Summary
For more than 50 years, ketamine has proven to be a safe anesthetic drug with potent analgesic properties. The active enantiomer is S(+)‐ketamine. Ketamine is mostly metabolized in norketamine, an active metabolite. During “dissociative anesthesia”, sensory inputs may reach cortical receiving areas, but fail to be perceived in some association areas. Ketamine also enhances the descending inhibiting serotoninergic pathway and exerts antidepressive effects. Analgesic effects persist for plasma concentrations ten times lower than hypnotic concentrations. Activation of the (N‐Methyl‐D‐Aspartate [NMDA]) receptor plays a fundamental role in long‐term potentiation but also in hyperalgesia and opioid‐induced hyperalgesia. The antagonism of NMDA receptor is responsible for ketamine's more specific properties. Ketamine decreases the “wind up” phenomenon, and the antagonism is more important if the NMDA channel has been previously opened by the glutamate binding (“use dependence”). Experimentally, ketamine may promote neuronal apoptotic lesions but, in usual clinical practice, it does not induce neurotoxicity. The consequences of high doses, repeatedly administered, are not known. Cognitive disturbances are frequent in chronic users of ketamine, as well as frontal white matter abnormalities. Animal studies suggest that neurodegeneration is a potential long‐term risk of anesthetics in neonatal and young pediatric patients.
Keywords: Alzheimer's disease, Behavioural neurology, Neuropsychopharmacology, Stroke
Introduction
In the 1950s, Parke‐Davis industries were searching, among cyclohexamine drugs, an ideal anesthetic agent with analgesic properties. CI‐395 (phencyclidine or N‐1‐phenyl‐cyclohexyl‐piperidine [PCP] chlorhydrate) and CI‐400 (N‐ethyl‐1‐phenyl‐cyclohexamine chlorhydrate) were initially developed 1. If these two drugs had no respiratory or cardiovascular depressive effects, severe psychodysleptic effects were observed (vivid dreams, hallucinations, sometimes involving outer space) 2. PCP was commercialized in the US (Sernyl®), but its abusive use as a recreational drug (“angel dust”) stopped its production in 1978. Because of the severe psychodysleptic effects of cyclohexamine, further research in the 1960s ultimately led to the synthesis and development of ketamine (CI‐581, 2‐O‐chloro‐phenyl‐2‐methylamino‐cyclohexanone, Ketalar®). First clinical studies were published in 1965 3. Ketamine demonstrated a lower and shorter effect than PCP, but the “psychic effects” were also less marked.
Physicochemical Characteristics
Ketamine is a hydrosoluble aryl‐cyclo‐alkylamine (Figure 1) with a molecular mass of 238 g/mol and a pKa 7.5. Used as a chlorhydrate in a slightly acid (pH 3.5–5.5) aqueous solution, ketamine sometimes includes benzethonium chloride or chlorobutanol as preservatives. The second carbon of the cyclohexanone radical is asymmetrical. Ketalar® is the racemic mixture (optically inactive) of 2 enantiomers of equal quantity (isomers that diverge light in opposite ways). The active enantiomer is S(+)‐ketamine (“S” spatial structure, light diverged to the right), two times stronger than the racemic form, and four times than the R(−)‐ketamine isomer. S(+)‐Ketamine is available (Ketanesth®) in some European countries (Germany, Austria, Italy, and the Netherlands).
Figure 1.
Metabolism. Ketamine is metabolized mainly to norketamine (80%), itself secondarily transformed into hydroxy‐norketamine (15%), mainly 6‐hydroxy‐norketamine. Accessory pathway passes directly through the transformation of ketamine in hydroxy‐ketamine (5%).
Pharmacokinetics
Ketamine Metabolism
Ketamine metabolism is characterized by a low binding to plasma proteins, about 10–30% 4. Because of a liposolubility five times higher than thiopental, ketamine has an extensive distribution. Central compartment volume is about 70 l, and the distribution volume at steady state is around 200 l 5, or 2.3 l/kg 6. Because of an oxidation by a microsomal enzyme system (N‐demethylation), ketamine is mostly metabolized in norketamine (80%), an active metabolite that is itself principally hydroxylized in 6‐hydroxy‐norketamine (15%), finally excreted in bile and urine after glucuronoconjugation. Three other less important metabolites are also formed (Figure 1). Another way directly transforms ketamine into hydroxy‐ketamine (5%) 7. This metabolism does not simply involve the liver 8, particularly in animals: the kidneys, the intestine, and the lungs are the site of significant metabolism 9. R(−)‐ketamine can be transformed in S(+)‐norketamine but it seems there is, in vivo, no connection between enantiomers. Ketamine elimination clearance is high (1000–1600 ml/min or 12–20 ml/min/kg), equal to liver blood flow, and then dependent on this flow 5. Ketamine elimination half‐life is 2–3 h. Its pharmacokinetics can be described as a tree compartment model 10. Its clearance may be 20% higher in women than in men 11.
Cytochromes, belonging to the cytochromes P450 system and responsible for ketamine metabolism, are not totally identified 12, but in humans, several microsomal enzymes are responsible for norketamine demethylation. Ketamine has an inhibiting action on some cytochromes belonging to P450 complex, and this could partly explain the tachyphylaxis observed during the repeated use of the molecule 13.
S(+) isomer demethylation is superior to that of R(−) isomer, which explains a 22% higher clearance compared to R(−)ketamine 14. S(+) isomer distribution volume is also higher 15. Racemic mixture pharmacokinetics is less favorable than that of S(+)‐ketamine 16, because R(−) isomer otherwise inhibits S(+) isomer demethylation 17 to a proportion of 30% 18. This interaction seems to exist in both ways; S(+) isomer also inhibits R(−) metabolism, probably by the same enzyme competition. However, differences in enantiomers are essentially pharmacodynamic, because their cerebral and blood concentrations are similar 19. For an i.v. perfusion (50 mg/min), racemic and S(+)‐ketamine induce narcosis in 3 ± 1 min 20 for a duration of 6 ± 2 min, but S(+)‐ketamine has a better pharmacokinetics than the racemic mixture, with a shorter emergence phase 15.
Norketamine Pharmacokinetics
Norketamine appears in blood 2–3 min after a ketamine i.v. bolus administration and reaches a peak about 30 min later. Norketamine is an analgesic molecule, whose power is about 20–30% compared with ketamine 21. Its pharmacokinetics is not well known, but time plasma concentration analysis after a unique ketamine administration demonstrates a slow elimination: norketamine persists more than 5 h after administration 21. Ketamine elimination half‐time is inferior to that of norketamine 22, which strongly participates in the pharmacological effects observed in the elimination phase, particularly the analgesic effect. Because of norketamine accumulation, the need for ketamine, when administered in continuous perfusion, decreases over time 23.
Kinetics and Metabolism's Modification Due to Other Drugs
Enzyme inducers, such as rifampicin, increase metabolism and clearance 7, not only for ketamine (13%), but essentially for norketamine (200%). Enzyme inhibitors, such as clarithromycin, have the opposite effect. Benzodiazepines seem capable of inhibiting ketamine N‐demethylation.
Administration Routes
By i.v. route, the study of the transfer to the action site demonstrates that ketamine reaches its receptors very quickly with a transfer half‐life of less than 1 min. Ketamine can also be administered through intramuscular (i.m), intrarectal, oral, or intranasal routes. Ketamine i.m. administration has a high bioavailability (93%), with a plasma peak obtained in 5 min. Per os, its bioavailability is limited (20%) because of the hepatic metabolism 24. Concentration peak occurs in 20–30 min 18. Intrarectal and intranasal ketamine bioavailabilities are, respectively, about 25 and 50% 21. After a unique epidural dose, ketamine rapidly goes to the systemic circulation 25: this can explain the high frequency of psychodysleptic effects observed after intrathecal or epidural injection 26.
Relationship with Renal and Liver Functions
The influence of kidney function on ketamine pharmacokinetics and on its active metabolite is low. For patients with kidney dysfunction, ketamine concentrations (obtained after the same dose) are 20% higher than for patients with normal kidney function: at a steady state, this corresponds to a moderate decrease in clearance. Only dehydronorketamine concentrations are statistically higher in patients with kidney dysfunction. Experimentally, ketamine has very little influence on the arterial or portal liver blood flow 27.
Pharmacokinetics in Children
Intramuscular absorption is faster in children than in adults. This phenomenon could be linked to muscle weakness in children and to differences in regional flows. Distribution volume is slightly lower (1.9 l/kg) but plasma clearance is more important (16.8 ml/kg/min) than in adults. Elimination half‐life is also shorter in children: 100 min 6. After an identical dose expressed in mg/kg, norketamine plasma concentrations are higher in children. However, in children from 4 to 10 years old and after an i.v. 2 mg/kg or i.m. 6 mg/kg injection, ketamine plasma concentrations are similar to those observed in adults. On the other hand, in the first 3 months of life, ketamine plasma clearance is shorter, probably due to a diminution of liver transformation and of kidney excretion. In this case, there is an increase in the elimination half‐life in newborns and infants. Distribution volume seems to be comparable to older children.
Pharmacodynamics
Neurophysiological Effects
Clinical Aspects, Site of Action, and Modification of EEG
Ketamine provides a totally different state of anesthesia compared with other anesthetic drugs (barbiturates, propofol, benzodiazepines, halogenated volatile anesthetics, etc.), the so‐called “dissociative anesthesia” 2. This is a cataleptic state in which the eyes stay open, with a typical nystagmus and conservation of laryngeal, corneal, and papillary reflexes. Some muscle hypertonia and motions are also observed. The anesthesia depth is compatible with the performance of a surgical procedure, with the absence of motor reaction orientated toward the nociceptive stimulus 28.
There is a functional and electrophysiological dissociation between thalamo‐neocortical and limbic systems: sensory inputs may reach cortical receiving areas, but fail to be observed in some of the association areas 2.
Thanks to modern functional magnetic resonance imaging, it has been shown in humans that ketamine plasma concentrations of 200 ng/ml, which reduce pain scores, concomitantly decrease insular cortex and thalamus activities, usually activated by a nociceptive stimulation 29. Sprenger et al. investigated the effects of subanesthetic IV S(+)‐ketamine doses (0.05–0.15 mg/kg/h) on the perception of painful heat stimuli in healthy volunteers. During placebo administration, a typical pain activation network (thalamus, insula, cingulate, and prefrontal cortex) was found, whereas decreased pain perception with ketamine was associated with a dose‐dependent reduction of pain‐induced cerebral activation. Analysis of the dose‐dependent ketamine effects on pain processing showed a decreasing activation of the secondary somatosensory cortex (S2), insula, and anterior cingulate cortex, which has been linked to the affective pain component that underlines the potency of ketamine in modulating affective pain processing 30. Honey et al. 31 showed that, even when overt behavior is unimpaired, ketamine has an impact on episodic memory task performance.
Although supraspinal action is preponderant 32, the spinal action is significant 33. Ketamine blocks afferent signals from the spino‐reticular pathways without modifying the conduction of the spino‐thalamic one. The medial reticular formation, relay of the pain perception emotional side, is selectively depressed, as well as medial thalamic nuclei 28. It has been demonstrated in cats that ketamine stops the signal from the reticularis formation, considered as an important relay in the nociceptive transmission between the spine and the supraspinal level 34. Contrary to other anesthetic drugs, ketamine enhances the descending inhibiting serotoninergic pathway 35, 36.
Ketamine modifies electroencephalogram (EEG) differently from other anesthetic drugs. The most typical EEG aspect is the diminution of alpha rhythm amplitude (even its abolition) without modification of its frequency 37 and the appearance of theta waves, without a measurable relationship with the depth of narcosis 38. Moreover, ketamine does not decrease the amplitude of mid‐latency auditory evoked potentials 39 or somatosensory evoked potentials 40. Finally, S(+)‐ketamine also provides a diminution of alpha rhythm amplitude 41, but R(−)‐ketamine has no action on EEG. Ketamine does not decrease the bispectral index (BIS) 42 and can even increase it 43. This effect is dose dependent: in a randomized controlled study, 0.5 mg/kg significantly increased the BIS (from 40 to 63) of patients undergoing general anesthesia with propofol and fentanyl, while 0.2 mg/kg did not increase it 44.
Doses and Concentration‐Effect Relationship
Ketamine alters memorization for a concentration of 70 ng/ml and provokes a lateral nystagmus for about 200 ng/ml 45. ED50 for narcosis (absence of verbal response) is 0.4–0.7 mg/kg. ED50 and ED95 for anesthesia (absence of response to the nociceptive stimulus) are, respectively, 0.6 and 1.3 mg/kg 46, 47. These ED correspond to an efficient concentration of about 10 μm 48. The ketamine analgesic effect persists for steady‐state plasma concentrations superior to 100–160 ng/ml 49 (about 0.5 μm). After a peripheric neurological lesion, ketamine‐efficient analgesic concentrations to reduce hyperalgesia and allodynia are at least superior to 100 ng/ml 50. After oral administration, ketamine‐efficient analgesic concentrations are lower (40 ng/ml), because of the more important action of norketamine 51.
Psychodysleptic Effects
Psychodysleptic effects (also called psychedelic effects) are phenomena appearing during the “awakening phase” or “emergence phase” and are disturbances of the visual and auditive perceptions, mood, body image, and time. These disturbances may result in an impression of unreality, of floating, or depersonalization, conscious dreams, or hallucinations. During the awakening phase after anesthesia, concentrations are 600–1100 ng/ml. These effects can occur as early as 50 ng/ml. In healthy volunteers (concentrations between 50 and 200 ng/ml), a linear dose‐effect relationship was demonstrated with progressive effects related to ketamine concentration 45. More severe effects (anxiety and paranoid feelings) appear around 500 ng/ml 52. “Emergence phenomena” is not really an adequate description: hallucinations and psychodysleptic effects can also occur during the induction of a sedation using subanesthetic dose. High doses or a too fast i.v. administration of low doses (from 5 to 10 mg) can lead to acute delirium 53. These effects are less pronounced with the dextrogyre isomer 54.
Psychosis, Ketamine, and Antidepressive Effect
In a double‐blind study in fifteen healthy subjects, a continuous subanesthetic S‐ketamine infusion was administered while cortical activation was measured using functional magnetic resonance imaging. While being scanned, subjects performed an overt word generation task. Ketamine administration elicited difficulties in abstract thinking, lack of spontaneity, and flow of conversation as well as formal thought disorder. A score for formal thought disorder positively correlated to activation measures encompassing the left superior temporal gyrus, the right middle and inferior frontal gyrus, and the precuneus. Difficulty in abstract thinking was correlated with pronounced activations in prefrontal as well as in anterior cingulate regions, whereas hyperactivations in the left superior temporal gyrus were found in association with a lack of spontaneity and flow of conversation. In the absence of behavioral impairments during verbal fluency, NMDAR blockade evoked psychopathological symptoms and cortical activations in regions previously reported in schizophrenia patients 55.
Ketamine‐induced psychic disorders in healthy volunteers and in schizophrenia have showed some similarities 56 and have led to pharmacological research.
Ketamine could allow the identification of a biophysical or biochemical process implicated in psychosis or the evaluation of the antipsychotic efficiency of a molecule susceptible to inhibit ketamine effects in healthy volunteers 57. On a neurophysiological basis, prefrontal cortex 58 and locus ceruleus 59 activation has been demonstrated. From a pharmacological point of view, there is an alteration 60, also a possible hypofunction 61 of NMDA receptors, with a hyperglutamatergic state 62.
NMDA receptor is implicated in depression state, which is strongly related to the glutamatergic system as recently demonstrated 63. Studies clearly showed ketamine antidepressive properties 64, 65, 66, with a rapid resolution of suicidal ideation, even during bipolar disorder 64, 67. Small ketamine doses also improve the postoperative mood of depressed patients 68, and this antidepressive effect has been suspected of participating in the antalgic effect in chronic pain 69.
Mechanisms of Action
Ketamine neuropharmacology is complex. Ketamine essentially acts on glutamate binding sites, NMDA (N‐Methyl‐D‐Aspartate), and non‐NMDA receptors 70. The antagonism of NMDA receptor is responsible for the specific ketamine properties (amnesic and psychosensory effects, analgesia, and neuroprotection). There are also other glutamate‐independent mechanisms.
Glutamate‐Independent Mechanisms
Ketamine interacts with many binding sites such as opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors.
γ‐Amino‐butyrique acid (GABA) is the most prevalent inhibiting neurotransmitter, responsible for an increase of chlorine conductance. Like other anesthetic agents, ketamine potentializes the GABA inhibition (GABA‐A complex) 71 but this interaction does not really account for the analgesic effects. A ketamine agonism on spinal GABA receptors, which plays a role in spinal analgesia, is established, but only for high concentrations (more than 500 μM) that are much more higher than those obtained in human practice 72.
Ketamine binds to mu, delta, and kappa opioid receptors. The affinity of S(+)‐ketamine for opioid receptors is two to three times higher than that of the R(−)isomer, but this interaction is not really responsible for its analgesic effect: in humans, this analgesic effect is not antagonized by naloxone 73. However, some psychic effects may involve kappa opioid receptors 73.
The action on the monoaminergic system is clearly essential. With the stimulation of noradrenergic neurons and the inhibition of catecholamines uptake, ketamine provokes a hyperadrenergic state (release of norepinephrine, dopamine, and serotonin). Inhibition of norepinephrine uptake is stereo specific: R(−) isomer only inhibits its neuronal uptake, while S(+) isomer also inhibits extra‐neuronal uptake. There is a prolonged synaptic action, leading to an increased transfer of norepinephrine in the circulation 70. Alpha‐2 agonists are able to decrease this hyperadrenergic state, but also psychic phenomena induced by ketamine 74. Because of its interfaction with the serotonin transporter 75, ketamine also inhibits dopamine and serotonin uptake 76. Moreover, ketamine emetic properties are inhibited by ondansetron, which suggests a serotoninergic mechanism. These interactions involving noradrenergic neurons are partially implicated in the hypnotic, psychic, and analgesic effects of the molecule.
In the hippocampus and in the striatum, cholinergic neurons control the liberation of acetylcholine. In prefrontal cortex, these cholinergic neurons could be activated by nicotinic and muscarinic receptors. Ketamine has a direct inhibiting effect on these receptors, which plays an important role in the occurrence of psychic phenomena. Thus, an anticholinesterasic agent, physostigmine, is able to reverse the central anticholinergic effects and also antagonize ketamine hypnotic effects 77. In this way, Balmer and Wyte have demonstrated, while injecting a ketamine perfusion (50 μg/kg/min) and physostigmine (0.5 mg) afterward, that the latter molecule antagonized ketamine sedative and hypnotic effects but respected its analgesic effects 78. Ketamine could also facilitate acetylcholine liberation in the hippocampus, because of a dopamine increase. However, at clinical efficient concentrations, ketamine could, in some models, inhibit acetylcholine liberation initiated by NMDA receptors 70. Ketamine, for clinical efficient concentrations (2.8 ± 0.6 mm), inhibits nicotinic receptors 79. It has an antagonist activity on muscarinic receptors 80, S(+)ketamine affinity being two times greater than that of the R(−) enantiomer.
Some ketamine effects involve the purinergic system, like toxic effects on the urinary tract 81.
Ketamine also has other effects because of its interactions with sodium channels (local anesthetic properties), L‐type calcium channels, and potassium channels.
Some psychodysleptic effects, which can be antagonized by nimodipine, could be initiated by L‐type calcium channels 82. The inhibition of sodium currents in the cardiac parasympathic neurons in nucleus ambiguus would be another explanation for the tachycardia induced by ketamine 83. An antagonism on sodium channels is linked to local anesthetic properties 84. Ketamine is similar to lidocaïne in terms of pKa and molecular weight. It may bind to the same site inside the sodium channels as local anesthetics 85 and is efficient as a local analgesic agent used in topical application 86. Ketamine inhibits neuron potassium channels 87: this mechanism could explain a part of (S)+isomer neuroprotective properties 88.
Glutamate‐Dependant Mechanisms
NMDA Receptor
The NMDA receptor, blocked by ketamine for concentrations between 2 and 50 μm, is responsible for ketamine's most important pharmacological properties. Glutamate is the most prevalent amino acid in the central nervous system (CNS), involving glutaminergic synapses. Its liberation activates several pre‐ and postsynaptic receptors located on ion channels. Ionotropic glutamate receptors are usually classified as NMDA (specifically activated by N‐methyl‐D‐aspartate) and non‐NMDA 89 (such as AMPA [alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid] and KA [kainate]) receptors. NMDA receptors are present on nearly all the cells of the CNS, particularly in the structures implicated in nociception, such as primary afferents or spinal dorsal horn. When glutamate is released in the synaptic cleft, there is an activation of the postsynaptic ionotropic receptors, which leads to the opening of ion channels, and is then responsible for a membrane depolarization 90. Permeable to sodium–potassium exchanges, the NMDA receptor is especially remarkable for its calcic conductance. Some have a presynaptic location. Like the AMPA receptor, the NMDA receptor is a heteromeric multimer. The most likely structure is tetrameric, the basic structure being constituted by two subunits (dimers of dimers). Most of the NMDA receptors of the CNS are constituted by two NR1 subunits and by two NR2 subunits. They are anchored in the plasmic membrane (Figure 3A) through the PSD‐95 protein (postsynaptic density 95). The subunits, which share common sequences with those of the AMPA and kainate receptors, are of three types: NR1, NR2, and NR3, also called GluN1, GluN2, and GluN3. These subunits possess four hydrophobic segments (M1 to M4) in their central region, with an arrangement in three transmembrane domains (M1, M3, and M4). The M2 segment that faces the cytoplasm represents the ionic channel of the receptor (Figure 2). Two wide domains are extracellular: the NTD N‐terminal extremity (N‐terminal domain) and the ABD domain (agonist‐binding domain), which allows, on NR2, the glutamate binding and, on NR1, glycine binding. These two domains, NTD and ABD, have a spatial clam structure (Figure 2).
Figure 3.
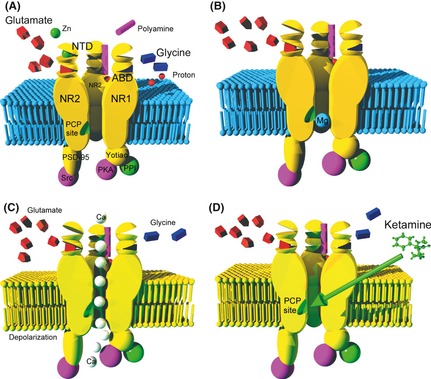
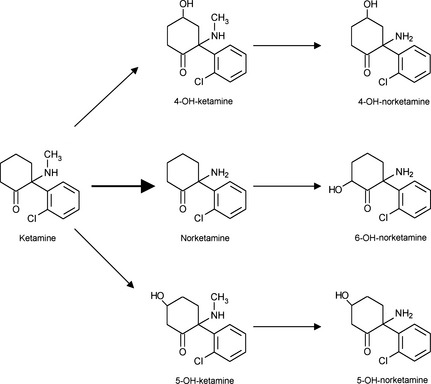
The NMDA receptor is anchored in the membrane (sky blue) by the PSD‐95 protein, linked to the Src tyrosine kinase. The four sub‐units (2 NR1 and 2 NR2) form an NMDA receptor channel selective for the cathions, which is shown open in (A). (A) The binding site of glutamate (red polyhedron), selectively activated by NMDA, is in ABD clam shaped NR2 subunit. The site for glycine (dark blue polyhedron), which acts as a co‐agonist of glutamate, is located in the ABD area of the NR1 subunit. We do not know exactly where polyamines (magenta cylinder) bind, but certainly in close connection with one or the other clam‐form fields in NR2 unit. Zinc ion (green spheres) binds to the NTD domain of NR2 subunit. Protons (red sphere) are an essential regulator mechanism that promotes closed state of the channel. The site of the proton detector is unknown, but it is assumed that it is ane area near ABD domain. Protein phosphatase type I (PP1) and cAMP‐dependent protein kinase (PKA) are attached to the NR1 subunit by an anchor protein named Yotiao. (B) When the membrane is not depolarized, even when agonists occupy ABD sites, the channel is blocked by Mg2+ ion (sky blue sphere). The binding site of magnesium is near the intracellular part of the receptor. (C) In contrast, a membrane depolarization (phospholipids represented in yellow) causes the departure of the Mg2+ ion (voltage‐dependent block) and allows a massive influx of calcium (white spheres), if the two coagonists occupy their binding site. (D) The molecule of ketamine or other derivatives of phencyclidine inactivate the receptor by binding to the intraductal PCP site (green slot), which partially covers the magnesium binding site.
Figure 2.
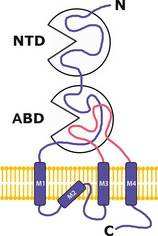
Structure of the four subunits of the NMDA receptor. NTD, N‐terminal domain, ABD, agonist‐binding domain. The M2 segment faces the cytoplasm and would be the receptor ion channel.
Agonists and competitive antagonists bind in the slot of this structure 91. Seven subcategories of NR1 subunit exist (h–g). The NR2 subunits, incorporated into NR1/NR2 heteromeric complex, seem to play an important role in pathological processes associated with abnormal NMDA channel function 91, including schizophrenia. Four subtypes, designated by the letters from A through D, determine the type of receptor. A and B types are the most common. NR2A subtype is ubiquitous. The NR2B subtype is particularly located in the limbic system, the place for emotions and memory. It is found in the anterior part of the brain, particularly the cingulate cortex, but also in the hippocampus, the amygdala, and the olfactory bulb. NR2B type also participates in the transmission of pain messages to the thalamus, spinal cord, and extrasynaptic locations, for example, at the level of primary afferents. Spinal cord is also rich in NR2D type and cerebellum in NR2C type. NR2 subunit plays a fundamental role in the spontaneous opening probability (independent of ligands) of the channel. The N‐terminal domains of the NR2A and NR2B subunit control the opening of the channel. This extracellular portion controls the sensitivity of the receptor to its endogenous inhibitors (especially zinc and protons) and has the property of binding allosteric inhibitors 91.
The activation of NMDA receptors is rather complex, involving multiple agonists interacting in cooperation and regulatory mechanisms, which, on the contrary, favor the closed state of the channel. NMDA receptors have a binding site for glutamate, which is located on the ABD area of the NR2 unit (Figure 3A). This is the site that is selectively activated by N‐methyl‐D‐aspartate. Receptor activation requires the simultaneous binding of one glycine molecule at a separate site in the ABD area on NR1 unit. Glutamate and glycine are thus described as “coagonists” of the system (Figure 3A).
Magnesium Channel Block
The main regulatory mechanism that opposes the opening of the channel is the voltage‐dependent magnesium block of the NMDA receptor 92. At the resting membrane potential (approximately 70 mV), extracellular Mg2+ blocks the receptor‐associated channel, even if the coagonists (glutamate and glycine) are bound to their respective sites. The binding site of Mg2+ is located quite low in the intracellular channel side (Figure 3B). The dissociation constant of magnesium is an exponential function of membrane voltage. In the case of neuronal depolarization, the negative electrostatic forces that fasten ion Mg2+ at the NR2 subunit collapse, and the cation is released. This ejection of the plug allows, under the action of the coagonists, a calcium influx linked to the importance of the depolarization (Figure 3C). Although magnesium administered alone does not seem to reduce postoperative pain, these phenomena could explain lesser amounts of propofol required for narcosis and some antihyperalgesic effects of magnesium 93. In addition, ketamine and magnesium have a synergistic effect 94.
Receptor Phosphorylation
Phosphorylation of the NMDA receptor plays a fundamental role in its activation. These phosphorylation mechanisms are the basis of long‐term potentiation but also of hyperalgesia and morphine interactions with NMDA receptors 95. Two phosphoproteins also modulate the NMDA receptor: phosphatase protein type I (PP1) and cAMP‐dependent protein kinase (PKA). These two regulatory proteins are attached to the NR1 unit by another anchor protein called Yotiao (Figure 3A). This type of control (simultaneous presence of a kinase and a phosphatase that control the phosphorylation of a receptor) is typical of many ion channels 96, 97.
Allosteric Inhibitors
Allosteric sites of the NR2 subunit also allow positive or negative modulation of the activity of the NMDA receptor. Some ions play an important role in the regulation of the channel opening by altering the spatial conformation of the receptor. These are protons and zinc ion (Zn2+).
Protons (H+ ions) are potent non‐competitive inhibitors of NMDA receptors 98. We do not know precisely the location of the proton detector, but it is known that protons act by stabilizing the closed state of the channel, independently of membrane polarization. Thus, tissue acidosis that accompanies ischemia or epileptic discharges reduces damage to neurons 99. Receptors composed of NR2A or NR1a subunits have an intermediate reactivity to pH with an IC50 (concentration inhibiting 50% of the receptors) of 6.9 pH units, while the NR2C units provide the receptor with virtual insensitivity. In contrast, receptors composed of NR1a/NR2B or NR1a/NR2D dimers are extremely sensitive to pH. Their IC50 close to pH 7.40 explains that, under normal conditions, half of these receptors are under the influence of a tonic inhibition of opening by protons. Thus, even moderate changes in pH can participate in the opening of these NMDA channels, which illustrates an additional pejorative aspect of alkalosis.
The NTD domain of NR2A and NR2B subunits also plays an important role in NMDA receptor function modulation by selectively binding to non‐competitive antagonists.
For the moment, only the zinc ion, often coreleased with glutamate in synaptic vesicles, has been identified as a ligand for NR2A and NR2B subunits 100 (Figure 3A). Zn2+ binds to the opening area of the NTD clam‐shaped domain with an IC50 of 15 nmol and causes its closure. This closure relaxes a tension in the connections between the ABD and NTD domains, which in turn causes the separation of the interface between the two ABD domains of the receptor. This separation relaxes a stress exerted on a membrane segment, and this conformational change allows, with the bond to a proton, the closure of the channel. Zinc thus potentiates the channel closure, regulated by protons. It is possible that these mechanisms explain part of the pathophysiology of near‐death experiences (NDE). In situations of cerebral ischemia or hypoglycemia, the NMDA channel is opened by neuronal depolarization and would hypothetically be modulated by zinc ions or protons. Prodils such as ifenprodil selectively inhibit NMDA receptors containing the NR2B subunit by binding in the slot of NTD domains, a site that partially overlaps with that of zinc binding 101. Some synthetic compounds highly selective for the NTD domain of NR2B subunits, as traxoprodil, besonprodil, or radiprodil and other more recently described compounds, are used as pharmacological tools and may, in the future, become therapeutic agents as analgesics (including for chronic pain), neuroprotective agents, anticonvulsants, antidepressants or treatments for Parkinson's disease, and other neurodegenerative diseases. In the same way as zinc, they promote occlusion of the NMDA channel under the influence of protons. They are promising in the sense that they inhibit the receptor most involved in pathological phenomena, but also because they are much more active when the channel has been previously opened.
Polyamines: Endogenous Allosteric Activators
Polyamines, putrescine, spermine, and spermidine, are basic aliphatic amines, positively charged at normal pH. They are synthesized from ornithine, a metabolite in the urea cycle, and spread throughout the body. They are released in neurons in a calcium‐dependent manner. Without being critical, they potentiate channel opening under the joint action of glutamate and glycine (Figure 3A). Polyamine deprivation has an analgesic effect in some animal models 102.
Other endogenous substances may allosterically modulate NMDA receptors; it is the case of neurosteroids, such as pregnenolone sulfate. The release of these substances can be triggered by stress.
Consequences of the Opening of the NMDA Channel
The increase in intracellular calcium concentration is the starting point for the synthesis of second and third messengers, prostaglandins and nitric oxide (NO), which facilitate the presynaptic release of glutamate, thus initiating the amplification of a vicious circle.
Binding protein PSD‐95 appears to be essential to the sequence, which links the increased synthesis of NO and calcium influx 103. The NR2 unit, rich in tyrosine residues on the cytoplasmic side, is linked to an intracellular tyrosine kinase of the Src family, by means of the anchoring PSD‐95 protein (Figure 3A). A major function of Src in the adult CNS is to regulate glutamatergic transmission and synaptic plasticity 104. PSD‐95 is also linked to other intracellular enzymes, in particular NO‐synthase 105.
The increase in intraneuronal calcium causes secondary activation of kinases that regulate the activity of receptors and modulate downstream early gene expression like c‐fos. These transcriptional processes support long‐term memory.
Because of an increase of intracellular calcium, the NMDA receptor stimulation by glutamate liberated in the nociceptive afferents leads to the activation of a neuronal NO‐synthase (NO‐s) and the production of NO from L‐arginine 106. NO stimulates the synthesis of cyclic guanosine monophosphate 3′5′ (c‐GMP), which plays a role in the central transmission of pain messages (“nitroxidergic” transmission) 107. It has been demonstrated that NO and c‐GMPc participate in the central sensitization and hyperalgesia processes, after a peripheral inflammation for example 108.
Mechanism of Action of Ketamine at the NMDA Receptor Level
Ketamine and other NMDA receptor non‐competitive antagonists (PCP, MK801, dextromethorphan, and memantine 109) are fastened to an intrachannel site called phencyclidine site (Figure 3D). Ketamine intrachannel binding decreases the channel opening time. Ketamine decreases the amplification of the response to a repeated stimulation (stimuli summation called “wind up”, considered as an elementary form of sensitization of the CNS) 110, 111. The antagonism is more important if the NMDA channel has been previously opened by the glutamate fixation. This “use dependence” concept can explain why ketamine analgesic properties are efficient if the pain is important or chronic 112.
Fixing of ketamine at a second site located in the hydrophobic domain of the NMDA receptor decreases the frequency of channel opening 113. Ketamine is also an allosteric antagonist of the receptor, with a marked tropism for NR2B unity, particularly involved in the phenomena of emotional perception and memory of pain. Independently of its action on NMDA receptors, ketamine might directly inhibit NO‐synthase, which could act in its analgesic and anesthetic effects 114.
S(+)‐ketamine affinity for the PCP site could be three times higher than that of R(−)‐ketamine 94, which confers to S(+)‐ketamine a strong analgesic and anesthetic effect, at least two times stronger compared to the racemic mixture 115.
NMDA Receptors and Opioid‐Induced Hyperalgesia
Interactions between opioid and NMDA receptors explain the antihyperalgesic properties of ketamine 116. Relations between ketamine and opioid system are not unequivocal 117. On the one hand, morphine has a low affinity for NMDA receptors. Morphine also decreases glutamatergic transmission in the cortex 118. Conversely, it has become clear, in recent years, that opioids favor phenomena of acute and chronic tolerance, termed opioid‐induced hyperalgesia 119, 120, 121. These effects, which are not universally found 122, but are dose dependent, result from the involvement of NMDA receptors in the CNS 123. There are cross‐talks between NMDA and opioid receptors on the surface of the same cells. Following the activation of opioid receptors and protein kinase C 124, especially its γ isoform 125, 126, phosphorylation of the NMDA receptor suppresses the magnesium plug of the channel, allowing the entry of Ca2+ into the cell, starting point of a cascade of events (activation of protein kinase C, of prostaglandin and nitric oxide systems, transcriptional changes) that leads to a down‐regulation (underlying tolerance) and a blunted response of opioid receptors (underlying hyperalgesia). The antagonism of NMDA receptors allows ketamine to exert a preventive action of these phenomena, one of the molecules most explored aspects for more than 10 years 127, 128, 129.
Excitotoxicity
NMDA receptors are involved in neuronal tissue physiology and in synaptic plasticity, but in certain circumstances, also in acute or chronic neurotoxic effects. The concept of excitotoxicity, issued from the work of John Olney 130, confers to glutamate the status of “excitotoxin.” While excitotoxicity could be mediated by any of the ionotropic receptors, the calcium conductance of the NMDA receptor makes it the privileged mediator of these phenomena. Indeed, a massive increase in calcium concentration inside the neuron is likely to produce a cascade of deleterious events, whose ultimate outcome is cell death. The cytoplasmic Ca2+ activates many enzymes such as protein kinase C (PKC), phospholipases A2 and C (PLA2 and PLC), protein kinase II Ca2+, and calmodulin‐dependent NO‐synthase as well as proteases and endonucleases. This sequence of reactions has been proposed as a pathogenic model of cerebral ischemia and traumatic brain injury 131. In case of hypoxia or ischemia, the collapse of the activity (or an activity in reverse mode) of the high‐affinity transporter, which normally removes glutamate from the synaptic cleft, provokes a significant increase in extracellular glutamate 132. The collapse of ATP‐dependent ion pumps (Na/K‐ATPase) increases extracellular potassium concentrations, which in turn causes a depolarization of neurons that terminates the magnesium block and reduces the effectiveness of high‐affinity transporter glutamate whose energy source is the transmembrane sodium gradient 130. In addition, potassium stimulates the secretion of glutamate and inhibits its glial capture. Finally, an injured cell glutamate content is an important source of excitatory amino acids. A potassium current, independent of calcium, also intervenes in the apoptotic phenomena 133. Ischemia is finally associated with the stimulation of ornithine decarboxylase, which leads to the synthesis of polyamines. Thus, excitotoxicity is the efferent common pathway of pathological processes, all compromising cellular energy intake. The glutamatergic system would thus be involved in chronic neurological pathologies, such as amyotrophic lateral sclerosis and Huntington's, Alzheimer's, or Parkinson's diseases. NMDA antagonists experimentally have neuroprotective properties because they are able to reduce neuronal apoptosis or brain injury induced by hypoxia or brain trauma 131. There exists a window of opportunity with respect to the ischemic penumbra, during the hours following cerebral infarction, because the effectiveness of NMDA antagonists in this area is highly dependent on the precocity of their administration 134, 135. Regarding neurodegenerative diseases, amantadine 136, originally used in the treatment of influenza, and its derivative memantine 137 are NMDA antagonists, which improve the symptoms of Parkinson's disease. A recent study reported improved Parkinsonian symptoms (dyskinesia and tremor) in the minutes following the injection of low doses of ketamine (10 mg two bolus IV) 138.
Ketamine Neurotoxicity
Experimentally, NMDA antagonists and ketamine are clearly known to exhibit a potential neurotoxicity 139, 140 and may promote neuronal apoptotic lesions 141. On the contrary, in usual anesthetic practice, ketamine does not induce neurotoxicity. Possible but rare neurotoxicity cases have scarcely been reported 142. The consequences of high doses, repeatedly administered for more than 24 h, are not known. Psychodysleptic effects could be linked to transient neurotoxic effects 139. Cognitive disturbances are frequent in ketamine chronic users 143, as well as frontal white matter abnormalities 144.
Recently, several experimental studies have raised the possibility of neurotoxic effects in the developing brain 145. Animal studies suggest that neurodegeneration, with possible cognitive sequelae, is a potential long‐term risk of anesthetics in neonatal and young pediatric patients. The existing experimental data implicate not only NMDA receptor antagonists, but also drugs that potentiate gamma‐aminobutyric acid signal transduction, as potentially neurotoxic to the developing brain 146.
Conclusions
Having been in use for more than 50 years, ketamine has proven to be a safe anesthetic drug with potent analgesic properties. It has been widely used to induce narcosis, because of the preservation of cardiovascular and respiratory functions, and in the context of an emergency, because it allows the preservation of pharyngeal and laryngeal reflexes.
The so‐called psychodysleptic effects explain a fall in ketamine use during the 1980s. But during the 90s, its peculiar antihyperalgesic properties renewed the interest for this agent, which allows a reduction of opioid tolerance in the context of postoperative pain. More recently, an antidepressive activity has been demonstrated that may participate in the recovery from the disability resulting from chronic pain syndromes. Ketamine and other anti‐NMDA derivatives are being explored in the prevention of the excitotoxicity phenomenons involved in acute and chronic brain damage. Some safety concerns have been advocated, especially in the context of chronic administration, or in the case of administration during pregnancy and in a pediatric setting.
Conflict of Interest
The authors declare no conflict of interest.
Pr Georges Mion is the recipient of a master in anesthesiology (1993). The paper is not based on a previous communication to a society or meeting.
References
- 1. Johnstone M, Evans V, Baigel S. Sernyl (CI‐395) in clinical anaesthesia. Br J Anaesth 1959;31:433–439. [DOI] [PubMed] [Google Scholar]
- 2. Corssen G, Domino EF. Dissociative anesthesia: Further pharmacologic studies and first clinical experience with the phencyclidine derivative CI‐581. Anesth Analg 1966;45:29–40. [PubMed] [Google Scholar]
- 3. Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI‐581, a new dissociative anesthetic, in man. Clin Pharmacol Ther 1965;6:279–291. [DOI] [PubMed] [Google Scholar]
- 4. Dayton PG, Stiller RL, Cook DR, Perel JM. The binding of ketamine to plasma proteins: Emphasis on human plasma. Eur J Clin Pharmacol 1983;24:825–831. [DOI] [PubMed] [Google Scholar]
- 5. Schuttler J, Stanski DR, White PF, et al. Pharmacodynamic modeling of the EEG effects of ketamine and its enantiomers in man. J Pharmacokinet Biopharm 1987;15:241–253. [DOI] [PubMed] [Google Scholar]
- 6. Grant IS, Nimmo WS, McNicol LR, Clements JA. Ketamine disposition in children and adults. Br J Anaesth 1983;55:1107–1111. [DOI] [PubMed] [Google Scholar]
- 7. Noppers I, Olofsen E, Niesters M, al. Effect of Rifampicin on S‐ketamine and S‐norketamine Plasma Concentrations in Healthy Volunteers after Intravenous S‐ketamine Administration. Anesthesiology 2011;114:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park GR, Manara AR, Mendel L, Bateman PE. Ketamine infusion. Its use as a sedative, inotrope and bronchodilator in a critically ill patient. Anaesthesia 1987;42:980–983. [DOI] [PubMed] [Google Scholar]
- 9. Edwards SR, Mather LE. Tissue uptake of ketamine and norketamine enantiomers in the rat: Indirect evidence for extrahepatic metabolic inversion. Life Sci 2001;69:2051–2066. [DOI] [PubMed] [Google Scholar]
- 10. Domino EF, Domino SE, Smith RE, Domino LE, Goulet JR, Domino KE. Ketamine kinetics in unmedicated and diazepam premedicated subjects. Clin Pharmacol Ther 1984;36:645–653. [DOI] [PubMed] [Google Scholar]
- 11. Sigtermans M, Dahan A, Mooren R, et al. S(+)‐ketamine effect on experimental pain and cardiac output: A population pharmacokinetic‐pharmacodynamic modeling study in healthy volunteers. Anesthesiology 2009;111:892–903. [DOI] [PubMed] [Google Scholar]
- 12. Persson J, Hasselstrom J, Maurset A, et al. Pharmacokinetics and non‐analgesic effects of S‐and R‐ketamine in healthy volunteers with normal and reduced metabolic capacity. Eur J Clin Pharmacol 2002;57:869–875. [DOI] [PubMed] [Google Scholar]
- 13. Chen JT, Chen RM. Mechanisms of ketamine‐involved regulation of cytochrome P450 gene expression. Expert Opin Drug Metab Toxicol 2010;6:273–281. [DOI] [PubMed] [Google Scholar]
- 14. Geisslinger G, Menzel‐Soglowek S, Kamp HD, Brune K. Stereoselective high‐performance liquid chromatographic determination of the enantiomers of ketamine and norketamine in plasma. J Chromatogr 1991;568:165–176. [DOI] [PubMed] [Google Scholar]
- 15. Geisslinger G, Hering W, Kamp HD, Vollmers KO. Pharmacokinetics of ketamine enantiomers. Br J Anaesth 1995;75:506–507. [DOI] [PubMed] [Google Scholar]
- 16. Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology 1992;77:1201–1207. [DOI] [PubMed] [Google Scholar]
- 17. Ihmsen H, Geisslinger G, Schuttler J. Stereoselective pharmacokinetics of ketamine: R(−)‐ketamine inhibits the elimination of S(+)‐ketamine. Clin Pharmacol Ther 2001;70:431–438. [DOI] [PubMed] [Google Scholar]
- 18. Noppers I, Niesters M, Aarts L, Smith T, Sarton E, Dahan A. Ketamine for the treatment of chronic non‐cancer pain. Expert Opin Pharmacother 2010;11:2417–2429. [DOI] [PubMed] [Google Scholar]
- 19. Henthorn TK, Krejcie TC, Niemann CU, Enders‐Klein C, Shanks CA, Avram MJ. Ketamine distribution described by a recirculatory pharmacokinetic model is not stereoselective. Anesthesiology 1999;91:1733–1743. [DOI] [PubMed] [Google Scholar]
- 20. White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth 1985;57:197–203. [DOI] [PubMed] [Google Scholar]
- 21. Malinovsky JM, Servin F, Cozian A, Lepage JY, Pinaud M. Ketamine and norketamine plasma concentrations after IV, nasal and rectal administration in children. Br J Anaesth 1996;77:203–207. [DOI] [PubMed] [Google Scholar]
- 22. Wieber J, Gugler R, Hengstmann JH, Dengler HJ. Pharmacokinetics of ketamine in man. Anaesthesist 1975;24:260–263. [PubMed] [Google Scholar]
- 23. Idwall J, Ahlgren I, Aronsen KR, Stenberg P. Ketamine infusions: Pharmacokinetics and clinical effects. Br J Anaesth 1979;51:1167–1173. [DOI] [PubMed] [Google Scholar]
- 24. Clements JA, Nimmo WS. The pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth 1981;53:27–30. [DOI] [PubMed] [Google Scholar]
- 25. Pedraz JL, Lanao JM, Calvo MB, Muriel C, Hernandez‐Arbeiza J, Dominguez‐Gil A. Pharmacokinetic and clinical evaluation of ketamine administered by IV and epidural routes. Int J Clin Pharmacol Ther Tox 1987;25:77–80. [PubMed] [Google Scholar]
- 26. Wong CS, Liaw WJ, Tung CS, Su YF, Ho ST. Ketaminepotentiates analgesic effect of morphine in postoperative epidural pain control. Reg Anesth 1996;21:534–541. [PubMed] [Google Scholar]
- 27. Thomson IA, Fitch W, Campbell D, Watson R. Effects of ketamine on liver blood flow and hepatic oxygen consumption. Studies in the anaesthetised greyhound. Acta Anaesthesiol Scand 1988;32:10–14. [DOI] [PubMed] [Google Scholar]
- 28. White PF, Way WL, Trevor AJ. Ketamine its pharmacology and therapeutic uses. Anesthesiology 1982;56:119–136. [DOI] [PubMed] [Google Scholar]
- 29. Rogers R, Wise RG, Painter DJ, Longe SE, Tracey I. An investigation to dissociate the analgesic and anesthetic properties of ketamine using functional magnetic resonance imaging. Anesthesiology 2004;100:292–301. [DOI] [PubMed] [Google Scholar]
- 30. Sprenger T, Valet M, Woltmann R, et al. Imaging pain modulation by subanesthetic S‐(+)‐ketamine. Anesth Analg 2006;103:729–737. [DOI] [PubMed] [Google Scholar]
- 31. Honey GD, Honey RA, O'Loughlin C, et al. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: An fMRI study. Cereb Cortex 2005;15:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okuda T. Comparison of direct and indirect depressant actions of ketamine on dorsal horn cells in rabbits. Neuropharmacology 1986;25:433–440. [DOI] [PubMed] [Google Scholar]
- 33. Denda S, Shimoji K, Tomita M, et al. Central nuclei and spinal pathways in feedback inhibitory spinal cord potentials in ketamine‐anaesthetized rats. Br J Anaesth 1996;76:258–265. [DOI] [PubMed] [Google Scholar]
- 34. Ohtani M, Kikuchi H, Kitahata LM, et al. Effects of ketamine on nociceptive cells in the medial medullary reticular formation of the cat. Anesthesiology 1979;51:414–417. [DOI] [PubMed] [Google Scholar]
- 35. Larson AA. Interactions between ketamine and phencyclidine and dorsal root potentials (DRPs), evoked from the raphe nuclei. Neuropharmacology 1984;23:785–791. [DOI] [PubMed] [Google Scholar]
- 36. Koizuka S, Obata H, Sasaki M, Saito S, Goto F. Systemic ketamine inhibits hypersensitivity after surgery via descending inhibitory pathways in rats. Can J Anaesth 2005;52:498–505. [DOI] [PubMed] [Google Scholar]
- 37. Oga K, Kojima T, Matsuura M, et al. Effects of low‐dose ketamine on neuropathic pain: An electroencephalogram‐electrooculogram / behavioral study. Psychiatry Clin Neurosci 2002;56:355–363. [DOI] [PubMed] [Google Scholar]
- 38. Plourde G, Baribeau J, Bonhomme V. Ketamine increases the amplitude of the 40‐Hz auditory steady‐state response in humans. Br J Anaesth 1997;78:524–529. [DOI] [PubMed] [Google Scholar]
- 39. Schwender D, Klasing S, Madler C, Poppel E, Peter K. Mid‐latency auditory evoked potentials during ketamine anaesthesia in humans. Br J Anaesth 1993;71:629–632. [DOI] [PubMed] [Google Scholar]
- 40. Langeron O, Lille F, Zerhouni O, et al. Comparison of the effects of ketamine‐midazolam with those of fentanyl‐midazolam on cortical somatosensory evoqued potentials during major spine surgery. Br J Anaesth 1997;78:701–706. [DOI] [PubMed] [Google Scholar]
- 41. Freye E, Latasch L, Schmidhammer H. Pharmacodynamic effects of S‐(+)‐ketamine on EEG, evoked potentials and respiration. A study in the awake dog. Anaesthesist 1992;41:527–533. [PubMed] [Google Scholar]
- 42. Morse Z, Kaizu M, Sano K, Kanri T. BIS monitoring during midazolam and midazolam‐ketamine conscious intravenous sedation for oral surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:420–424. [DOI] [PubMed] [Google Scholar]
- 43. Morioka N, Ozaki M, Matsukawa T, Sessler DI, Atarashi K, Suzuki H. Ketamine causes a paradoxical increase in the Bispectral index. Anesthesiology 1997;87:A502. [Google Scholar]
- 44. Sengupta S, Ghosh S, Rudra A, Kumar P, Maitra G, Das T. Effect of ketamine on bispectral index during propofol‐fentanyl anesthesia: A randomized controlled study. Middle East J Anesthesiol 2011;21:391–395. [PubMed] [Google Scholar]
- 45. Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy‐Byrne PP. Psychedelic effects of ketamine in healthy volunteers: Relationship to steady‐state plasma concentrations. Anesthesiology 1998;88:82–88. [DOI] [PubMed] [Google Scholar]
- 46. Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J. Additive interactions between propofol and ketamine when used for anesthesia induction in female patients. Anesthesiology 1995;82:641–648. [DOI] [PubMed] [Google Scholar]
- 47. White PF, Dworsky WA, Horai Y, Trevor AJ. Comparison of continuous infusion fentanyl or ketamine versus thiopental determining the mean effective serum concentrations for outpatient surgery. Anesthesiology 1983;59:564–569. [DOI] [PubMed] [Google Scholar]
- 48. Yamakura T, Chavez‐Noriega LE, Harris A. Subunit‐dependant inhibition of human neuronal nicotinic acetylcholine receptors and other ligand‐gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology 2000;92:1144–1153. [DOI] [PubMed] [Google Scholar]
- 49. Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci 1982;71:539–542. [DOI] [PubMed] [Google Scholar]
- 50. Leung A, Wallace MS, Ridgeway B, Yaksh T. Concentration‐effect relationship of intravenous alfentanil and ketamine on peripheral neurosensory thresholds, allodynia and hyperalgesia of neuropathic pain. Pain 2001;91:177–187. [DOI] [PubMed] [Google Scholar]
- 51. Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of IM and oral ketamine. Br J Anaesth 1981;53:805–810. [DOI] [PubMed] [Google Scholar]
- 52. Hartvig P, Valtysson J, Lindner KJ, et al. Central nervous system effects of subdissociative doses of (S)‐ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther 1995;58:165–173. [DOI] [PubMed] [Google Scholar]
- 53. Mion G, Granry JC, Villevieille T. Nuove applicazioni della ketamina nell'anesthesia moderna (Capitolo 33) In: Romano Ezio, editor. Anestesia generale e clinica, tomo I, 2d edn Torino, Milano: UTET, 2004;515–531. [Google Scholar]
- 54. Pfenninger EG, Durieux ME, Himmelsehers S. Cognitive impairment after small‐dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology 2002;96:357–366. [DOI] [PubMed] [Google Scholar]
- 55. Nagels A, Kirner‐Veselinovic A, Wiese R, Paulus FM, Kircher T, Krach S. Effects of ketamine‐induced psychopathological symptoms on continuous overt rhyme fluency. Eur Arch Psychiatry Clin Neurosci 2012;262:403–414. [DOI] [PubMed] [Google Scholar]
- 56. Adler CM, Malhotra AK, Elman I, et al. Comparison of ketamine‐induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 1999;156:1646–1649. [DOI] [PubMed] [Google Scholar]
- 57. Carpenter WT. The schizophrenia ketamine challenge study debate. Biol Psychiatry 1999;46:1081–1091. [DOI] [PubMed] [Google Scholar]
- 58. Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickard D. Association of ketamine‐induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 1997;154:805–811. [DOI] [PubMed] [Google Scholar]
- 59. Aghadjanian GK, Marek GJ. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res Rev 2000;31:302–312. [DOI] [PubMed] [Google Scholar]
- 60. Malhotra AK, Pinals DA, Adler CM, et al. Ketamine‐induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic‐free schizophrenics. Neuropsychopharmacology 1997;17:141–150. [DOI] [PubMed] [Google Scholar]
- 61. Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 1999;33:523–533. [DOI] [PubMed] [Google Scholar]
- 62. Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco‐magnetic resonance imaging study. Arch Gen Psychiatry 2008;65:154–164. [DOI] [PubMed] [Google Scholar]
- 63. Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N‐methyl‐D‐aspartate (NMDA) receptors following antidepressant treatment: Implications for the pharmacotherapy of depression. Pharmacopsychiatry 1996;29:23–26. [DOI] [PubMed] [Google Scholar]
- 64. DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N‐methyl‐D‐aspartate antagonist in patients with treatment‐resistant major depressive disorder. J Clin Psychiatry 2010;71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mathew SJ, Shah A, Lapidus K, et al. Ketamine for treatment‐resistant unipolar depression: Current evidence. CNS Drugs 2012;26:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: Rationale and progress to date. Drugs 2012;72:1313–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zarate CA Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add‐on trial. Biol Psychiatry 2012;71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kudoh A, Takahira Y, Katagai H, Takazawa T. Small‐dose ketamine improves the postoperative state of depressed patients. Anesth Analg 2002;95:114–118. [DOI] [PubMed] [Google Scholar]
- 69. Romero‐Sandoval EA. Depression and pain: Does ketamine improve the quality of life of patients in chronic pain by targeting their mood? Anesthesiology 2011;115:687–688. [DOI] [PubMed] [Google Scholar]
- 70. Kohrs R, Durieux ME. Ketamine: Teaching an old drug new tricks. Anesth Analg 1998;87:1186–1193. [DOI] [PubMed] [Google Scholar]
- 71. Miller KW. The nature of sites of general anaesthetic action. Br J Anaesth 2002;89:17–31. [DOI] [PubMed] [Google Scholar]
- 72. Flood P, Kransowski MD. Intravenous anesthetics differentially modulate ligand‐gated ion channels. Anesthesiology 2000;92:1418–1425. [DOI] [PubMed] [Google Scholar]
- 73. Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, and muscarinic receptors. Pharmacol Toxicol 1995;77:355–359. [DOI] [PubMed] [Google Scholar]
- 74. Leväen J, Muffelman M, Scheinin H. Dexmedetomidine premedication attenuates ketamine‐induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology 1995;82:1117–1125. [DOI] [PubMed] [Google Scholar]
- 75. Martin DC, Introna RP, Aronstam RS. Inhibition of neuronal 5‐HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett 1990;112:99–103. [DOI] [PubMed] [Google Scholar]
- 76. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology 1982;21:113–118. [DOI] [PubMed] [Google Scholar]
- 77. Toros‐Matos A, Rendon‐Platas AM, Avila‐Valdez E, Villanreal‐Guzman RA. Physostigmine antagonizes ketamine. Anesth Analg 1980;59:764–767. [PubMed] [Google Scholar]
- 78. Balmer HGR, Wyte SR. Antagonism of ketamine by physostigmine. Br J Anaesth 1979;49:510. [Google Scholar]
- 79. Furuya R, Oka K, Watanabe I, Kamiya Y, Itoh H, Andoh T. The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P2X purinoceptors in PC12 cells. Anesth Analg 1999;88:174–180. [DOI] [PubMed] [Google Scholar]
- 80. Durieux ME. Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth Analg 1995;81:57–62. [DOI] [PubMed] [Google Scholar]
- 81. Meng E, Chang HY, Chang SY, Sun GH, Yu DS, Cha TL. Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J Urol 2011;186:1134–1141. [DOI] [PubMed] [Google Scholar]
- 82. Krupitsky EM, Burakov AM, Romanova TN, et al. Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: Psychopharmacologic implications of the interaction of NMDA and L‐type calcium channel antagonists. Neuropsychopharmacology 2001;25:936–947. [DOI] [PubMed] [Google Scholar]
- 83. Irnaten M, Wang J, Chang KS, Andresen MC, Mendelowitz D. Ketamine inhibits sodium currents in identified cardiac parasympathetic neurons in nucleus ambiguus. Anesthesiology 2002;96:659–666. [DOI] [PubMed] [Google Scholar]
- 84. Frenkel C, Urban BW. Molecular actions of racemic ketamine on human CNS sodium channels. Br J Anaesth 1992;69:292–297. [DOI] [PubMed] [Google Scholar]
- 85. Wagner LE, Gingrich KJ, Kulli JC, Yang J. Ketamine blockade of voltage‐gated sodium channels. Evidence for a shared receptor site with local anesthetics. Anesthesiology 2001;95:1406–1413. [DOI] [PubMed] [Google Scholar]
- 86. Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: A double‐blind placebo‐controlled trial of topical ketamine. Pain 2009;146:18–25. [DOI] [PubMed] [Google Scholar]
- 87. Friederich P, Urban BW. Interaction of intravenous anesthetics with human neuronal potassium currents in relation to clinical concentrations. Anesthesiology 1999;91:1853–1860. [DOI] [PubMed] [Google Scholar]
- 88. Proescholdt M, Heiman A, Kempski O. Neuroprotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res 2001;904:245–251. [DOI] [PubMed] [Google Scholar]
- 89. Brennan TJ. AMPA/Kainate receptor antagonists as novel analgesic agents. Anesthesiology 1998;89:1049–1051. [DOI] [PubMed] [Google Scholar]
- 90. Greenamyre JT, Porter HP. Anatomy and physiology of glutamate in the CNS. Neurology 1994;44:S7–S13. [PubMed] [Google Scholar]
- 91. Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B‐containing NMDA receptors: Molecular mechanisms and therapeutic potential. Br J Pharmacol 2009;157:1301–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Herroeder S, Schönherr ME, De Hert SG, Hollmann MW. Magnesium ‐ essentials for anesthesiologists. Anesthesiology 2011;114:971–993. [DOI] [PubMed] [Google Scholar]
- 93. Lysakowski C, Dumont L, Czarnetzki C, Tramèr MR. Magnesium as an adjuvant to postoperative analgesia: A systematic review of randomized trials. Anesth Analg 2007;104:1532–1539. [DOI] [PubMed] [Google Scholar]
- 94. Hollmann MW, Liu HT, Hoenemann CW, Liu WH, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium. Part II: Interactions with volatile anesthetics. Anesth Analg 2001;92:1182–1191. [DOI] [PubMed] [Google Scholar]
- 95. Guo W, Zou S, Guan Y, et al. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci 2002;22:6208–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Piggott LA, Bauman AL, Scott JD, Dessauer CW. The A‐kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci USA 2008;105:13835–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salter MW, Dong Y, Kalia LV, et al. Regulation of NMDA Receptors by Kinases and Phosphatases In: Van Dongen AM, editor. Biology of the NMDA Receptor. Boca Raton, FL: CRC Press, 2009. Chapter 7. Available at: http://www.ncbi.nlm.nih.gov/books/NBK5288/. [PubMed] [Google Scholar]
- 98. Traynelis SF, Cull‐Candy SG. Proton inhibition of N‐methyl‐D‐aspartate receptors in cerebellar neurons. Nature 1990;345:347–350. [DOI] [PubMed] [Google Scholar]
- 99. Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen‐glucose deprivation neuronal injury in cortical cultures. Brain Res 1990;506:339–342. [DOI] [PubMed] [Google Scholar]
- 100. Rachline J, Perin‐Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc‐binding domain on the NMDA receptor subunit NR2B. J Neurosci 2005;25:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Williams K. Ifenprodil, a novel NMDA receptor antagonist: Site and mechanism of action. Curr Drug Targets 2001;2:285–298. [DOI] [PubMed] [Google Scholar]
- 102. Kergozien S, Bansard JY, Delcros JG, Havouis R, Moulinoux JPh. Polyamine deprivation provokes an antalgic effect. Life Sci 1996;58:2209–2215. [DOI] [PubMed] [Google Scholar]
- 103. Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N‐methyl‐D‐aspartate (NMDA) receptors in pain: A review. Anesth Analg 2003;97:1108–1116. [DOI] [PubMed] [Google Scholar]
- 104. Kalia LV, Pitcher GM, Pelkey KA, Salter MW. PSD‐95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. EMBO J 2006;18:4971–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sala C, Sheng M. The fyn art of N‐methyl‐D‐aspartate receptor phosphorylation. Proc Natl Acad Sci USA 1999;96:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Garthwaite J, Charles SL, Chess‐Williams R. Endothelium‐derived relaxing factor release on activationof receptor suggests role as intercellular messenger in the brain. Nature 1988;336:385–388. [DOI] [PubMed] [Google Scholar]
- 107. Nakamura K, Mori K. Nitric oxide and anesthesia. Anesth Analg 1993;77:877–879. [DOI] [PubMed] [Google Scholar]
- 108. Omote K, Kawamata T, Kawamata M, Nakayama Y, Hazama K, Namiki A. Activation of peripheral NMDA‐nitric oxide cascade in formalin test. Anesthesiology 2000;93:173–178. [DOI] [PubMed] [Google Scholar]
- 109. Lipton SA. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open‐channel blockers like memantine in the treatment of acute and chronic neurologic insults. Neuro Rx 2004;1:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arendt‐Nielsen L, Petersen‐Felix S, Fischer M, Bak P, Bjerring P, Zbinden AM. The effect of N‐methyl‐D‐aspartate antagonist (ketamine) on single and repeated nociceptive stimuli: A placebo‐controlled experimental human study. Anesth Analg 1995;81:63–68. [DOI] [PubMed] [Google Scholar]
- 111. Guirimand F, Dupont X, Brasseur L, Chauvin M, Bouhassira D. The effects of ketamine on the temporal summation (wind‐up) of the R(III) nociceptive flexion reflex and pain in humans. Anesth Analg 2000;90:408–414. [DOI] [PubMed] [Google Scholar]
- 112. De Kock M, Lavand'homme P, Waterloos H. « Balanced analgesia » in the perioperative period: Is there a place for ketamine? Pain 2001;92:373–380. [DOI] [PubMed] [Google Scholar]
- 113. Schmid RL, Sandler AN, Katz J. Use and efficacy of low‐dose ketamine in the management of acute postoperative pain: A review of current techniques and outcomes. Pain 1999;82:111–125. [DOI] [PubMed] [Google Scholar]
- 114. Gordh T, Karlsten R, Kristensen J. Intervention with spinal NMDA, adenosine and NO systems for painmodulation. Ann Med 1995;27:229–234. [DOI] [PubMed] [Google Scholar]
- 115. Yamakura T, Shimoji K. Subunit‐ and site‐specific pharmacology of the NMDA receptor channel. Prog Neurobiol 1999;59:279–298. [DOI] [PubMed] [Google Scholar]
- 116. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and tolerance: A current view of their possible interactions. Pain 1995;62:259–274. [DOI] [PubMed] [Google Scholar]
- 117. Mao J. NMDA and opioid receptors: Their interactions in antinociception, tolerance and neuroplasticity. Brain Res Brain Res Rev 1999;30:289–304. [DOI] [PubMed] [Google Scholar]
- 118. Ostermeier AM, Schlösser B, Schwender D, Sutor B. Activation of μ‐ and δ‐opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology 2000;93:1053–1063. [DOI] [PubMed] [Google Scholar]
- 119. Chia YY, Liu K, Wang JJ, Kuo MC, Ho ST. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can J Anaesth 1999;46:872–877. [DOI] [PubMed] [Google Scholar]
- 120. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance; intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000;93:409–417. [DOI] [PubMed] [Google Scholar]
- 121. Cooper DW. Can epidural fentanyl induce a selective spinal hyperalgesia ? Anesthesiology 2000;93:1153. [DOI] [PubMed] [Google Scholar]
- 122. Cortinez LI, Brandes V, Munoz HR, Guerrero ME, Mur M. No clinical evidence of acute opioid tolerance after remifentanil‐based anaesthesia. Br J Anaesth 2001;87:866–869. [DOI] [PubMed] [Google Scholar]
- 123. Eisenach JC. Preemptive hyperalgesia, not analgesia? Anesthesiology 2000;92:308–309. [DOI] [PubMed] [Google Scholar]
- 124. Chen L, Huang LY. Sustained potentiation of NMDA receptor‐mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron 1991;7:319–326. [DOI] [PubMed] [Google Scholar]
- 125. Martin WJ, Malmberg AB, Basbaum AI. PKCgamma contributes to a subset of the NMDA‐dependent spinal circuits that underlie injury‐induced persistent pain. J Neurosci 2001;21:5321–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xiaoping G, Xiaofang Z, Yaguo Z, Juan Z, Junhua W, Zhengliang M. Involvement of the spinal NMDA receptor/PKCγ signaling pathway in the development of bone cancer pain. Brain Res 2010;1335:83–90. [DOI] [PubMed] [Google Scholar]
- 127. Elliott K, Kest B, Man A, Kao B, Inturrisi CE. N‐methyl‐D‐aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology 1995;13:347–356. [DOI] [PubMed] [Google Scholar]
- 128. Shimoyama N, Shimoyama M, Inturrisi CE, Elliott KJ. Ketamine attenuates and reverses morphine tolerance in rodents. Anesthesiology 1996;85:1357–1366. [DOI] [PubMed] [Google Scholar]
- 129. Célèrier E, Rivat C, Jun Y, et al. Long‐lasting hyperalgesia induced by fentanyl in rats: Preventive effects of ketamine. Anesthesiology 2000;92:465–472. [DOI] [PubMed] [Google Scholar]
- 130. Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994;330:613–622. [DOI] [PubMed] [Google Scholar]
- 131. Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989;244:798–800. [DOI] [PubMed] [Google Scholar]
- 132. Sakai F, Amaha K. Midazolam and ketamine inhibit glutamate release via a cloned human brain glutamate transporter. Can J Anaesth 2000;47:800–806. [DOI] [PubMed] [Google Scholar]
- 133. Ichinose T, Yu S, Wang XQ, Yu SP. Ca2+‐independent, but voltage‐ and activity‐dependent regulation of the NMDA receptor outward K+ current in mouse cortical neurons. J Physiol 2003;551(Pt 2):403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wood AM, Bristow DR. N‐methyl‐D‐aspartate receptor desensitisation is neuroprotective by inhibiting glutamate‐induced apoptotic‐like death. J Neurochem 1998;70:677–687. [DOI] [PubMed] [Google Scholar]
- 135. Giroux Ch, Carter Ch, Scatton B. Les antagonistes NMDA: Une nouvelle perspective thérapeutique pour l'infarctus cérébral humain ? Sang Thrombose Vaisseaux 1990;2:257–261. [Google Scholar]
- 136. Hubsher G, Haider M, Okun MS. Amantadine: The journey from fighting flu to treating Parkinson disease. Neurology 2012;78:1096–1099. [DOI] [PubMed] [Google Scholar]
- 137. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: A randomised, double‐blind, placebo‐controlled trial. Lancet Neurol 2010;9:969–977. [DOI] [PubMed] [Google Scholar]
- 138. Wright JJ, Goodnight PD, McEvoy MD. The utility of ketamine for the preoperative management of a patient with Parkinson's disease. Anesth Analg 2009;108:980–982. [DOI] [PubMed] [Google Scholar]
- 139. Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: Mechanism and prevention. Science 1991;254:1515–1518. [DOI] [PubMed] [Google Scholar]
- 140. Vranken JH, Troost D, de Haan P, et al. Severe toxic damage to the rabbit spinal cord after intrathecal administration of preservative‐free S(+)‐ketamine. Anesthesiology 2006;105:813–818. [DOI] [PubMed] [Google Scholar]
- 141. Olney JW, Wozniak DF, Jevtovic‐Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug‐induced apoptotic neurodegeneration in the developing brain. Brain Pathol 2002;12:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ubogu EE, Sagar SM, Lerner AJ, Maddux BN, Suarez JI, Werz MA. Ketamine for refractory status epilepticus: A case of possible ketamine‐induced neurotoxicity. Epilepsy Behav 2003;4:70–75. [DOI] [PubMed] [Google Scholar]
- 143. Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long‐term effects of ketamine: Evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend 2004;75:301–308. [DOI] [PubMed] [Google Scholar]
- 144. Liao Y, Tang J, Ma M, et al. Frontal white matter abnormalities following chronic ketamine use: A diffusion tensor imaging study. Brain 2010;133(Pt 7):2115–2122. [DOI] [PubMed] [Google Scholar]
- 145. Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long‐lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 2011;33:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg 2007;104:509–520. [DOI] [PubMed] [Google Scholar]


