Summary
Background
Ambulatory arterial stiffness index (AASI) has been proposed as a new measure of arterial stiffness for predicting cardio–cerebro–vascular morbidity and mortality. However, there has been no research on the direct relationships between AASI and arterial stiffness‐determining factors.
Methods
We utilized beat‐to‐beat intra‐aortic blood pressure (BP) telemetry to characterize AASI in Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHR). By determination of aortic structural components and analysis of their correlations with AASI, we provided the first direct evidence for the associations between AASI and arterial stiffness‐determining factors including the collagen content and collagen/elastin.
Results
Ambulatory arterial stiffness index was positively correlated with pulse pressure in both WKY and SHR, less dependent on BP and BP variability than pulse pressure, and relatively stable, especially the number of BP readings not less than ~36. The correlations between AASI and aortic components were comparable for various AASI values derived from BP readings not less than ~36. Not only AASI but also BP variability and pulse pressure demonstrated a direct relationship with arterial stiffness.
Conclusions
These findings indicate AASI may become a routine measure in human arterial stiffness assessment. It is recommended to use a cluster of parameters such as AASI, BP variability, and pulse pressure for evaluating arterial stiffness.
Keywords: Aorta, Arterial stiffness, Blood pressure telemetry, Collagen, Elastin, Heart disease, Stroke
Introduction
Arterial stiffness is universally acknowledged as an important risk factor and independent predictor of cardio–cerebro–vascular events 1. Decreased elasticity of the arterial wall is implicated in the development of hypertension and increases the risk of cardiovascular mortality and fatal strokes 2. Cerebral and carotid arteries remodeling including changes in composition and structure of the vascular wall play an important role in the development of various cerebro–vascular diseases 3, 4. Effective monitoring of arteriosclerosis has clinical significance for cardio–cerebro–vascular accident. In spite of the increased awareness on the pathophysiological and clinical value of arterial stiffness, the assessment of arterial stiffness is by no means a routine procedure in clinical practice because most techniques to measure arterial properties require expensive equipment and highly trained observers.
Recently, ambulatory arterial stiffness index (AASI) was proposed as a simple and useful surrogate measure for the assessment of arterial stiffness through 24‐h ambulatory blood pressure (BP) recordings 5. This novel index is defined as one minus the regression slope of 24‐h diastolic on systolic BP in individual subjects. AASI shows a strong correlation with other classical measures of arterial stiffness, including pulse wave velocity, central and peripheral pulse pressure, and systolic augmentation index 5. Further studies have shown that AASI is associated with target organ damage in hypertension 6, 7, 8 and is recognized as a prognostic marker for cardio–cerebro–vascular morbidity and mortality 9, 10, 11, 12, particularly for fatal stroke 10, 11, 12. Several large sample clinical investigations showed that AASI predicted stroke mortality over and beyond traditional cardiovascular risk factors including the mean arterial pressure and pulse pressure in both European (Dublin and Copenhagen) and Japanese (Ohasama) population 10, 11, 12.
However, several reports have questioned the ability of AASI to represent an accurate measure of arterial stiffness 13, 14, 15, 16. It has been demonstrated that AASI is strongly influenced by factors unrelated to arterial stiffness, including nocturnal BP fall and the correlation coefficient between diastolic and systolic BP values 17, 18. The actual relationship between AASI and arterial stiffness remains unclear. The structural properties of the arterial wall, especially the concentration of collagen and elastin as well as the ratio of collagen/elastin, are important determining factors of vascular stiffness 16, 19, which play a significant role in the pathogenesis of cerebro–vascular disease 2 and should not change with BP fluctuations over 24 h. However, there has been no study investigating the relationships between AASI and arterial structural components (wall composition) presumably due to difficulty in harvesting human vascular samples.
Also, in previous human AASI studies, the intervals between BP readings are variety, ranged from 15 min 6 to 60 min 20. Much of the studies take a shorter interval (15–30 min) during daytime and a longer interval (30–60 min) during nighttime to limit interference with sleep. One study has been carried out to show the changes of AASI with BP readings at different interval time and the influence of these AASI changes on the predictive accuracy for cardiovascular mortality 21. However, this human study did not evaluate the interval time less than 30 min, due to the original BP readings obtained at the programmed interval time of 30 min. Moreover, in human studies, AASI is derived from intermittently noninvasive brachial BP data, and it is very difficult to more accurately and reliably evaluate the stability of AASI values using continuously beat‐to‐beat intra‐arterial BP data.
This first animal study on AASI was designed to address the above‐mentioned clinical issues using intra‐aortic BP telemetry and aortic structural component analysis. The aim was (1) to characterize the AASI in animals, including normotensive and hypertensive rats, (2) to provide the direct evidence for the relationship between AASI and aortic structure, and lastly (3) to evaluate the stability of AASI values derived from BP readings at different interval time based on the beat‐to‐beat intra‐aortic BP data.
Methods
Animals and Experimental Protocol
Spontaneously hypertensive rats and WKY rats were provided by the Animal Center of the Second Military Medical University (Shanghai, China). All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethical Committee of the Second Military Medical University.
At the average age of 27 weeks, male WKY and SHR were anaesthetized with an intraperitoneal injection of ketamine (50 mg/kg) and diazepam (5 mg/kg) and underwent intraperitoneal implantation of a BP telemeter (DSI model TA11PAC40) with the catheter situated in the abdominal aorta immediately above the iliac bifurcation as we previously described 22. Rats were housed in separate cages in a hemodynamic monitoring room (23 ± 2°C; 12‐h light‐dark cycle, lights from 08.00 to 20.00 h) with free access to tap water and rat chow, and hemodynamic monitoring was performed by telemetry for 28 h during 13–14 days after telemeter implantation. Next day after telemetry, some WKY and SHR were anaesthetized with sodium pentobarbitone (60 mg/kg, i.p.) for tissue sampling by our previous methods 23, 24; whole thoracic aorta and left ventricle were isolated and weighed, and descending thoracic aorta was stored at −80°C and used for assay of collagen and elastin.
Blood Pressure Telemetry and Data Analysis
Blood pressure was continuously recorded beat by beat at 500 Hz using a DSI DataQuest ART Gold 3.1 acquisition system (Data Sciences, Inc., St. Paul, MN, USA). Telemetry recording was started at 16.00 h p.m. and maintained for a period of 28 h. Systolic BP, diastolic BP, pulse pressure, and heart rate values were online computed from the BP waveform data every 2 seconds. The number of BP readings during 24 h was 43,200. Locomotor activity was also measured. In off‐line analysis, the mean values of these parameters over the last 24‐h period were calculated and served as systolic BP, diastolic BP, pulse pressure, heart rate, and activity, respectively. AASI was expressed as 1 minus regression slope of diastolic BP over systolic BP during the last 24‐h period 5. The standard deviation values of systolic BP, diastolic BP, and heart rate over the last 24‐h period were calculated and defined as the variability of systolic BP, diastolic BP, and heart rate, respectively 23. Systolic BP, diastolic BP, and heart rate values during the 12‐h dark period and during the 12‐h light period were calculated, and the dark‐light difference values were named as Δsystolic BP, Δdiastolic BP, and Δheart rate, respectively, indicating diurnal variations 25.
Determination of Collagen and Elastin Concentrations
Hot alkali method was used to separate elastin and collagen as described elsewhere 26. Briefly, 10 mg tissue sample was minced in PBS and defatted in alcohol–ether (3:1). The intracellular protein was removed by 0.3% SDS treatment. After lyophilization, the sample was placed in 1 mL 0.1 N NaOH and boiled for 45 min. The supernatant and precipitate were separated by centrifugation. The precipitate was washed with hot distilled water twice.
The aortic collagen content was determined by assaying hydroxyproline in NaOH solution and washing water and treated as described previously 27. Briefly, NaOH solution was lyophilized and hydrolyzed in 6 mol/L hydrochloric acid for 24 h at 110°C. Hydroxyproline was determined by colorimetric assay after hydrochloric acid neutralized. Hydroxyproline values were then converted to collagen content by multiplying by a factor of 7.46. The aortic elastin content was determined by weighing the precipitate after lyophilization using a scale accurate to 0.01 mg.
Statistics
Data are presented as mean ± SEM. The differences between two groups were evaluated by a two‐tailed Student's unpaired t‐test. The correlations were assessed by linear regression analysis. Statistical significance was judged at P < 0.05.
Results
AASI in Normotensive and Hypertensive Rats
To first determine AASI in animals, we included a relatively large population (n = 59) of normotensive WKY (n = 29) and hypertensive SHR (n = 30) in this study and used the most advanced and accurate BP telemetry to monitor hemodynamics directly (intra‐aortic BP) and continuously (beat‐to‐beat BP) in conscious freely moving rats for 28 h (Figure 1). This ambulatory BP monitoring provided 43,200 readings over 24 h (12‐h dark period from 20:00 to 8:00 and 12‐h light period from 8:00 to 20:00). Compared with WKY, SHR demonstrated a higher level in BP and BP variability, a slightly lower level in heart rate, and no significant change in heart rate variability (Figures 1 and 2) and locomotor activity (3.10 ± 0.24 vs. 2.93 ± 0.35; P = 0.69). Both WKY and SHR exhibited diurnal variations (Δ, dark–light difference) in BP, heart rate, and locomotor activity (Figures 1 and 2), indicating a circadian rhythm for these parameters. Among them, only diurnal variation in heart rate was significantly blunted in SHR.
Figure 1.
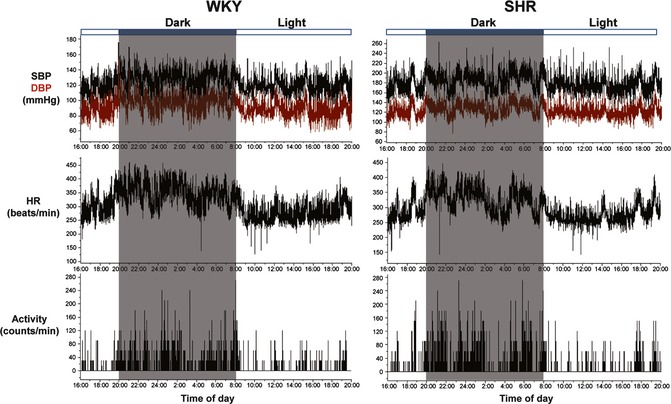
Representative recordings of telemetric systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR) and locomotor activity in Wistar–Kyoto (WKY), and spontaneously hypertensive rats (SHR).
Figure 2.
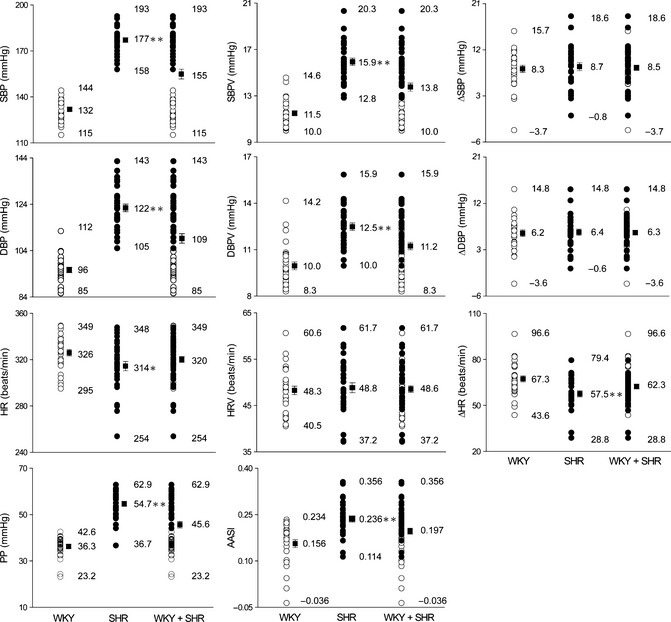
Distribution of haemodynamic parameters in Wistar–Kyoto (WKY, n = 29) and spontaneously hypertensive rats (SHR, n = 30). SBP and SBPV, systolic blood pressure and its variability; DBP and DBPV, diastolic blood pressure and its variability; HR and HRV, heart rate and its variability; Δ, dark–light difference; PP, pulse pressure; AASI, ambulatory arterial stiffness index. Maximal, minimal, and mean values are shown. *P < 0.05, **P < 0.01 SHR versus WKY.
As the parameters for arterial stiffness, both AASI and pulse pressure were markedly increased in SHR compared with WKY (Figure 2). AASI mean value was 0.156 in normotensive WKY and 0.236 in hypertensive SHR. The distribution of AASI was partially overlapped for WKY and SHR (Figure 2), similar with diastolic BP, BP variability, and pulse pressure, and different from systolic BP (total separation) and BP diurnal variation (almost total overlap). The distribution of heart rate and its variability and diurnal variation was also shown in Figure 2. Overall, the hemodynamic data except for systolic BP data were continuously distributed in the whole population between WKY and SHR.
Further, AASI demonstrated a positive correlation with pulse pressure, a classical parameter for arterial stiffness, in separated and combined WKY and SHR (Figure 3). The correlations between AASI and other hemodynamic parameters were also examined (Table 1). Both AASI and pulse pressure were positively correlated with BP and BP variability, negatively correlated with heart rate diurnal variation, and not correlated with BP diurnal variation. Of note, from view of correlation coefficients, AASI was less dependent on BP and BP variability than pulse pressure, although both AASI and pulse pressure are derived from systolic and diastolic BP data.
Figure 3.
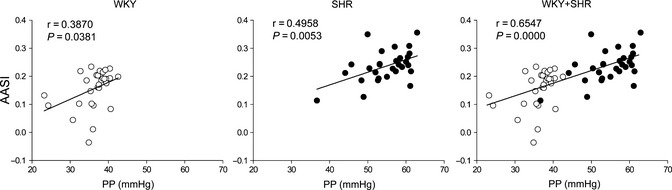
Scatter plots showing the relationships between ambulatory arterial stiffness index (AASI) and pulse pressure (PP) in Wistar–Kyoto (WKY, n = 29) and spontaneously hypertensive rats (SHR, n = 30).
Table 1.
Correlation coefficients between AASI and hemodynamic parameters in Wistar–Kyoto and spontaneously hypertensive rats
| PP, mmHg | AASI | |
|---|---|---|
| SBP, mmHg | 0.8798** | 0.5489** |
| DBP, mmHg | 0.6768** | 0.4013** |
| HR, beats/min | −0.3001* | −0.1481 |
| SBP variability, mmHg | 0.7658** | 0.5501** |
| DBP variability, mmHg | 0.5426** | 0.0799 |
| HR variability, beats/min | 0.0331 | −0.2515* |
| ΔSBP, mmHg | 0.0775 | 0.1784 |
| ΔDBP, mmHg | −0.0054 | 0.0658 |
| ΔHR, beats/min | −0.4131** | −0.4580** |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Δ, dark–light difference; PP, pulse pressure; AASI, ambulatory arterial stiffness index.
n = 59. *P < 0.05, **P < 0.01.
AASI is Correlated with Aortic Structure
To provide direct evidence whether AASI is associated with arterial stiffness, we examined aortic structure in a subgroup of the experimental animals containing 9 WKY and 10 SHR. Aortic hypertrophy index, expressed as the ratio of aortic weight/body weight (AW/BW), and left ventricular hypertrophy index, expressed as the ratio of left ventricular weight/body weight (LVW/BW), were both elevated in SHR compared with WKY (Table 2). In aortic structural components related to vascular stiffness, the collagen concentration tended to increase (P = 0.06), the elastin concentration decreased, and the ratio of collagen/elastin increased in SHR compared with WKY.
Table 2.
Aortic structure in Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHR)
| WKY (n = 10) | SHR (n = 9) | |
|---|---|---|
| BW, g | 391 ± 14 | 315 ± 4** |
| AW/BW, ‰ | 0.19 ± 0.01 | 0.25 ± 0.01** |
| LVW/BW, ‰ | 2.18 ± 0.03 | 3.19 ± 0.04** |
| Aortic collagen, % | 15.02 ± 0.90 | 17.26 ± 0.82 |
| Aortic elastin, % | 39.70 ± 0.85 | 36.85 ± 0.66* |
| Aortic collagen/elastin | 0.38 ± 0.03 | 0.47 ± 0.02* |
BW, body weight; AW, aortic weight; LVW, left ventricular weight.
*P < 0.05, **P < 0.01 versus WKY.
The relationships between aortic structure and hemodynamic parameters were determined (Table 3). Aortic and left ventricular hypertrophy were correlated with most of hemodynamic parameters including BP, heart rate, BP variability, heart rate diurnal variation, pulse pressure, and AASI. Aortic structural components were correlated with three parameters including BP variability, pulse pressure, and AASI (Table 3, Figure 4). Of note, systolic BP and systolic BP variability were the best predictors for cardiovascular hypertrophy. AASI and systolic BP variability were the better predictors for vascular stiffness than pulse pressure. Of interest, AASI was more related with collagen, while systolic BP variability was more related with elastin, although both were similarly related with the ratio of collagen/elastin. Actually, based on our data, systolic BP variability is the most appropriate parameter for predicting both cardiovascular hypertrophy and vascular stiffness in this study model.
Table 3.
Correlation coefficients between aortic structure and hemodynamic parameters in Wistar–Kyoto and spontaneously hypertensive rats
| AW/BW, ‰ | LVW/BW, ‰ | Elastin, % | Collagen, % | Collagen/Elastin | |
|---|---|---|---|---|---|
| SBP, mmHg | 0.8504** | 0.9394** | −0.4491 | 0.3132 | 0.3880 |
| DBP, mmHg | 0.7061** | 0.8520** | −0.4004 | 0.0900 | 0.2105 |
| HR, beats/min | −0.5147* | −0.5245* | 0.1688 | −0.4125 | −0.3705 |
| SBP variability, mmHg | 0.8310** | 0.9007** | −0.5803** | 0.4637* | 0.5329* |
| DBP variability, mmHg | 0.6523** | 0.7447** | −0.5094* | 0.2195 | 0.3169 |
| HR variability, beats/min | −0.2326 | −0.2094 | 0.0456 | −0.1767 | −0.1720 |
| ΔSBP, mmHg | 0.1554 | 0.1108 | −0.3308 | 0.3548 | 0.3789 |
| ΔDBP, mmHg | 0.0456 | 0.0885 | −0.2779 | 0.1403 | 0.1934 |
| ΔHR, beats/min | −0.5035* | −0.5529* | 0.3279 | −0.3566 | −0.3879 |
| PP, mmHg | 0.7989** | 0.7997** | −0.3904 | 0.4902* | 0.4932* |
| AASI | 0.6411** | 0.6146** | −0.3695 | 0.5624* | 0.5482* |
Figure 4.
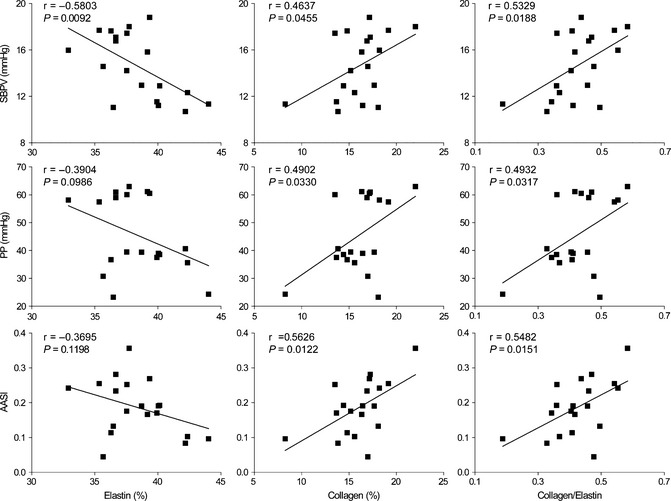
Representative scatter plots showing the relationships between aortic components and hemodynamic parameters in Wistar–Kyoto and spontaneously hypertensive rats (n = 19). SBPV, systolic blood pressure variability; PP, pulse pressure; AASI, ambulatory arterial stiffness index.
Evaluation for the Stability of AASI Values
Previously, AASI was computed from 24‐h BP data determined at different interval time in different human studies. To know whether the interval time for BP readings, in another word, the number of BP readings influences the calculated AASI value, we extracted systolic and diastolic BP readings at various intervals to obtain various AASI values for each animal in a subgroup of 9 WKY and 10 SHR (Figure 5). The interval time of 20 or 30 min in 12‐h dark period and 40 or 60 min in 12‐h light period (indicated as 20/40 min and 30/60 min) in rats mimicked the shorter interval during daytime and the longer interval during nighttime in humans as described in the previous reports on AASI 5, 20. Other intervals were designed as the same during the dark and light periods, including 2 seconds, 10, 20, 30, 40, 50, and 60 min. The AASI values appeared relatively stable for each animal, especially when the interval time for BP readings was within 40 min or the number of BP readings was not less than 36 in spite of the interval time at 30/60 min (Figure 5). Further, the correlations between aortic structural components and AASI were comparable for the interval time of BP readings from 2 seconds to 40 min (including 30/60 min), or for the number of BP readings from 36 to 43,200 (Table 4). These indicate that the usually programmed interval time of 15–30 min during daytime and 30–60 min during nighttime for ambulatory BP monitoring in humans 28 is reliable for determination of AASI values.
Figure 5.
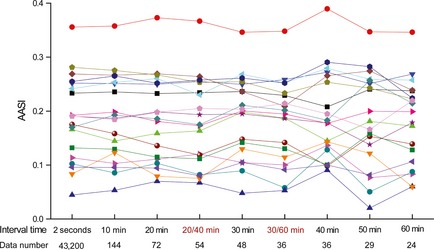
Ambulatory arterial stiffness index (AASI) derived from blood pressure readings at different interval time in Wistar–Kyoto and spontaneously hypertensive rats (n = 19). The interval time for blood pressure readings is indicated, and the number of blood pressure readings during 24 h is shown. Each line represents AASI values of an individual animal, calculated from blood pressure readings at different interval time. 20/40 min, the interval time of 20 min in 12‐h dark period and 40 min in 12‐h light period; 30/60 min, the interval time of 30 min in 12‐h dark period and 60 min in 12‐h light period.
Table 4.
Correlation coefficients between aortic components and ambulatory arterial stiffness index (AASI) derived from blood pressure readings at different interval time in Wistar–Kyoto and spontaneously hypertensive rats
| AASI | Elastin, % | Collagen, % | Collagen/Elastin | |
|---|---|---|---|---|
| Interval time | Data number | |||
| 2 seconds | 43,200 | −0.3696 | 0.5625* | 0.5483* |
| 10 min | 144 | −0.3630 | 0.5470* | 0.5386* |
| 20 min | 72 | −0.3824 | 0.5903** | 0.5781** |
| 20/40 min | 54 | −0.3864 | 0.5963** | 0.5789** |
| 30 min | 48 | −0.3462 | 0.5345* | 0.5245* |
| 30/60 min | 36 | −0.3724 | 0.5298* | 0.5264* |
| 40 min | 36 | −0.3535 | 0.5687* | 0.5595* |
| 50 min | 29 | −0.3425 | 0.4788* | 0.4789* |
| 60 min | 24 | −0.3317 | 0.4871* | 0.4805* |
Interval time as indicated in Figure 5.
n = 19. *P < 0.05, **P < 0.01.
Discussion
To our knowledge, the present study is the first animal investigation on the recently proposed AASI as well as the first report for the direct evidence of the relationship between AASI and vascular wall composition. We characterized AASI in normotensive (WKY) and hypertensive (SHR) rats; the AASI values were higher in SHR compared with WKY, partially overlapped between WKY and SHR, positively correlated with pulse pressure in both WKY and SHR, less dependent on BP and BP variability than pulse pressure, and relatively stable, especially when the number of ambulatory 24‐h BP readings not less than ~36. AASI was associated with arterial stiffness‐determining factors, including the collagen content and the ratio of collagen/elastin. In terms of translation of our work to clinical practice, using our telemetric BP data (continuously beat‐to‐beat intra‐aortic BP) to determine various AASI values and their correlations with aortic components, we confirmed that the usually programmed intervals of 15–30 min during the day and every 30–60 min at night for ambulatory BP monitoring in humans are reliable for measurement of AASI.
In fact, the present study identified three hemodynamic parameters associated with aortic stiffness. They are BP variability, pulse pressure, and AASI. All of these indexes are also associated with cardio–cerebro–vascular disease. Pulse pressure is one of classical indirect measures for arterial stiffness and has long been extensively used as a predictor of cerebro–vascular complications 4, 5. Compared with pulse pressure, AASI was more correlated with the arterial stiffness‐determining factors, including aortic collagen concentration and the ratio of aortic collagen/elastin. These findings are consistent with the clinical research, which showed AASI was a stronger predictor of stroke than pulse pressure 10, 11, 12. BP variability has been demonstrated a strong and negative association with carotid distensibility in SHR 29. Aortic distensibility is decreased in sinoaortic‐denervated rats 30, an animal model of high BP variability without hypertension 31. Our previous study shows the increased collagen and decreased elastin in aortas of sinoaortic‐denervated rats 32. These previous results indicate that increased BP variability may reduce arterial distensibility and enhance arterial stiffness. Meanwhile, numerous studies demonstrate that BP variability is related with poor outcome in many cardio–cerebro–vascular diseases including stroke 33, 34, 35. In addition, the present study further demonstrated the relationships between BP variability and aortic stiffness‐determining components, particularly the aortic elastin concentration (a negative correlation) and the ratio of aortic collagen/elastin (a positive correlation). Compared with systolic BP variability, AASI demonstrated a comparable association with the ratio of aortic collagen/elastin, indicating they both have the same predictive significance for arterial stiffness. However, there existed an obvious difference for their association with individual aortic components; AASI was more related with collagen, while BP variability was more related with elastin. Although the exact explanations for the difference are unclear, these results imply that AASI is more sensitive to the change of collagen, whereas BP variability is more sensitive to the change of elastin. Therefore, the ratio of collagen/elastin may represent as an overall parameter for arterial stiffness.
The present study also identified systolic BP and systolic BP variability as the best predictors for cardiovascular hypertrophy in aortas and left ventricles. As BP was not significantly related with any of three aortic stiffness‐determining factors, and AASI was less related to cardiovascular hypertrophy than systolic BP variability, we believe that systolic BP variability is the best predictor for both cardiovascular hypertrophy and stiffness in the present study model. It is also reasonable to deduce that systolic BP variability may be the best predictor among the present examined hemodynamic parameters for cardio–cerebro–vascular events, because both vascular hypertrophy and stiffness are risk factors for cardio–cerebro–vascular events 1, 36. Furthermore, the present study has extended our previous conclusion that BP variability is more important than BP level in determination of end‐organ damage 23, 36, although our previous study only included hemodynamic parameters of BP, heart rate, and their variability, and did not examine pulse pressure, AASI, and diurnal variations in BP, and heart rate 23, 36.
Numerous clinical studies have demonstrated that both age and gender are determinants of AASI 5, 6, 17. The relationship between these factors and AASI in animal model should also be investigated in further work to provide insight into AASI clinical application. High levels of aldosterone can reduce vascular compliance through multiple mechanisms including collagen synthesis, vascular inflammation, and autophagy. Its blocker attenuates myocardial fibrosis and is approved for treatment of chronic heart failure after myocardial infarction 37. Further studies should explore the relationship between aldosterone and AASI, which could be helpful to clarify whether aldosterone blocker can also be used to improve the arterial stiffness in hypertensive patients.
Perspectives
Arterial stiffness is increasingly recognized as an important harbinger of future cardio–cerebro–vascular morbidity and mortality in different clinical settings, and already included in the guidelines for the management of arterial hypertension as an index of subclinical organ damage 18. However, assessment of arterial stiffness in humans is limited by requirement of specialized equipment and dedicated personnel, and using routine ambulatory BP monitoring to obtain AASI for evaluating arterial stiffness is questionable for its trade‐off between simplicity and accuracy. This first animal study on AASI provides the first direct evidence that AASI is associated with aortic stiffness‐determining factors and confirms the AASI stability and reliability by mimicking ambulatory BP monitoring in humans. Our findings indicate AASI is a simple measure of arterial stiffness and can be used in the clinical practice as well as epidemiological survey. We prefer to recommend a cluster of BP‐derived parameters for more accurately evaluating arterial stiffness, because BP variability and pulse pressure, in addition to AASI, may predict arterial stiffness in our study model, and pathogenesis for arterial stiffness is various in different clinical settings. It remains to further study the relationship between AASI and carotid artery stiffness to clarify why AASI is a prognostic marker, especially for fatal stroke that has become the second most common cause of death worldwide 38. Intervention study is also needed to understand whether AASI is a prognostic marker for both disease and treatment, which may help to establish new preventive and therapeutic target.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2009CB521902 to C.‐Y.M.), the National Natural Science Foundation of China (81130061 to C.‐Y.M. and 81202572 to Z.‐Y.L.), the Program of Shanghai Subject Chief Scientist (10XD1405300 to C.‐Y.M.), and the Shanghai “Shu Guang” Project (10GG19 to C.‐Y.M.).
The first two authors contributed equally to this work.
References
- 1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: A systematic review and meta‐analysis. J Am Coll Cardiol 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2. Bian PD, Pan HH, Li XY, Lin W, Hu SJ. Associated factors of brachial‐ankle pulse wave velocity in hypertensive patients aged 80 and over. CNS Neurosci Ther 2012;18:188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang C, Zhang X, Song SW, Yu JG, Cai GJ. Cerebral artery remodeling in stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17:785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: Mechanisms and potential prevention strategies. CNS Neurosci Ther 2011;17:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Wang JG, Dolan E, et al. Ambulatory arterial stiffness index derived from 24‐hour ambulatory blood pressure monitoring. Hypertension 2006;47:359–364. [DOI] [PubMed] [Google Scholar]
- 6. Leoncini G, Ratto E, Viazzi F, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension 2006;48:397–403. [DOI] [PubMed] [Google Scholar]
- 7. Ratto E, Leoncini G, Viazzi F, et al. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens 2006;24:2033–2038. [DOI] [PubMed] [Google Scholar]
- 8. Mule G, Cottone S, Cusimano P, et al. Inverse relationship between ambulatory arterial stiffness index and glomerular filtration rate in arterial hypertension. Am J Hypertens 2008;21:35–40. [DOI] [PubMed] [Google Scholar]
- 9. Muxfeldt ES, Cardoso CR, Dias VB, Nascimento AC, Salles GF. Prognostic impact of the ambulatory arterial stiffness index in resistant hypertension. J Hypertens 2010;28:1547–1553. [DOI] [PubMed] [Google Scholar]
- 10. Dolan E, Thijs L, Li Y, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the dublin outcome study. Hypertension 2006;47:365–370. [DOI] [PubMed] [Google Scholar]
- 11. Hansen TW, Staessen JA, Torp‐Pedersen C, et al. Ambulatory arterial stiffness index predicts stroke in a general population. J Hypertens 2006;24:2247–2253. [DOI] [PubMed] [Google Scholar]
- 12. Kikuya M, Staessen JA, Ohkubo T, et al. Ambulatory arterial stiffness index and 24‐hour ambulatory pulse pressure as predictors of mortality in ohasama, japan. Stroke 2007;38:1161–1166. [DOI] [PubMed] [Google Scholar]
- 13. Kips JG, Vermeersch SJ, Reymond P, et al. Ambulatory arterial stiffness index does not accurately assess arterial stiffness. J Hypertens 2012;30:574–580. [DOI] [PubMed] [Google Scholar]
- 14. Laurent S. Surrogate measures of arterial stiffness: Do they have additive predictive value or are they only surrogates of a surrogate? Hypertension 2006;47:325–326. [DOI] [PubMed] [Google Scholar]
- 15. Westerhof N, Lankhaar JW, Westerhof BE. Ambulatory arterial stiffness index is not a stiffness parameter but a ventriculo‐arterial coupling factor. Hypertension 2007;49:e7; author reply e8–9. [DOI] [PubMed] [Google Scholar]
- 16. Gavish B. Correlating ambulatory blood pressure measurements with arterial stiffness: A conceptual inconsistency? Hypertension 2006;48:e108; author reply e109. [DOI] [PubMed] [Google Scholar]
- 17. Schillaci G, Parati G, Pirro M, et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension 2007;49:986–991. [DOI] [PubMed] [Google Scholar]
- 18. Schillaci G, Parati G. Ambulatory arterial stiffness index: Merits and limitations of a simple surrogate measure of arterial compliance. J Hypertens 2008;26:182–185. [DOI] [PubMed] [Google Scholar]
- 19. Adiyaman A, Dechering DG, Thien T, et al. Putting a spin on the ambulatory arterial stiffness index. J Hypertens 2008;26:1266–1267; author reply 1267–1269. [DOI] [PubMed] [Google Scholar]
- 20. Jerrard‐Dunne P, Mahmud A, Feely J. Ambulatory arterial stiffness index, pulse wave velocity and augmentation index–interchangeable or mutually exclusive measures? J Hypertens 2008;26:529–534. [DOI] [PubMed] [Google Scholar]
- 21. Kikuya M, Staessen JA, Ohkubo T, et al. How many measurements are needed to provide reliable information in terms of the ambulatory arterial stiffness index? The ohasama study Hypertens Res 2011;34:314–318. [DOI] [PubMed] [Google Scholar]
- 22. Naelten G, Liu KL, Lo M. Durable improvement of renal function after perindopril withdrawal in lyon hypertensive rats. J Cardiovasc Pharmacol 2011;57:240–245. [DOI] [PubMed] [Google Scholar]
- 23. Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end‐organ damage in rats. J Hypertens 2006;24:1125–1135. [DOI] [PubMed] [Google Scholar]
- 24. Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue‐derived visfatin is a vascular smooth muscle cell growth factor: Role of nicotinamide mononucleotide. Cardiovasc Res 2009;81:370–380. [DOI] [PubMed] [Google Scholar]
- 25. Van Vliet BN, Chafe LL. Maternal endothelial nitric oxide synthase genotype influences offspring blood pressure and activity in mice. Hypertension 2007;49:556–562. [DOI] [PubMed] [Google Scholar]
- 26. Mercier N, Kakou A, Challande P, Lacolley P, Osborne‐Pellegrin M. Comparison of the effects of semicarbazide and beta‐aminopropionitrile on the arterial extracellular matrix in the brown norway rat. Toxicol Appl Pharmacol 2009;239:258–267. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi K, Luo M, Zhang Y, et al. Secreted frizzled‐related protein 2 is a procollagen c proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 2009;11:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Committee National Heart Foundation and High Blood Pressure Research Council of Australia Ambulatory Blood Pressure Monitoring Consensus Committee . Ambulatory blood pressure monitoring. Aust Fam Physician 2011;40:877–880. [PubMed] [Google Scholar]
- 29. Dabire H, Lacolley P, Chaouche‐Teyara K, Fournier B, Safar ME. Relationship between arterial distensibility and low‐frequency power spectrum of blood pressure in spontaneously hypertensive rats. J Cardiovasc Pharmacol 2002;39:98–106. [DOI] [PubMed] [Google Scholar]
- 30. Lacolley P, Bezie Y, Girerd X, et al. Aortic distensibility and structural changes in sinoaortic‐denervated rats. Hypertension 1995;26:337–340. [DOI] [PubMed] [Google Scholar]
- 31. Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002;20:1865–1872. [DOI] [PubMed] [Google Scholar]
- 32. Miao CY, Tao X, Gong K, Zhang SH, Chu ZX, Su DF. Arterial remodeling in chronic sinoaortic‐denervated rats. J Cardiovasc Pharmacol 2001;37:6–15. [DOI] [PubMed] [Google Scholar]
- 33. Gui H, Guo YF, Liu X, et al. Effects of combination therapy with levamlodipine and bisoprolol on stroke in rats. CNS Neurosci Ther 2013;19:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu AJ, Zang P, Guo JM, et al. Involvement of acetylcholine‐alpha7nachr in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther 2012;18:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu W, Su BL, Wang ZS, Zhang X, Gao YS, Song SW. Gastrodin improved baroreflex sensitivity and increased gamma‐amino butyric acid content in brains without decreasing blood pressure in spontaneously hypertensive rats. CNS Neurosci Ther 2012;18:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Su DF, Miao CY. Reduction of blood pressure variability: A new strategy for the treatment of hypertension. Trends Pharmacol Sci 2005;26:388–390. [DOI] [PubMed] [Google Scholar]
- 37. Kosmala W, Przewlocka‐Kosmala M, Szczepanik‐Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: A randomised controlled trial of aldosterone blockade. Heart 2013;99:320–326. [DOI] [PubMed] [Google Scholar]
- 38. Wang P, Xu TY, Guan YF, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through sirt1‐dependent adenosine monophosphate‐activated kinase pathway. Ann Neurol 2011;69:360–374. [DOI] [PubMed] [Google Scholar]


