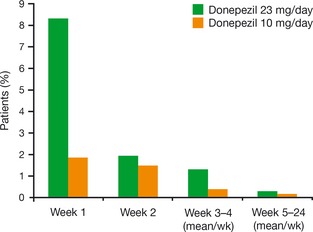
Discontinuations due to adverse events among patients treated with donepezil 23 mg/day or donepezil 10 mg/day during the double‐blind trial. Data presented are derived from the study safety population (all randomized patients who took at least one dose of study drug and had at least 1 postbaseline safety assessment) 28.
Post hoc analyses to explore the influence of several demographic factors on the safety and tolerability of higher‐dose donepezil showed that in the donepezil 23 mg/day group, but not the 10‐mg group, more women than men experienced anorexia (6.3 vs. 3.7%) and weight loss (5.8 vs. 2.8%). Higher incidences of fatigue, somnolence, and urinary incontinence were also observed in successively older age groups exclusively among subjects who increased their dose to 23 mg/day. Subjects with the lowest body weight at baseline (≤55 kg) experienced more AEs than other subjects in both treatment groups. A higher rate of AEs was also seen in subjects taking concomitant memantine, irrespective of the donepezil dose, possibly because of their more advanced AD stage at study baseline. Serious AEs occurred in a similar proportion of subjects in the 23 and 10 mg/day groups (8.3 vs. 9.6%), with the majority in both groups considered by study physicians to be unrelated to treatment.
Longer‐term Safety of Donepezil 23 mg/day
Subjects completing the double‐blind study of donepezil 23 mg/day versus 10 mg/day were given the option of enrolling in a subsequent 12‐month, open‐label extension study during which they would all receive the higher dose of donepezil 32. The primary purpose of the open‐label extension was to evaluate the safety and tolerability of donepezil 23 mg/day in patients with moderate or severe AD during long‐term exposure. As noted about the double‐blind study, subjects transitioning from the 10 mg dose to the 23 mg dose experienced an increase in cholinergic‐related GI AEs and discontinuations in the first month of open‐label treatment. This increase was transient; the rates of newly occurring events were lower and similar in both groups for the remainder of the trial.
Weight loss was the most commonly observed AE over the course of the extension trial, regardless of the dose of donepezil received during the double‐blind study. Weight loss of ≥7% of body weight from extension study baseline occurred in 36 (11.0%) subjects transitioning from the 10 to the 23 mg dose, and in 64 (11.2%) subjects continuing on donepezil 23 mg. However, in the absence of a placebo control group, it is unclear whether the weight loss was related to treatment with donepezil or to disease progression. Anorexia and weight loss are important considerations in patients with advanced AD, and weight should be monitored closely in patients receiving long‐term donepezil therapy.
Rates of bradycardia (n = 11 [1.2% of safety population]) and GI bleeding (n = 7 [0.8% of safety population]) were very low among subjects treated for 12 months with high‐dose donepezil. Other common AEs and serious AEs observed during the open‐label extension were predictable and consistent with events observed in other long‐term studies of AChEIs 35, 36, 37, 38.
Using Donepezil 23 mg/day in Clinical Practice—Selecting the Patient
To be eligible for treatment with donepezil 23 mg, as stated in the label for donepezil 23 mg, a patient must have a diagnosis of moderate or severe AD dementia and have been taking a stable dose of donepezil 10 mg for at least 3 months. If taking another cholinesterase inhibitor, the patient should be switched to and/or advanced as appropriate to a stable dose of donepezil 10 mg for 3 months prior to increasing to the 23 mg dosage. Beyond this basic profile, patients with documented evidence of clinical worsening while receiving donepezil 10 mg are particularly suitable for a trial of donepezil 23 mg. It is also reasonable to consider this treatment for patients with more severe cognitive impairment (MMSE below 16) as, based on the clinical trial results, these patients appear to be most likely to experience the greatest incremental treatment benefits. Clinical trial data and our clinical experience also show that donepezil 23 mg can be prescribed concomitantly with memantine; that is, memantine may be added to donepezil 10 mg, then the dose increased to 23 mg, or patients taking memantine may be titrated with donepezil and advanced to the 23 mg dose.
Based on the clinical trial evidence and our clinical experience, patients with very low weight, very poor appetite, bradycardia, or a history of GI bleeding may not be appropriate candidates for donepezil 23 mg; extra caution is required in those patients who are advanced to a trial of 23 mg. Patients who had difficulty (many AEs or severe AEs) when increasing from 5 to 10 mg are likely to have difficulty when titrating to 23 mg, although most will be able to tolerate the higher dose after the first several weeks.
Using Donepezil 23 mg/day in Clinical Practice—Measuring Benefits
The Donepezil 23 mg EWG concluded that efficacy outcomes with donepezil 23 mg in real‐world clinical practice appear to mirror the results of the pivotal double‐blind clinical trial, with benefits primarily seen on cognitive symptoms and greater benefits seen in more severely impaired patients. However, a recognized issue associated with assessing the efficacy of donepezil 23 mg in clinical practice is the lack of an appropriate instrument for determining outcomes in patients with moderate or severe AD. Because treatment decisions are often based, in whole or in part, on efficacy outcomes, it is important that clinicians using donepezil 23 mg have the available tools and knowledge with which to adequately assess therapeutic benefits.
In the pivotal clinical trial of donepezil 23 mg/day versus 10 mg/day, cognition was evaluated using the SIB, which is widely used for assessing treatment‐mediated cognitive changes in clinical trials, but is not widely used in clinical practice. The most commonly used tool for measuring cognition in clinical practice is the MMSE; however, the SIB was created, in part, because the MMSE is not a reliable instrument for measuring cognitive change in patients with more advanced AD 39, 40, 41. Alternatives to assess cognition in patients with advanced AD include a detailed caregiver interview or a shortened version of the SIB 42. Language changes were improved by donepezil 23 mg as evidenced by the analysis of the language items of the SIB. Attention to language and communication skills may help the clinician to assess benefits of treatments.
Using Donepezil 23 mg/day in Clinical Practice—Managing the Transition from Donepezil 10 mg/day
An outcome of increasing the daily dose of donepezil from 10 to 23 mg in both the double‐blind study and the open‐label extension was the increased incidence of GI side effects, such as diarrhea, vomiting, and nausea 28, 29.
A first step in addressing the issue of the onset of these GI side effects is to manage patient and caregiver expectations. A transient increase in some cholinergic‐related GI events is an expected outcome when titrating the donepezil dose from 5 to 10 mg/day 20. Prescribers therefore must help patients and their caregivers recognize that the same expectations are associated with the transition from 10 to 23 mg/day and that this is a step toward full titration of the drug. Importantly, the prescriber must help the patient and caregiver to recognize that these side effects will generally not persist and usually end relatively quickly. Most subjects receiving 23 mg/day in the double‐blind trial had been taking donepezil 10 mg for a long period and, in the experience of the EWG, these patients tend to have less treatment‐emergent AEs than those who have been taking 10 mg only for the label‐specified minimum of 3 months.
As well as managing the expectations of the patient and caregiver, prescribers can also help maximize compliance by educating the patient's wider caregiving network (e.g., their own office staff, nursing home staff, visiting nurses) on the expected side effects, with an emphasis on their likely transient nature. With this knowledge, the network of individuals caring for the patient with AD can play an important role in supporting adherence to this therapy.
The EWG noted that use of a more stepwise titration strategy when transitioning from donepezil 10 mg/day to 23 mg/day can help mitigate the GI side effects and may improve adherence to treatment. The specific strategies used vary somewhat, but all involve an approximately 1‐ to 2‐month period during which the patient receives an interim donepezil dose, for example, by taking 1 × 10 mg tablet plus 1 × 5 mg tablet daily (i.e., 15 mg/day) or by taking a 10‐mg tablet and a 23 mg tablet on alternating days. Any dosing schedules adopted should be simple and easy for the patient and caregiver to follow. Such titration strategies are not approved in the label because they were not evaluated in the clinical trial, in which only an immediate switch from 10 to 23 mg was used. The EWG noted that pharmacotherapy for cognitive enhancement is only one dimension of guidelines for the care of patients with moderate to severe AD dementia. Exercise, social engagement, healthy diet, and care of the care partner are critically important to successful treatment of patients at this stage of the disease. Treatment of behavioral disturbances, evaluation and medical management of illnesses, and consideration of medical foods are all part of an integrated guideline for management. Table 2 summarizes the recommendations of the EWG for the use of donepezil 23 mg in patients with moderate to severe AD.
Table 2.
EWG recommendations for the use of high‐dose donepezil (23 mg/day) in treatment of moderate to severe AD
| Alzheimer's disease |
| Ensure an accurate diagnosis of AD |
| Determine patient dementia severity to be moderate to severe |
| Evaluate for contraindications for donepezil 23 mg/day, including very low weight, very poor appetite, bradycardia, history of gastrointestinal bleeding |
| General care |
| Review medical care to ensure appropriate management of all comorbid medical conditions |
| Evaluate preventive care to ensure that diet, exercise, and general health measures are optimized |
| Assess sleep hygiene measures to facilitate optimal sleep (avoid fluids or stimulants late in day, take any needed pain medications prior to going to bed, ensure a quiet environment, exercise during the day, and avoid excessive napping) |
| Discuss patient situation with caregiver to assess the patient's need for services (day care, etc.) |
| Discuss caregiver situation with caregiver to assess need for services for caregiver (in‐home assistance, respite care, etc.) |
| Donepezil history |
| Review the patient's history to ensure a minimum of 1 month's exposure to donepezil 10 mg/day |
| Evaluate the side effect history to determine whether donepezil 10 mg/day has been associated with side effects such as multiple stools, loss of appetite, or weight loss |
| Ensure that the patient is not taking more than one type of cholinesterase inhibitor |
| Introduction of donepezil 23 mg/day |
| Consider stepwise escalation to donepezil 15 mg/day for 1 month followed by dose increase to 23 mg/day |
| Escalate dose to donepezil 23 mg/day after 1 month of 15 mg/day or directly from donepezil 10 mg/day |
| Monitor side effects with dose increase, especially gastrointestinal side effects such as diarrhea, nausea, vomiting |
| Assess cognition to evaluate the effects of donepezil 23 mg/day |
EWG, European Working Group; AD, Alzheimer's disease.
Conclusion
Donepezil 23 mg is an efficacious therapy for patients with moderate to severe AD. It can be used in conjunction with memantine without significant impact on its efficacy, safety, or tolerability. Adverse effects are typically GI; they occur most frequently during the first month of therapy and usually abate. There are useful strategies to mitigate their impact on the patient. However, even with the use of more stepwise titration strategies, not all patients will benefit from and/or be able to tolerate a dose increase to 23 mg/day. If those patients appear to be no longer benefiting from 10 mg/day, treatment discontinuation is not supported by recent evidence 43, but options include addition of, or switching to, memantine, or switching to an alternative AChEI. Overall, donepezil 23 mg is a valuable therapeutic option to consider for patients with moderate to severe AD, for whom few other alternatives exist. The recommendations are integrated into a comprehensive care plan that addresses all aspects of the management of moderate to severe AD dementia.
Conflict of Interest
The listed authors were all attendees at the Donepezil 23 mg EWG meeting in Dallas, Texas, USA, on May 17 and 18, 2011. The donepezil 23 mg EWG meeting was an Eisai‐ and Pfizer‐sponsored initiative that provided a forum at which US‐based experts could debate clinical issues and provide feedback on their real‐world clinical practice experience.
All authors received honoraria from Eisai Inc. for their participation in the donepezil 23 mg EWG meeting; they did not receive remuneration for their part in the development of this manuscript. Dr. Cummings has provided consultation to Abbott, Acadia, ADAMAS, Anavex, Astellas, AstraZeneca, Avanir, Baxter, Bristol‐Myers Squibb, Eisai, Elan, EnVivo, Forest, Genentech, GlaxoSmithKline, Janssen, Lilly, Lundbeck, Medtronics, Merck, Neurokos, Neuronix, Novartis, Otsuka, Pain Therapeutics, Pfizer, Plexxicon, Prana, QR, Sanofi, Sonexa, Takeda, and Toyama pharmaceutical companies. He has also provided consultation on diagnostic assessment to Bayer, Avid, GE, MedAvante, Neurotrax, and UBC. Dr. Cummings owns stock in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, Neurokos, and QR Pharma. He is a speaker/lecturer for Eisai, Forest, Janssen, Novartis, Pfizer, and Lundbeck. Dr. Cummings owns the copyright of the Neuropsychiatric Inventory and has provided expert witness consultation regarding olanzapine and ropinirole. Dr. Geldmacher has received research support from Baxter, ADCS, Bayer, BMS, and Glaxo, and consultant/advisor honoraria from Eisai, Janssen, and Pfizer. Dr. Farlow has received research funding from Eisai Medical Inc., ADCS, Pfizer, Eli Lilly, Novartis, Sanofi Aventis, Genentech, and Roche. Dr. Farlow has received consultant/advisor honoraria from Eisai Medical Inc, Accera, Inc., ADAMAS, Adlyfe, Inc., Astellas Pharma US Inc., Baxter, Bayer, Bio RX, Eli Lilly, GE Healthcare, Inc., Helicon, Medavante, Inc., Medivation, Merck, Novartis, Octapharma, QR Pharma, Sanofi‐Aventis, Toyama Chemical Co. Ltd., and UCB. Dr. Sabbagh has been a consultant/advisor to Amerisciences, BMS, Takeda, Bayer, Eisai, and Pfizer; he has received royalties from Wiley and Amerisciences, and contract/grant support from Avid, Lilly, Bayer, Baxter, GE, Pfizer, Janssen, BMS, and Eisai. Dr. Christensen has received consultant fees from Pfizer, Eisai, and Medivation and has been on the Speakers' Bureau for Pfizer and Eisai; he holds stock in Pfizer, Eli Lily, BMS and Johnson & Johnson. Dr. Betz has received speaker fees from Pfizer and Eisai.
Acknowledgments
During the development of this article, editorial assistance was provided by Richard Daniel, PhD, at PAREXEL, and was funded by Eisai Inc. and Pfizer Inc. All authors were involved in the drafting and critical review of the manuscript at all stages of development, and all authors approved the final manuscript for submission.
References
- 1. Alzheimer's Association . 2011 Alzheimer's disease facts and figures. Alzheimer's Association, 2011. Available at: http://www.alz.org/downloads/facts_figures_2011.pdf. Accessed October 29, 2012. [Google Scholar]
- 2. Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60:1119–1122. [DOI] [PubMed] [Google Scholar]
- 3. Bergvall N, Brinck P, Eek D, et al. Relative importance of patient disease indicators on informal care and caregiver burden in Alzheimer's disease. Int Psychogeriatr 2011;23:73–85. [DOI] [PubMed] [Google Scholar]
- 4. Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS. Caregiver burden in Alzheimer disease: cross‐sectional and longitudinal patient correlates. Am J Geriatr Psychiatry 2010;18:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaugler JE, Yu F, Krichbaum K, Wyman JF. Predictors of nursing home admission for persons with dementia. Med Care 2009;47:191–198. [DOI] [PubMed] [Google Scholar]
- 6. Gustavsson A, Brinck P, Bergvall N, et al. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement 2011;7:318–327. [DOI] [PubMed] [Google Scholar]
- 7. Feldman H, Gauthier S, Hecker J, et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer's disease and the effect on caregiver burden. J Am Geriatr Soc 2003;51:737–744. [DOI] [PubMed] [Google Scholar]
- 8. Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer's disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry 2008;23:537–545. [DOI] [PubMed] [Google Scholar]
- 9. Herrmann N, Cappell J, Eryavec GM, Lanctot KL. Changes in nursing burden following memantine for agitation and aggression in long‐term care residents with moderate to severe Alzheimer's disease: an open‐label pilot study. CNS Drugs 2011;25:425–433. [DOI] [PubMed] [Google Scholar]
- 10. Geldmacher DS, Provenzano G, McRae T, Mastey V, Ieni JR. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. J Am Geriatr Soc 2003;51:937–944. [DOI] [PubMed] [Google Scholar]
- 11. Lopez OL, Becker JT, Wisniewski S, Saxton J, Kaufer DI, DeKosky ST. Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease. J Neurol Neurosurg Psychiatry 2002;72:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cappell J, Herrmann N, Cornish S, Lanctot KL. The pharmacoeconomics of cognitive enhancers in moderate to severe Alzheimer's disease. CNS Drugs 2010;24:909–927. [DOI] [PubMed] [Google Scholar]
- 13. Feldman H, Gauthier S, Hecker J, et al. Economic evaluation of donepezil in moderate to severe Alzheimer disease. Neurology 2004;63:644–650. [DOI] [PubMed] [Google Scholar]
- 14. Wimo A, Winblad B, Engedal K, et al. An economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a 1‐year, double‐blind, randomized trial. Dement Geriatr Cogn Disord 2003;15:44–54. [DOI] [PubMed] [Google Scholar]
- 15. Wimo A, Winblad B, Stoffler A, Wirth Y, Mobius HJ. Resource utilisation and cost analysis of memantine in patients with moderate to severe Alzheimer's disease. Pharmacoeconomics 2003;21:327–340. [DOI] [PubMed] [Google Scholar]
- 16. Exelon [US prescribing information]. East Hanover, NJ: Novartis Inc.; 2006. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/exelon.pdf. [Google Scholar]
- 17. Exelon Patch [US prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010. Available at: http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf. [Google Scholar]
- 18. Razadyne and Razadyne ER [US prescribing information]. Titusville, NJ: Ortho McNeil Neurologics; 2011. Available at: http://www.razadyneer.com/sites/default/files/shared/pi/razadyne_er.pdf#zoom=100. [Google Scholar]
- 19. Namenda [US prescribing information]. St. Louis, MO: Forest Laboratories, Inc.; 2007. Available at: http://www.frx.com/pi/namenda_pi.pdf. [Google Scholar]
- 20. Aricept [US prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; 2010. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=510. [Google Scholar]
- 21. Aisen PS, Gauthier S, Ferris SH, et al. Tramiprosate in mild‐to‐moderate Alzheimer's disease ‐ a randomized, double‐blind, placebo‐controlled, multi‐centre study (the Alphase Study). Arch Med Sci 2011;7:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Extance A. Alzheimer's failure raises questions about disease‐modifying strategies. Nat Rev Drug Discov 2010;9:749–751. [DOI] [PubMed] [Google Scholar]
- 23. Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 2009;302:2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer's disease: consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doody RS, Corey‐Bloom J, Zhang R, Li H, Ieni J, Schindler R. Safety and tolerability of donepezil at doses up to 20 mg/day: results from a pilot study in patients with Alzheimer's disease. Drugs Aging 2008;25:163–174. [DOI] [PubMed] [Google Scholar]
- 26. Brand‐Schieber E, Zou H, Zhu W, et al. Long‐term cognitive effect of increasing donepezil dose from 10 mg to 23 mg in moderate to severe Alzheimer's disease – impact of treatment delay. Presented at: 63rd Annual Meeting of the American Academy of Neurology (AAN); April 9–16, 2011; Honolulu, HI.
- 27. Doody RS, Geldmacher DS, Farlow MR, Sun Y, Moline M, Mackell J. Efficacy and safety of donepezil 23 mg versus donepezil 10 mg for moderate‐to‐severe Alzheimer's disease: a subgroup analysis in patients already taking or not taking concomitant memantine. Dement Geriatr Cogn Disord 2012;33:164–173. [DOI] [PubMed] [Google Scholar]
- 28. Farlow M, Veloso F, Moline M, et al. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer's disease. BMC Neurol 2011;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high‐dose (23 mg/d) versus standard‐dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24‐week, randomized, double‐blind study. Clin Ther 2010;32:1234–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferris SH, Schmitt FA, Saxton J, et al. Analyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer's disease. Alzheimers Res Ther 2011;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salloway S, Ramos H, Faison W, Zou H. Efficacy and safety of donepezil 23 mg/d versus donepezil 10 mg/d in moderate to severe Alzheimer's disease: subgroup analysis of US participants in a global study. Presented at: 63rd Annual Meeting of the American Academy of Neurology (AAN); April 9–16, 2011; Honolulu, HI.
- 32. Tariot P, Salloway S, Yardley J, Mackell J, Moline M. Long‐term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer's disease. BMC Res Notes 2012;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24‐week, randomized, double‐blind study of donepezil in moderate to severe Alzheimer's disease. Neurology 2001;57:613–620. [DOI] [PubMed] [Google Scholar]
- 34. Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer's disease: double‐blind, parallel‐group, placebo‐controlled study. Lancet 2006;367:1057–1065. [DOI] [PubMed] [Google Scholar]
- 35. Aupperle PM, Koumaras B, Chen M, Rabinowicz A, Mirski D. Long‐term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer's disease: results of a 52‐week open‐label study. Curr Med Res Opin 2004;20:1605–1612. [DOI] [PubMed] [Google Scholar]
- 36. Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open‐label, multicentre study in patients with Alzheimer's disease. Int J Geriatr Psychiatry 2007;22:806–812. [DOI] [PubMed] [Google Scholar]
- 37. Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD. Open‐label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 2001;58:427–433. [DOI] [PubMed] [Google Scholar]
- 38. Pirttila T, Wilcock G, Truyen L, Damaraju CV. Long‐term efficacy and safety of galantamine in patients with mild‐to‐moderate Alzheimer's disease: multicenter trial. Eur J Neurol 2004;11:734–741. [DOI] [PubMed] [Google Scholar]
- 39. Saxton J, McGonigle‐Gibson KL, Swihart AA, Miller VJ, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess 1990;2:298–303. [Google Scholar]
- 40. Schmitt FA, Ashford W, Ernesto C, et al. The Severe Impairment Battery: concurrent validity and the assessment of longitudinal change in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):S51–S56. [PubMed] [Google Scholar]
- 41. Schmitt FA, Cragar D, Ashford JW, et al. Measuring cognition in advanced Alzheimer's disease for clinical trials. J Neural Transm Suppl 2002;62:135–148. [DOI] [PubMed] [Google Scholar]
- 42. Schmitt FA, Mackell J, Richardson S, Xu Y. Evaluating cognitive benefits in patients with moderate to severe Alzheimer's disease treated with donepezil 23 mg/d: utility of the SIB‐8 scale. Presented at: The 63rd Annual Meeting of the American Academy of Neurology (AAN); April 9–16, 2011; Honolulu, HI.
- 43. Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate‐to‐severe Alzheimer's disease. N Engl J Med 2012;366:893–903. [DOI] [PubMed] [Google Scholar]


