SUMMARY
A multisite, double‐blind, placebo‐controlled trial of bupropion for methamphetamine dependence was reanalyzed using a novel, nonbinary method of evaluating success and failure. The original analysis focused on a group response endpoint (the change in percentage of participants with methamphetamine‐free urines each week over the course of the trial) and no significant bupropion effect was observed in the total population of study participants. In this reanalysis, individual participants were regarded as treatment success if they achieved multiple weeks of abstinence lasting through the end of the study, and their degree of success was quantified by calculating the number of beyond‐threshold weeks of success (NOBWOS). Thus, setting the threshold at 1 week of end‐of‐study abstinence (EOSA), treatment successes were assigned NOBWOS values ranging from 1 to 11, with 1 corresponding to 2 weeks EOSA and 11 corresponding to abstinence throughput the entire 12‐week trial. Treatment failures were assigned a value of 0. Comparison of NOBWOS values revealed a significant effect of bupropion to facilitate abstinence (P= 0.0176). In the bupropion group, 20% of participants achieved 2 or more weeks EOSA, 14% achieved 6 or more weeks EOSA, and 6% were abstinent throughout the trial; this compares with 7%, 4%, and 1% in the placebo group, respectively. On the basis of the NOBWOS analysis, bupropion seems to effectively facilitate the achievement of abstinence in methamphetamine‐dependent individuals.
Keywords: Abstinence, Bupropion, Methamphetamine, Responder‐based analysis
Introduction
Methamphetamine abuse and dependence are associated with well‐established morbidity and mortality risks because of the pharmacological actions of the drug [1, 2]. Moreover, methamphetamine use is linked to an increased risk of HIV transmission through needle sharing and risky sexual behavior [3]. Considering these adverse health consequences as well as methamphetamine‐associated crime, incarceration, and lost productivity, the annual societal cost of methamphetamine abuse has been estimated to exceed $23 billion in the United States [4]. Although methamphetamine abuse in the United States seemed to peak in 2005, abuse of the drug has continued to escalate in other countries, such as the Czech Republic [5]. No medication has either received regulatory approval or shown a clear profile of safety and effectiveness in treating methamphetamine dependence.
Among all medications evaluated in the treatment of methamphetamine dependence, bupropion has shown the most promising results. At the preclinical level, bupropion significantly reduced the self‐administration of methamphetamine in monkeys at a dose that did not affect operant responding for food [6]. In a placebo‐controlled human laboratory study [7], treatment with 150 mg sustained release bupropion two times a day significantly reduced subject‐reported drug craving in response to methamphetamine‐associated cues, and significantly reduced the reported subjective effects of 15–30 mg intravenous methamphetamine. Moreover, from a safety perspective, bupropion did not potentiate the cardiovascular effects of intravenous methamphetamine but rather significantly attenuated the ability of methamphetamine to increase heart rate and produced a nonsignificant trend in the direction of diminished methamphetamine effects on blood pressure [8]. On the basis of these findings, the National Institute on Drug Abuse (NIDA) initiated a five‐site, 151 subjects, double‐blinded, placebo‐controlled trial of bupropion for the treatment of methamphetamine dependence. The initial analysis of the trial [9] compared group responses in terms of the change in percentage of participants with a week of methamphetamine‐free urines over the course of the 12‐week treatment period. This analysis failed to demonstrate a significant effect of bupropion in the total population of study participants.
There are no published U.S. Food and Drug Administration (FDA) guidelines that address medications development for the treatment of methamphetamine dependence; however, responder analyses have been used in recent FDA reviews of New Drug Applications for other substance use disorders (e.g., [10, 11]). The efficacy of varenicline was judged by its ability to facilitate a period of abstinence from smoking that lasted through the end of study, hereafter referred to as “end‐of‐study abstinence” (EOSA). Similarly, the efficacy of depot naltrexone injection to treat alcoholism was judged by its ability to facilitate a similar period of no heavy drinking days. We now describe a new, nonbinary method for conducting success/failure analyses that allow for subject‐to‐subject variability in the onset and duration of EOSA. Using this method to reanalyze the multisite bupropion/methamphetamine trial [9], a significant bupropion effect to facilitate EOSA was revealed.
Methods
Study Design and Participants
The design and conduct of this clinical trial has been previously described in detail [9]. In brief, a total of 151 treatment‐seeking men and women who met DSM‐IV criteria for methamphetamine dependence participated in a two‐arm, double‐blind, placebo‐controlled study that was conducted at five sites (25–33 participants per site). Exclusion criteria were: seizure disorder, any psychiatric disorder that required ongoing medication, pregnancy or lactation, serious medical illness, and court‐mandated drug abuse treatment. Subsequent to screening, participants received 150 mg sustained release bupropion or matching placebo once daily for 3 days, then twice daily (corresponding to 300 mg bupropion per day) for approximately 11 weeks, followed by a reduction to 1 tablet daily during the last 3 days of the 12‐week treatment period. There were 79 participants in the bupropion group and 72 in the placebo group. Throughout the treatment period, all study participants received standardized cognitive behavioral therapy in 90‐minute group sessions three times per week. During their thrice weekly visits, participants provided urine samples that were initially screened by immunoassay with a cutoff level of 300 ng/mL methamphetamine/amphetamine. Samples that were positive by immunoassay were assayed for methamphetamine level by gas chromatography/mass spectrometry. Urine samples were regarded methamphetamine‐free if concentrations were below 300 ng/mL (the lower limit of quantification for the immunoassay).
Data Analysis
In this reanalysis, in order for a week to be regarded as methamphetamine‐free, two or more methamphetamine‐free urine samples (with no methamphetamine‐positive urines) were required. Study participants were judged to be treatment successess if they achieved 2 or more methamphetamine‐free weeks at the end of the 12‐week study (2 or more weeks of EOSA). They were judged to be treatment failures if they provided one or more methamphetamine‐positive urine samples or if they did not provide at least two urine samples per week during the last 2 weeks of treatment. Thus, 1 week of EOSA was not regarded as a success but rather the threshold level of abstinence that had to be exceeded. For treatment success, the degree of success was calculated by determining the extent to which EOSA exceeded the 1‐week threshold; these calculations resulted in a range of values for the “number of beyond‐threshold weeks of success” (NOBWOS). For example, a study participant who achieved 8 weeks of EOSA surpassed the 1‐week threshold by 7 weeks, resulting in a NOBWOS value of 7. Similarly, a participant who achieved the minimum level of success, 2 weeks of EOSA, was assigned a NOBWOS value of 1. Treatment failures did not exceed the threshold and received a NOBWOS value of 0. The ability of bupropion to facilitate EOSA was then evaluated by comparing NOBWOS values in the bupropion and placebo groups using a two‐sided Van der Waerden two‐sample test [12]. In addition, possible associations between baseline characteristics (race, gender, etc.) and treatment outcome (success or failure to achieve at least 2 weeks of EOSA) in the bupropion group were evaluated using a two‐sample t‐test for continuous variables and either a chi‐square or Fisher's exact test, as appropriate, for categorical variables. With regard to missing data, it follows from the above that two or more missing urine samples during any given week was assumed to reflect methamphetamine use. Also, study participants who dropped out of the study before week 12 were regarded as treatment failures.
Results
The percentage of participants who were in treatment and abstinent from methamphetamine until the end of the 12‐week study are show in Figure 1 as a function of study week. In the bupropion group (filled circles) 5 of 79 study participants (6%) successfully initiated EOSA during week 1 (these individuals provided two or more urine samples each week for 12 weeks, with no methamphetamine‐positive results). In comparison, just 1 of 72 study participants (1.4%) in the placebo group (open circles) initiated EOSA during week 1. Throughout the course of the study, the success rate in the bupropion group seemed to increase in a biphasic fashion, with a plateau at 11% (9/79) from study weeks 4–6, which then increased steadily to 20% (16/79). In the placebo group, only 7% (5/72) were able to achieve 2 or more weeks of EOSA.
Figure 1.
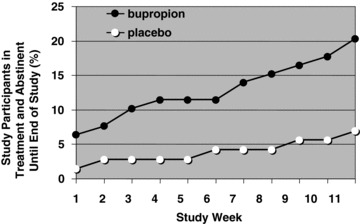
Effect of bupropion (150 mg twice daily) or placebo on the percentage of study participants who were in treatment and abstinence from methamphetamine through the end of the 12‐week study. In order for a week to be regarded as methamphetamine‐free, two or more methamphetamine‐free urine samples, with no methamphetamine‐positive samples, were required. Urine samples were regarded as methamphetamine‐free if they were below 300 ng/mL. This figure represents a reanalysis of data previously reported by Elkashef et al. [9]. Study participants (79 methamphetamine‐dependent individuals in the bupropion group and 72 in the placebo group) attended cognitive behavioral therapy counseling sessions and provided urine samples up to 3 times per week.
Study participants were required to surpass a threshold of 1 week EOSA to be regarded as a treatment success, and the extent of each participant's success was quantified by calculating the NOBWOS. The data are shown in Table 1. Because of the large number of treatment failures, 67 of 72 study participants in the placebo group and 63 of 79 in the bupropion group received NOBWOS values of 0. These values are not normally distributed, invalidating the use of a parametric statistical test to compare the bupropion and placebo groups. Among nonparametric tests, it has been suggested that the Van der Waerden two‐sample test is most sensitive for comparing data sets with highly skewed distributions [12]. Application of this test to the NOBWOS values in Table 1 revealed a significant effect of bupropion to facilitate abstinence (P= 0.0176). Thus, the current method of data analysis revealed a significant effect of bupropion in the entire population of study participants.
Table 1.
NOBWOS analysis of data represented in Figure 1, with study participants required to exceed a threshold of 1‐week end‐of‐study abstinence to qualify as a treatment success
| End‐of‐study abstinence (weeks) | NOBWOS | Breakdown of groups | |
|---|---|---|---|
| Placebo group | Bupropion groupa | ||
| 0‐1 | 0 | 67 | 63 |
| 2 | 1 | 1 | 2 |
| 3 | 2 | 0 | 1 |
| 4 | 3 | 1 | 1 |
| 5 | 4 | 0 | 1 |
| 6 | 5 | 0 | 2 |
| 7 | 6 | 1 | 0 |
| 8 | 7 | 0 | 0 |
| 9 | 8 | 0 | 1 |
| 10 | 9 | 0 | 2 |
| 11 | 10 | 1 | 1 |
| 12 | 11 | 1 | 5 |
aSignificantly different from placebo, P= 0.0176, two‐sided Van der Waerden two‐sample test.
NOBWOS, Number of beyond‐threshold weeks of success.
The baseline characteristics of study participants in the bupropion group who succeeded in achieving at least 2 weeks of EOSA are shown in Table 2. The only factor that was significantly associated with a successful outcome with bupropion treatment was the self‐reported level of methamphetamine use during the 30 days immediately before screening; the proportion of treatment successes reporting 18 days or less of baseline methamphetamine use (69%) was significantly greater than the proportion of treatment failures reporting this level of baseline use (40%; P= 0.04). The relationship between treatment success and level of methamphetamine use was further evaluated in both the bupropion and placebo groups, with the heavy baseline users subdivided into those who reported 19–29 of 30 days use and those who reported daily (30 of 30 days) use before screening (Figure 2). In the bupropion group, 31% (11/36) of those reporting 18 days or less of methamphetamine use, 15% (4/27) of those reporting 19–29 days of use, and only 6% (1/16) of those reporting daily use during the 30‐day baseline period were successful in achieving 2 or more weeks of EOSA, respectively. In contrast, success rates in the placebo group were low and showed no apparent relationship to baseline levels of methamphetamine use. In the placebo arm, 6% (2/35) of those reporting 18 days or less of baseline use were successful in achieving 2 or more weeks of EOSA compared to 6% (1/18) of those reporting 19–29 days of baseline use, and 11% (2/19) of those reporting daily use during the 30‐day baseline period.
Table 2.
Comparative baseline characteristics of treatment successes and failures in the bupropion group
| Treatment successes | Treatment failures | P value | |||
|---|---|---|---|---|---|
| Age | 36.1 | (9.9)a | 36.3 | (9.1)a | 0.96 |
| Gender | 0.61 | ||||
| Male | 11 | (69%) | 39 | (62%) | |
| Female | 5 | (31%) | 24 | (38%) | |
| Race | 0.62 | ||||
| White, not Hispanic | 10 | (63%) | 49 | (78%) | |
| Hispanic or Latino | 2 | (13%) | 3 | (5%) | |
| African American or Black | 1 | (6%) | 1 | (2%) | |
| Asian or Pacific Islander | 3 | (19%) | 8 | (13%) | |
| American Indian or Alaskan | 0 | (0%) | 1 | (2%) | |
| Other | 0 | (0%) | 1 | (2%) | |
| Self‐reported days of methamphetamine use | 0.04 | ||||
| 1‐18 of 30 days before screening | 11 | (69%) | 25 | (40%) | |
| > 18 of 30 days before screening | 5 | (31%) | 38 | (60%) | |
| Lifetime years of methamphetamine use | 9.81 | (6.42)a | 10.57 | (7.89)a | 0.72 |
| Route of lifetime methamphetamine use | 0.49 | ||||
| Nasal | 2 | (13%) | 13 | (21%) | |
| Smoking | 12 | (75%) | 37 | (59%) | |
| Injection | 2 | (13%) | 13 | (21%) | |
| Depression (HAM‐D Total > 12) | 0.98 | ||||
| No | 13 | (81%) | 51 | (81%) | |
| Yes | 3 | (19%) | 12 | (19%) | |
| Current Alcohol Dependence | 0.5 | ||||
| No | 15 | (94%) | 61 | (97%) | |
| Yes | 1 | (6%) | 2 | (3%) | |
| Adult ADD | 0.19 | ||||
| No | 16 | (100%) | 57 | (90%) | |
| Yes | 0 | (0%) | 6 | (10%) | |
aStandard deviation.
HAM‐D, Hamilton rating scale for depression; ADD, Attention deficit disorder.
All “treatment successes” achieved 2 or more weeks of end‐of‐study abstinence.
Figure 2.
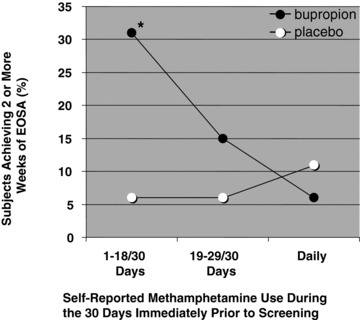
Success rates for achieving 2 or more weeks of EOSA as a function of self‐reported level of baseline methamphetamine use (n = 36, 27, and 15 in the bupropion group, and 35, 18, and 19 in the placebo group for study participants reporting 1–18, 19–29, and 30 days of baseline use, respectively; *P= 0.012 compared with placebo, two‐tailed Fisher's exact test).
Discussion
On the basis of the analysis described in this article, it seems that bupropion is a highly effective medication for the treatment of methamphetamine dependence. Any posthoc analysis raises the issue of a potential “fishing expedition.” Thus, it is important to note that the focus on success or failure of individuals to achieve EOSA was prompted by FDA evaluations of medications to treat tobacco and alcohol dependence [10, 11]. No other endpoints were considered. The development of a data analysis method that considers different levels of success (different durations of EOSA) as an alternative to a dichotomous success/failure analysis was stimulated by the gradual slope/biphasic nature of the line for the bupropion group in Figure 1. Given that the NOBWOS method was designed with the present data in mind, it is important to note that more conventional, dichotomous success/failure analyses also show a significant treatment effect for bupropion. When 2, 3, 4, 5, or 6 weeks of EOSA are used to define success, bupropion has a significant effect in all cases, with respective P values of 0.0199, 0.0245, 0.0407, 0.0293, or 0.0498 (individual applications of a two‐sided Fisher's exact test). None of these fixed‐period EOSA comparisons places an appropriate value on the longer periods of abstinence exhibited by some study participants. Thus, the P value (0.0176) resulting from the NOBWOS analysis is lower than any of the above P values, indicating that the method can be more powerful than a simple, dichotomous success/failure analysis. This finding is consistent with previous observations of reduced power in association with dichotomization [13, 14, 15].
The efficacy of bupropion may best be appreciated through the calculation of an odds ratio, and comparison with results from published smoking cessation trials. A recent meta‐analysis of randomized, placebo‐controlled smoking cessation trials [16] found odds ratios of 2.55 for varenicline, 2.37 for nicotine nasal spray, 2.18 for nicotine inhaler, 2.12 for bupropion, 1.88 for nicotine patch, and 1.65 for nicotine gum. From Table 1, it can be calculated that the odds of achieving 2 or more weeks of EOSA in the bupropion and placebo groups were 25.4 and 7.46%, respectively, yielding an odds ratio of 4.22. Similarly positive findings in a second multisite trial and evaluation of the safety of bupropion in the target population will likely be required for regulatory approval. Correspondingly, NIDA has initiated a second multisite, placebo‐controlled, double‐blind trail of bupropion for methamphetamine dependence, with completion targeted for late 2011.
In this reanalysis, the only striking determinant of success or failure within the bupropion group was the reported baseline level of methamphetamine use (Table 2). Compared to placebo, there was no trend toward increased success with bupropion treatment among those who reported daily methamphetamine use during the 30 days immediately before screening (Figure 2). The effect of bupropion was greatest in those who reported 18 days or less of baseline methamphetamine use (the odds ratio for bupropion vs. placebo in this subgroup of study participants was 7.26; P= 0.012) and there was a nonsignificant trend toward increased success among users who reported 19–29 days of baseline methamphetamine use (odds ratio 2.96; P= 0.63). One possible interpretation of these data is that patients who are highly dependent on methamphetamine may be less responsive to the pharmacological actions of bupropion. Alternatively, study participants abusing methamphetamine on a daily basis may have been below average in their medication adherence. This study was designed to rely on pill count and self‐report to estimate medication adherence; however, there were so many cases in which study participants either lost medication cards or forgot to bring them to appointments that compliance could not be estimated from pill counts. Self‐reported medication adherence has been shown to be extremely inaccurate [17]. Thus, we can draw no conclusions about the possible contribution of medication adherence (or lack thereof) to the low success rate observed in heavy baseline users. In the ongoing multisite trial, plasma levels of bupropion are being measured at weeks 4 and 12 to provide snapshots of medication adherence. If failure to initiate methamphetamine abstinence is found to be associated with poor medication adherence in study participants reporting heavy baseline use, then longer acting products and/or the addition of behavioral approaches to promote medication adherence could be considered.
The NOBWOS method of analysis may be especially appropriate for use in Phase 2 trials when the onset of a meaningful therapeutic action has not previously been characterized, and application of the method to other types of clinical trials where a delayed response is anticipated (e.g., antidepressant and anxiolytic studies) may be useful for determining if an agent has a rapid onset of action. For this reanalysis, the threshold for success was selected after an evaluation of baseline methamphetamine use. During the 2 weeks immediately before randomization, 94% of study participants (142/151) provided one or more urine samples that tested positive for methamphetamine. Thus, for all but 6% of study participants, achieving 2 or more weeks EOSA would constitute improvement. In contrast, only 85% of study participants (129/151) tested positive for methamphetamine during the week that immediately preceded randomization. To avoid the possibility of an unacceptably high success rate in the placebo group, 2 weeks EOSA was set as the minimum criterion for success. Beyond such practical considerations, it is desirable that success be defined in a way that reflects clinically meaningful improvement. It can be argued that any statistically significant increase in abstinence from methamphetamine use constitutes clinically meaningful improvement. This follows from the fact that, in addition to the well‐established morbidity and mortality risks associated with methamphetamine use, each dose of illicit methamphetamine carries with it the unknown health risks of contaminants, as well as the risk of incarceration. Thus, if the therapeutic effect of bupropion evinced in this reanalysis is confirmed in the second multisite trial, then bupropion may become an important component of therapy for methamphetamine dependence. The ongoing trial may also play an important role in the long‐term assessment of the NOBWOS method. Considering the posthoc nature of the current analysis, such prospective applications of the method will be essential to its validation.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The authors wish to thank both Dr. Daniel Falk (NIAAA) and Dr. Phil Skolnick (NIDA) for useful discussions during the preparation of this manuscript.
References
- 1. Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health 2010;31:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montoya ID, McCann DJ. Drugs of abuse: Management of intoxication and antidotes In: Luch A, editor. Molecular, clinical and environmental toxicology: Clinical toxicology, Vol. 2. Basel : Birkhäuser Verlag AG, 2009;519–541. [Google Scholar]
- 3. Shoptaw S, Reback CJ. Methamphetamine use and infectious disease‐related behaviors in men who have sex with men: Implications for interventions. Addiction 2007; 102:130–135. [DOI] [PubMed] [Google Scholar]
- 4. Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The economic costs of methamphetamine use in the United States, 2005. RAND Corporation [Internet]. 2009; MG‐829‐MPF/NIDA. Available from: http://www.rand.org/pubs/monographs/MG829/ [Accessed 30 January 2011.
- 5. United Nations Office on Drugs and Crime . World Drug Report 2009 [Internet]. 2009; http://www.unodc.org/unodc/en/data‐and‐analysis/WDR‐2009.html [Accessed 30 January 2011.
- 6. Schindler CW, Gilman JP, Panlilio LV, McCann DJ, Goldberg SR. Comparison of the effects of methamphetamine, bupropion, and methylphenidate on the self‐administration of methamphetamine by rhesus monkeys. Exp Clin Psychopharmacol 2011;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newton TF, Roache JD, De La Garza R 2nd, et al Bupropion reduces methamphetamine‐induced subjective effects and cue‐induced craving. Neuropsychopharmacology 2006;31:1537–1544. [DOI] [PubMed] [Google Scholar]
- 8. Newton TF, Roache JD, De La Garza R 2nd, et al Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology 2005; 182:426–435. [DOI] [PubMed] [Google Scholar]
- 9. Elkashef AM, Rawson RA, Anderson AL, et al Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 2008;33:1162–1170. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration , Center for Drug Evaluation and Research. Medical Review of Varenicline: 21–928 [Internet]. 2006; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021928_s000_ChantixTOC.cfm [Accessed 30 January 2011.
- 11. U.S. Food and Drug Administration , Center for Drug Evaluation and Research. Medical Review of Vivitrol: 21–897 [Internet]. 2006; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021897_toc_Vivitrol.cfm [Accessed 30 January 2011.
- 12. Permutt T, Berger VW. A new look at rank tests in ordered 2xk contingency tables. Commun Stat A-Theor 2000; 29:989–1033. [Google Scholar]
- 13. Deyi BA, Kosinski AS, Snapinn SM. Power considerations when a continuous outcome variable is dichotomized. J Biopharm Stat 1998;8:337–352. [DOI] [PubMed] [Google Scholar]
- 14. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snapinn SM, Jiang Q. Responder analyses and the assessment of a clinically relevant treatment effect. Trials 2007;8:31‐1–31‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenberg MJ, Filion KB, Yavin D, et al Pharmacotherapies for smoking cessation: A meta‐analysis of randomized controlled trials. CMAJ 2008;179:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Das M, Santos D, Matheson T, et al Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high‐risk men who have sex with men. AIDS 2010; 24:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]


