SUMMARY
Dabigatran etexilate is emerging as an alternative for vitamin K antagonists, but evidence‐based guidelines for management of intracerebral hemorrhage and acute ischemic stroke in patients taking this drug are nonexistent. This review summarizes current knowledge on key pharmacological features and the assessment of dabigatran activity. Pragmatic approaches are provided for individualized decision taking with regard to hemostatic therapy and reperfusion strategies in acute stroke patients.
Keywords: Acute stroke, Dabigatran etexilate, Hemostasis, Reperfusion
Introduction
Dabigatran is a small molecule that directly inhibits both free and clot‐bound thrombin, independently of endogenous antithrombin. It is the active moiety of the orally bioavailable prodrug, dabigatran etexilate (Pradaxa®, Boehringer Ingelheim, Ingelheim am Rhein, Germany) [1]. Based on positive results in pivotal trials, it has been approved for prophylaxis of venous thromboembolism in elective hip and knee surgery (150 or 220 mg od) [2, 3, 4, 5] and for the prevention of stroke and systemic embolism in patients with atrial fibrillation (110 or 150 mg bid) [6]. It has been shown that dabigatran 150 mg bid is as effective as warfarin for treatment of acute venous thromboembolism [7], and several clinical trials in other conditions are ongoing [8]. Given its favorable profile, it is to be expected that dabigatran will become a part of routine practice as an alternative for vitamin K antagonists. Despite the good efficacy/safety balance, clinicians will be confronted with patients under treatment with dabigatran presenting with intracerebral hemorrhage or acute ischemic stroke.
This article describes a practical approach for rapid decision taking with regard to acute stroke management in patients taking dabigatran. As clinical evidence and internationally accepted guidelines with regard to this situation currently are nonexistent, the options discussed in this article are based on the best available facts, which may involve off‐label use of diagnostics and therapeutics.
Key Pharmacological Features of Dabigatran
Dabigatran's anticoagulant effect is directly dependent on its plasma concentration and starts immediately after oral intake, with peak plasma concentrations and maximal effects within 2–3 h. The bioavailability after oral administration of dabigatran etexilate is 7.2%[1]. After multiple dose administrations in healthy men, the estimated half‐life is 14–17 h and steady‐state conditions are reached within 3 days [9, 10, 11]. The drug is predominantly excreted unchanged via the kidneys (∼80%) whereas the remainder is excreted via the bile [1]. Patients with poor renal function may have prolonged excretion rates and elevated plasma concentrations of dabigatran [12]. There is no interference with cytochrome P450 drug‐metabolizing enzymes. Dabigatran displays low (35%) plasma protein binding, implying that displacement interactions are unlikely to affect its pharmacokinetics and pharmacodynamics [1].
The bleeding risk is increased with concomitant use of P‐glycoprotein inhibitors (quinidine, systemic ketoconazole, verapamil, and amiodarone), antiplatelet drugs and nonsteroidal antiinflammatory drugs (NSAIDs). Ciclosporine, itraconazol, and tacrolimus are powerful P‐glycoprotein inhibitors that based on in vitro data may carry the same risk as ketoconazole. P‐glycoprotein inducers (rifampicin, carbamazepin, phenytoin, and St. John's Wort) may result in decreased plasma levels of dabigatran [13].
Assessment of Dabigatran Activity
In patients with acute stroke, urgent evaluation of dabigatran plasma concentration is essential for guiding the management strategy. Currently, neither plasma levels of dabigatran nor its effect on hemostasis can rapidly and reliably be measured in routine clinical practice. The assessment of dabigatran activity relies on the estimation of residual activity based on both pharmacological data and hemostatic tests.
Estimation of Residual Anticoagulant Activity Based on Pharmacological Data
When the time since the last drug intake is known, the residual anticoagulant effect can be estimated based on the half‐life and bioavailability of the drug. The half‐life is about 13, 15, 18, and 27 h in volunteers with creatinine clearance >80, 50–80, 30–50, and <30 mL/min, respectively [12]. Other important determinants of the bleeding risk include advanced age, comorbidities, and concomitant medications (P‐glycoprotein inhibitors or inducers, antiplatelet drugs, NSAIDs, and anticoagulants) [13].
Laboratory Analyses of Hemostasis
At clinically relevant plasma concentrations, the prothrombin time (PT) and its derived international normalized ratio (INR) are only little and variably affected by dabigatran and therefore are of no use for evaluating its anticoagulant effect [9].
Prolongation of the activated partial thromboplastin time (aPTT) occurs with increasing dabigatran plasma concentration, but is less sensitive at supratherapeutic levels [9, 14]. Thus, aPTT measurement may provide a qualitative indication of dabigatran's anticoagulant activity, but is not suitable for precise quantification [15, 16].
The thrombin time (TT) displays a linear dose–response curve for therapeutic concentrations of dabigatran. TT tests are widely available in hospital laboratories, but cut‐off limits cannot be defined because of the wide variation in methodology. The TT is a sensitive method for determining if any dabigatran is present [14].
Clinical studies on the activated clotting time (ACT) in patients taking dabigatran have not yet been undertaken. In vitro studies showed a concentration‐dependent increase in ACT which was linear with dabigatran concentrations up to 250 ng/mL but flattened at higher concentrations (≥500 ng/mL), limiting the utility of this assay [14].
Clinical experience supports the ecarin clotting time (ECT) as a sensitive test for measuring anticoagulant activity with superiority over the aPTT [17], but this assay is not generally available in hospital laboratories and commercial kits have not been validated for dabigatran [17]. Therefore, ECT cannot yet be recommended for this indication.
The Hemoclot® Thrombin Inhibitor assay (Hyphen BioMed, Neuville‐sur‐Oise, France) is a sensitized TT that allows quantitative measurement of direct thrombin inhibitor activity in plasma, thanks to a direct linear relationship between dabigatran concentration and the clotting time [14].
Taken together, the aPTT and TT are the most accessible qualitative methods for determining the presence or absence of anticoagulant effect in dabigatran‐treated patients. If available, the Hemoclot® Thrombin Inhibitor assay can be used for quantitative assessments.
Acute Intracerebral Hemorrhage in Patients Taking Dabigatran
In the RE‐LY trial, the annual incidence of life‐threatening hemorrhage was 1.2 and 1.5% for patients treated with 110 and 150 mg dabigatran, respectively, but information on how the bleedings were managed is not available [6]. In contrast to heparins and vitamin K antagonists, a specific antidote for direct thrombin inhibitors is not yet available [18]. The management of patients with intracerebral hemorrhage while taking dabigatran should be individualized based on the extent of dabigatran‐related coagulopathy, stroke severity, and the availability of medical and surgical treatments (Figure 1).
Figure 1.
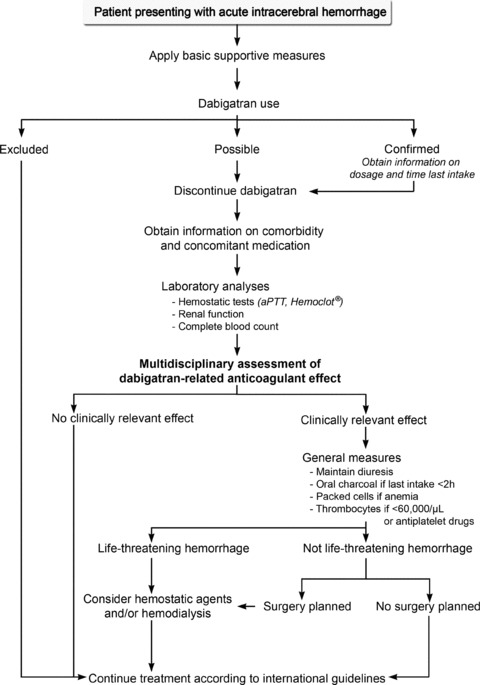
A pragmatic approach for management of patients with intracerebral hemorrhage while taking dabigatran. aPTT, activated partial thromboplastin time; Hemoclot, Hemoclot® Thrombin Inhibitor assay.
Assessment of Dabigatran‐Related Coagulopathy
Patients or their relatives should be questioned with regard to recent anticoagulant use. Detailed information on dabigatran dosage, time since last intake, relevant comorbidity (especially renal dysfunction), and concomitant medication (P‐glycoprotein inhibitors or inducers, antiplatelet drugs, and NSAIDs) should be obtained. A routine blood analysis including complete blood count, creatinine clearance, and aPTT should urgently be performed. The Hemoclot® Thrombin Inhibitor assay can be used for more reliable quantitative assessments, if available [14].
Based on the clinical information and the blood analysis, a multidisciplinary team consisting of an experienced stroke specialist, a neurosurgeon, and a coagulation expert should decide on the presence of a clinically relevant dabigatran‐related coagulopathy.
General Measures
Basic supportive measures should be applied according to internationally accepted guidelines [19, 20]. Dabigatran administration should be discontinued and adequate diuresis should be maintained. Preliminary in vitro data indicate that dabigatran etexilate can be successfully adsorbed by standard activated charcoal therapy [14]. It may be advised to orally administer charcoal (1g/kg body weight) if the time to last intake of dabigatran does not exceed 2 h. Because of lack of clinical data on removal of dabigatran from plasma via hemoperfusion over a charcoal filter, this technique cannot be recommended currently. In case of clinically relevant anemia, transfusion of packed red cells may be indicated. Thrombocyte transfusion may be considered for patients with thrombocyte count <60,000 per μL or the combined use of dabigatran and an antiplatelet drugs [16, 21]. In accordance with international guidelines, surgical intervention may be an option, preferably after normalization of the coagulation [19, 20].
Hemostatic Agents for Reversal of Dabigatran‐Related Coagulopathy
The use of hemostatic therapies in dabigatran‐related bleeding is merely based on preclinical data and case reports [22]. Hemostatic agents are not registered for this indication and have thrombogenic potential [23]. They should only be used in patients with life‐threatening hemorrhages after thorough risk–benefit evaluation [14].
Recombinant activated factor VII at a dose of 80 μg/kg (Novo‐ Seven®, Novo Nordisk, Bagsvaerd, Denmark) reduces hematoma growth in patients with acute intracerebral hemorrhage, however, without improvement of clinical outcome and at a small increased risk of thromboembolic events [24, 25]. Preclinical data suggest that recombinant activated factor VII may antagonize the anticoagulant effect of dabigatran [26].
There are several types of prothrombin complex concentrates, but data with regard to dabigatran are only available for Cofact® (Sanquin, Amsterdam, The Netherlands), Confidex® (CSL Behring, Marburg, Germany), and Feiba VH® (Baxter, Vienna, Austria). Administration of a single bolus of Cofact® 50 IU/kg in six healthy volunteers taking dabigatran 150 mg bid did not reverse its anticoagulant effect, as measured by aPTT, ECT, and TT [27]. Confidex® showed reduced bleeding with high dose dabigatran in a rabbit model [18]. Feiba VH® reduced bleeding due to high dose dabigatran in a rat model and in vitro studies using human plasma showed complete reversal of the dabigatran‐inhibited endogenous thrombin potential [14]. There is neither expected benefit nor experience with the use of protamine sulfate, vitamin K, desmopressin, aprotinin, tranexamic acid, and aminocaproic acid in patients receiving dabigatran. There is no presumptive evidence that fresh frozen plasma can reverse the anticoagulant effect of dabigatran and the risk of complications (transfusion‐related acute lung injury, circulatory overload, and transmission of infectious diseases) further argue against its use [28].
Hemodialysis
Dabigatran is characterized by low protein binding and is dialyzable. Results from an open‐label study in patients with end‐stage renal failure on maintenance hemodialysis show a decline of systemic dabigatran concentrations by 60–69% in a 4 h dialysis session [12]. The clinical utility of this approach is unknown, but it can be an option in life‐threatening situations, especially in patients with renal dysfunction.
Restart of Dabigatran
Based on the results of a decision analysis, it is advised that survivors of intracerebral hemorrhage with atrial fibrillation should not be offered long‐term anticoagulation with vitamin K‐antagonists, except those at a particularly high risk for thromboembolic stroke or low risk of hemorrhage recurrence [29]. In the absence of clinical data, it seems acceptable to advise against restart of dabigatran after intracerebral hemorrhage.
Management of Acute Infarction in Patients Taking Dabigatran
Despite treatment with dabigatran, the annual risk of ischemic stroke remains about 1% in patients with atrial fibrillation [6]. Care of patients presenting with acute ischemic stroke while taking dabigatran should be based on an individualized risk–benefit analysis, taking the bleeding risk and the stroke severity into account (Figure 2).
Figure 2.
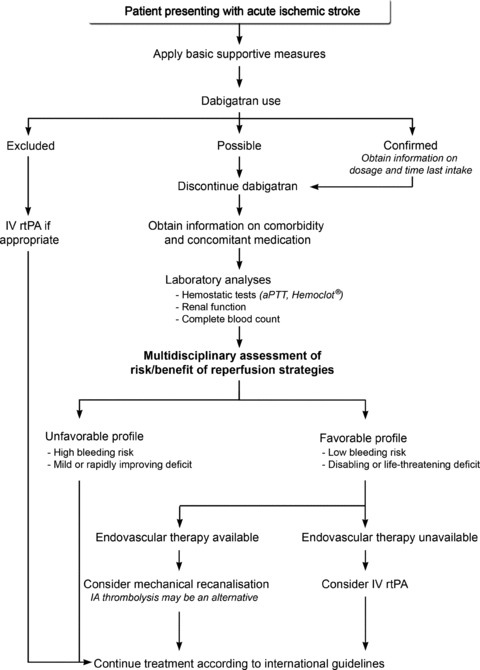
A pragmatic approach for management of patients with acute ischemic stroke while taking dabigatran. aPTT, activated partial thromboplastin time; Hemoclot, Hemoclot® Thrombin Inhibitor assay; IA; intraarterial; IV rtPA, intravenous recombinant tissue plasminogen activator.
Assessment of Dabigatran‐Related Coagulopathy
The presence of a clinically relevant coagulopathy should be evaluated by a multidisciplinary team, based on available pharmacological information (dosage, time since last intake, renal function, and concomitant medication) and coagulation tests (aPTT, Hemoclot® Thrombin Inhibitor assay).
General Measures
Concordant with international guidelines, patients should be hospitalized in stroke units providing care through standardized orders. Special attention should be given to rapid detection and correction of hypoxia, hypoglycemia, fever, cardiac arrhythmias, severe arterial hypotension or hypertension. Decompressive craniectomy for space occupying hemispheric or cerebellar ischemic strokes may be considered after normalization of the coagulation [30, 31].
Reversal of Dabigatran's Anticoagulant Effect
In case of an acute thromboembolic event, it could be an option to first administer hemostatic agents for reversal of the coagulopathy before initiating thrombolytic therapy. However, the prothrombotic risks of these agents are likely to outweigh the potential benefits in patients (taking dabigatran) with acute ischemic stroke [32], and their use may therefore not be advised for this indication until more clinical data become available. Relevant declines in dabigatran concentration have been obtained by hemodialysis [12], but this technique is time consuming and will prevent initiation of thrombolytic therapy within the appropriate time window in most patients.
Reperfusion Strategies
The clinical experience with reperfusion therapy in patients taking dabigatran is limited to intravenous administration of recombinant tissue plasminogen activator (IV rtPA) in three cases—two with good outcome [33, 34] and one patient suffering from fatal intracerebral hemorrhage [35]. IV rtPA is the only specific treatment that has been approved for acute ischemic stroke with class I recommendation and evidence level A [36]. As patients receiving therapeutic doses of anticoagulants before stroke onset were excluded from fibrinolytic therapy in all randomized clinical trials, little is known about the bleeding risk and efficacy of this combination [37]. Conflicting results can be found with regard to the safety of IV rtPA in patients taking vitamin K antagonists [38, 39], but it is generally accepted that this therapy is contraindicated in those with INR ≥ 1.7 because of a supposedly higher risk of hemorrhage [36]. In vitro studies indicate that dabigatran enhances the effect of thrombolytics [40], and the combined treatment of the direct thrombin inhibitor argatroban and rtPA resulted in higher recanalization rates at the expense of a moderately increased bleeding risk [41].
According to international guidelines, patients without relevant coagulopathy should receive IV rtPA if the criteria are met [30, 31, 32], but in case of dabigatran‐related coagulopathy, stroke severity and the expected outcome of spontaneous evolution should be weighed against the risks and benefits of reperfusion therapies. Comorbidity (e.g., renal dysfunction) or concomitant medication associated with increased bleeding diathesis (P‐glycoprotein inhibitors, antiplatelet drugs, NSAIDs, and anticoagulants) are reasons to refrain from thrombolysis. In patients with rapidly improving stroke or mild deficits, the suspected risk of fibrinolytic therapy may exceed the clinical benefit [42]. The bleeding risk may even be higher in severe stroke, but so is the probability of unfavorable spontaneous evolution [43]. Multimodal computed tomography or magnetic resonance imaging can be useful for confirmation of acute cerebral ischemia, identification of predictors of intracranial hemorrhage, and selection of patients suffering from large artery occlusion [44].
Endovascular treatment by intra‐arterial thrombolysis, thrombectomy, thromboaspiration, or thrombus disruption can be used in patients who are ineligible for IV rtPA [30, 31, 45]. Mechanical recanalization could be the treatment of choice in patients with coagulopathy because these techniques do not interfere with the coagulation cascade. It may also be reasonable to consider intraarterial thrombolysis, because this is associated with a lower risk of symptomatic intracerebral hemorrhage than IV rtPA [46].
Although there is no proof that reperfusion procedures are safe in patients taking dabigatran, IV rtPA may be an option in disabling or life‐threatening stroke if endovascular approaches are unavailable [33, 34]. Adjunctive use of anticoagulants or antiplatelet drugs should be avoided and patients should be monitored closely [16]. Retrospective study of the three cases treated with IV rtPA while taking dabigatran underlines the need for a multidisciplinary assessment [33, 34, 35]. Although good outcome was obtained in two patients with a favorable risk/benefit profile, the patient with the fatal intracerebral hemorrhage indeed exhibited a high bleeding risk: initiation of IV rtPA at 6 h after last dose of dabigatran (therapeutic plasma concentrations must have been present) and pretreatment with enoxaparin. On the other hand, secondary intracerebral hemorrhage might have occurred in this patient even without rtPA or dabigatran, because of hyperglycemia and extensive cerebral infarction [35, 47].
Restart of Dabigatran
In patients suffering from ischemic stroke despite treatment with dabigatran, the risk of recurrent cardio‐embolism has to be balanced against the risk of hemorrhagic transformation of the infarction. Until data on the latter become available for dabigatran, it may be advisable to withhold anticoagulants for 10–14 days in major stroke and low risk of stroke recurrence, as is common practice for patients treated with vitamin K antagonists [16, 48]. Data with regard to the choice of anticoagulants (vitamin K antagonists, direct thrombin inhibitors, or factor Xa inhibitors) in patients suffering from ischemic stroke despite taking dabigatran are not available. Differences in therapeutic efficacy and safety between direct thrombin inhibitors and factor Xa inhibitors are matter of on‐going debate. For this reason, we would not routinely change the anticoagulant regime but rather restart dabigatran.
Conclusion
Alternatives for vitamin K antagonists are emerging under the form of direct thrombin inhibitors (dabigatran etexilate) and direct factor Xa inhibitors [49, 50, 51]. It is to be expected that clinicians will increasingly be faced with intracerebral hemorrhage and acute ischemic stroke in patients taking these new anticoagulants. Pragmatic approaches for tackling these situations are needed until evidence‐based guidelines become available. Individualized decisions should rapidly be obtained by multidisciplinary teams evaluating the extent of the coagulopathy and the risks and benefits of various therapeutic options.
Several actions can be taken to improve acute stroke care in patients taking dabigatran. First of all, we should engage in a registry of clinical data, therapeutic interventions, and outcome of patients suffering from stroke under treatment with dabigatran. Second, assays for rapid qualitative evaluation of dabigatran plasma levels should be available in any routine laboratory, and standardized quantitative measurements should be provided by laboratories with experience in hemostasis. Third, research on antagonists for dabigatran is to be encouraged. Fourth, safety data are needed with regard to thrombolysis for acute ischemic stroke.
Disclosures
Kristin Jochmans is member of the Pradaxa Advisory Board for Boehringer Ingelheim Belgium.
Conflicts of Interest
The authors declare no conflict of interest.
The first two authors contributed equally to this work.
References
- 1. Blech S, Ebner T, Ludwig‐Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008;36:386–399. [DOI] [PubMed] [Google Scholar]
- 2. Eriksson BI, Dahl OE, Rosencher N, et al Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double‐blind, non‐inferiority trial. Lancet 2007;370:949–956. [DOI] [PubMed] [Google Scholar]
- 3. Ginsberg JS, Davidson BL, Comp PC, et al Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Eriksson BI, Dahl OE, Rosencher N, et al Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE‐MODEL randomized trial. J Thromb Haemost 2007;5:2178–2185. [DOI] [PubMed] [Google Scholar]
- 5. Friedman RJ, Dahl OE, Rosencher N, et al Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: A pooled analysis of three trials. Thromb Res 2010;126:175–182. [DOI] [PubMed] [Google Scholar]
- 6. Connolly SJ, Ezekowitz MD, Yusuf S, et al Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Kearon C, Kakkar AK, et al Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342‐2352. [DOI] [PubMed] [Google Scholar]
- 8. Health USNIo . Available from: http://www.clinicaltrials.gov. [accessed 15 April 2012].
- 9. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47–59. [DOI] [PubMed] [Google Scholar]
- 11. Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009;15(Suppl 1):9S–16S. [DOI] [PubMed] [Google Scholar]
- 12. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: An open‐label, parallel‐group, single‐centre study. Clin Pharmacokinet 2010;49:259–268. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency . Pradaxa. Summary of product characteristics 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000829/WC500041059.pdf. [accessed 15 April 2012].
- 14. van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–1127. [DOI] [PubMed] [Google Scholar]
- 15. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285–295. [DOI] [PubMed] [Google Scholar]
- 16. Alberts MJ, Bernstein RA, Naccarelli GV, Garcia DA. Using dabigatran in patients with stroke: A practical guide for clinicians. Stroke 2012;43:271–279. [DOI] [PubMed] [Google Scholar]
- 17. Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003;33:173–183. [DOI] [PubMed] [Google Scholar]
- 18. Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev 2007;21:37–48. [DOI] [PubMed] [Google Scholar]
- 19. Steiner T, Kaste M, Forsting M, et al Recommendations for the management of intracranial haemorrhage – part I: Spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006;22:294–316. [DOI] [PubMed] [Google Scholar]
- 20. Broderick J, Connolly S, Feldmann E, et al Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group Stroke 2007;38:2001–2023. [DOI] [PubMed] [Google Scholar]
- 21. Büller HRPJ, Alings AMW, van Gelder IC, et al Zakboek dabigatran (Pradaxa). Alphen aan den Rijn : Van Zuiden Communications, 2011. [Google Scholar]
- 22. Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009;7(Suppl 1):107–110. [DOI] [PubMed] [Google Scholar]
- 23. Dusel CH, Grundmann C, Eich S, Seitz R, Konig H. Identification of prothrombin as a major thrombogenic agent in prothrombin complex concentrates. Blood Coagul Fibrinolysis 2004;15:405–411. [DOI] [PubMed] [Google Scholar]
- 24. Mayer SA, Brun NC, Begtrup K, et al Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 25. Diringer MN, Skolnick BE, Mayer SA, Steiner T, Davis SM, Brun NC, Broderick JP. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: Results from the factor seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 2010;41:48–53. [DOI] [PubMed] [Google Scholar]
- 26. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 2009;48:1–22. [DOI] [PubMed] [Google Scholar]
- 27. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo‐controlled, crossover study in healthy subjects. Circulation 2011;124:1573–1579. [DOI] [PubMed] [Google Scholar]
- 28. MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma 2006;60:S46–S50. [DOI] [PubMed] [Google Scholar]
- 29. Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716. [DOI] [PubMed] [Google Scholar]
- 30. Adams HP, Jr. , del Zoppo G, Alberts MJ, et al Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:e478–e534. [DOI] [PubMed] [Google Scholar]
- 31. Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 32. Dempfle CE, Hennerici MG. Dabigatran and stroke thrombolysis. Cerebrovasc Dis 2010;30:203–205. [DOI] [PubMed] [Google Scholar]
- 33. Matute MC, Guillan M, Garcia‐Caldentey J, Buisan J, Aparicio M, Masjuan J, Alonso de Lecinana M. Thrombolysis treatment for acute ischaemic stroke in a patient on treatment with dabigatran. Thromb Haemost 2011;106:178–179. [DOI] [PubMed] [Google Scholar]
- 34. De Smedt A, De Raedt S, Nieboer K, De Keyser J, Brouns R. Intravenous thrombolysis with recombinant tissue plasminogen activator in a stroke patient treated with dabigatran. Cerebrovasc Dis 2010;30:533–534. [DOI] [PubMed] [Google Scholar]
- 35. Casado Naranjo I, Portilla‐Cuenca JC, Jimenez Caballero PE, Calle Escobar ML, Romero Sevilla RM. Fatal intracerebral hemorrhage associated with administration of recombinant tissue plasminogen activator in a stroke patient on treatment with dabigatran. Cerebrovasc Dis 2011;32:614–615. [DOI] [PubMed] [Google Scholar]
- 36. Adams HP, Jr. , Brott TG, Furlan AJ, et al Guidelines for thrombolytic therapy for acute stroke: A supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation 1996;94:1167–1174. [DOI] [PubMed] [Google Scholar]
- 37. Cronin CA. Intravenous tissue plasminogen activator for stroke: A review of the ECASS III results in relation to prior clinical trials. J Emerg Med 2010;38:99–105. [DOI] [PubMed] [Google Scholar]
- 38. Vergouwen MD, Casaubon LK, Swartz RH, Fang J, Stamplecoski M, Kapral MK, Silver FL. Subtherapeutic warfarin is not associated with increased hemorrhage rates in ischemic strokes treated with tissue plasminogen activator. Stroke 2011;42:1041–1045. [DOI] [PubMed] [Google Scholar]
- 39. Miedema I, Gosselt A, de Keyser J, Koopman K, Kremer B, Luijckx GJ, Uyttenboogaart M. Prior anticoagulant therapy, subtherapeutic international normalized ratio and thrombolytic therapy in acute ischemic stroke. Int J Stroke 2011;6:568–569. [DOI] [PubMed] [Google Scholar]
- 40. Ammollo CT, Semeraro F, Incampo F, Semeraro N, Colucci M. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin‐activatable fibrinolysis inhibitor. J Thromb Haemost 2010;8:790–798. [DOI] [PubMed] [Google Scholar]
- 41. Sugg RM, Pary JK, Uchino K, et al Argatroban tPA stroke study: Study design and results in the first treated cohort. Arch Neurol 2006;63:1057–1062. [DOI] [PubMed] [Google Scholar]
- 42. Chong CA, Chiu L. Dabigatran and acute stroke thrombolysis. Cerebrovasc Dis 2010;30:202. [DOI] [PubMed] [Google Scholar]
- 43. Cucchiara B, Kasner SE, Tanne D, et al Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: Pooled analysis of placebo data from the Stroke‐Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke 2009;40:3067–3072. [DOI] [PubMed] [Google Scholar]
- 44. Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol 2011;7:400–409. [DOI] [PubMed] [Google Scholar]
- 45. Grigoryan M, Qureshi AI. Acute stroke management: Endovascular options for treatment. Semin Neurol 2010;30:469–476. [DOI] [PubMed] [Google Scholar]
- 46. Goldstein JN, Marrero M, Masrur S, et al Management of thrombolysis‐associated symptomatic intracerebral hemorrhage. Arch Neurol 2010;67:965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dempfle CE, Hennerici MG. Fibrinolytic treatment of acute ischemic stroke for patients on new oral anticoagulant drugs. Cerebrovasc Dis 2011;32:616–619. [DOI] [PubMed] [Google Scholar]
- 48. Mudd PD, James MA. Anticoagulation for atrial fibrillation: Should warfarin be temporarily stopped or continued after acute cardioembolic stroke? Age Ageing 2010;39:670–673. [DOI] [PubMed] [Google Scholar]
- 49. Patel MR, Mahaffey KW, Garg J, et al Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 50. Granger CB, Alexander JH, McMurray JJ, et al Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 51. Connolly SJ, Eikelboom J, Joyner C, et al Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]


