Neuropathic pain associated with peripheral nerve injury is characterized by the sensory abnormalities such as spontaneous pain, hyperalgesia, allodynia, and radiation of pain 1. Pain after injury to the nervous system is a major chronic pain condition. The traditional analgesics like opioids or nonsteroidal antiinflammatory drugs (NSAIDs) are not very satisfactory because of their significant side effects and ineffectiveness 2. Therefore, the treatment of neuropathic pain remains a challenge. Chronic constriction injury (CCI) of sciatic nerve induced neuropathy is a widely employed model for the induction of neuropathic pain in experimental animals 3. CCI‐induced neuropathy in experimental animals mimics complex regional pain syndrome in humans 4. More recently, various studies have reported herbal medicine to produce the beneficial effect in the management of painful neuropathy.
Sophora alopecuroides L. is a predominant herbal plant resource in Northwest China. Among various alkaloids from Sophora alopecuroids L., oxymatrine (OMT) has been identified as the major bioactive component contributing to a variety of pharmacological effects. In the recent years, a large number of pharmacological and clinical studies have found that oxymatrine itself has a wide spectrum of effect in antivirus, liver fibrosis, immunoinhibition 5. Studies have shown that oxymatrine has analgesic effect 6, while there has been no published support that oxymatrine is beneficial for the treatment on neuropathic pain. In the present study, we tested the hypothesis that the analgesic effect of oxymatrine on neuropathic pain induced by CCI of sciatic nerve in mice.
Male Institute of Cancer Research (ICR) mice (21–25 g) were injured using the CCI model, as previously described 3. Sham‐operated mice were subjected to all surgical procedures except for the nerve ligation. OMT was obtained from Ningxia Institute of Drug (Ningxia, China, purity>98.3%). The doses of OMT (40–160 mg/kg) were selected based on the results of our preliminary experiments 6. For analysis of behavior, mechanical allodynia test, thermal hyperalgesia test, and cold allodynia test were performed 7. To objectively analyze the antinociception effect of OMT, GABAAR agonist and antagonist were administrated via Intrathecal injection to observe the behavioral changes. To further confirm the analgesic effect of OMT, we performed the immunofluorescence and western blot analyses to determine the expression of GABAARα2 and GABA transporter‐1 (GAT‐1). Data are expressed by mean ± SD and analyzed with one‐way analysis of variance (ANOVA) followed by least‐significant difference post hoc test and Tamhane's T2 test.
In this study, 7 days after CCI, the mice showed a relatively high degree of similarity with other studies published on neuropathic pain in terms of the degrees of allodynia and hyperalgesia against mechanical, hot, and cold stimuli. The behavioral alterations started on postoperative day 7 and lasted throughout the experimental period. Intraperitoneal injection of OMT (160, 80 mg/kg) increased the paw withdrawal threshold and paw withdrawal latency when compared with CCI group. However, the lowest dose (40 mg/kg, i.p.) of OMT was essentially inactive in the mechanical allodynia and thermal hyperalgesia tests. Intraperitoneal injection of OMT (160, 80, 40 mg/kg) was found to produce a marked, dose‐dependent effect in cold allodynia test (Figure 1).
Figure 1.
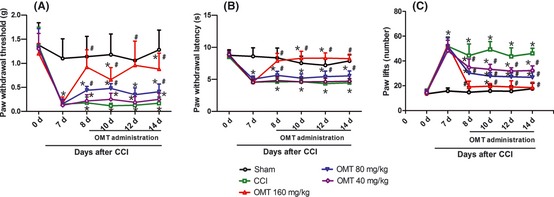
The antinociceptive effects of OMT on behavioral tests in the hind paw ipsilateral to the chronic constrictive injury (CCI) of the sciatic nerve in mice. (A) mechanical allodynia test; (B) thermal hyperalgesia test; (C) cold allodynia test. Data were presented as mean ± SD *P < 0.05 versus sham group; # P < 0.05 versus CCI group.
The paw withdrawal threshold after the combination of OMT (35 mg/kg, i.p.) and γ‐aminobutyric acid (GABA) (2 mg/kg, i.t.), muscimol (GABAA receptor agonist, MUS), (0.1 μg/site, i.t.), or NO‐711 (GABA transporter‐1 inhibitor) (0.125 mg/kg, i.t.) also showed a significantly larger increase than that seen after OMT (35 mg/kg, i.p.) alone. I.P treatment with OMT (160 mg/kg) produced significant increase in the paw withdrawal threshold. Bicuculline (GABAA receptor antagonist, BIC) (0.75 μg/site, i.t.) significantly reduced the OMT‐induced antinociception. Compared with CCI group, the expression of GABAARα2 immunostaining positive neurons in the OMT‐treated CCI mice was significantly increased (F(2,12) = 67.671, P < 0.001). Compared with sham group, the GABAARα2 protein level was decreased in CCI group. However, the GABAARα2 protein levels showed a increase in OMT group compared with those in CCI group (F(2,6) = 148.048, P < 0.001). Compared with sham group, the expression of GAT‐1 immunostaining positive neurons was increased in CCI group. Compared with CCI group, the expression of GAT‐1 immunostaining positive neurons in the OMT‐treated CCI mice was significantly decreased (F(2,18) = 44.018, P < 0.001). Western blot analysis showed that compared with sham group, the GAT‐1 protein level was increased in CCI group. However, the GAT‐1 protein levels showed a decrease in OMT group compared with those in CCI group (F(2,6) = 49.492, P < 0.001) (Figure 2).
Figure 2.
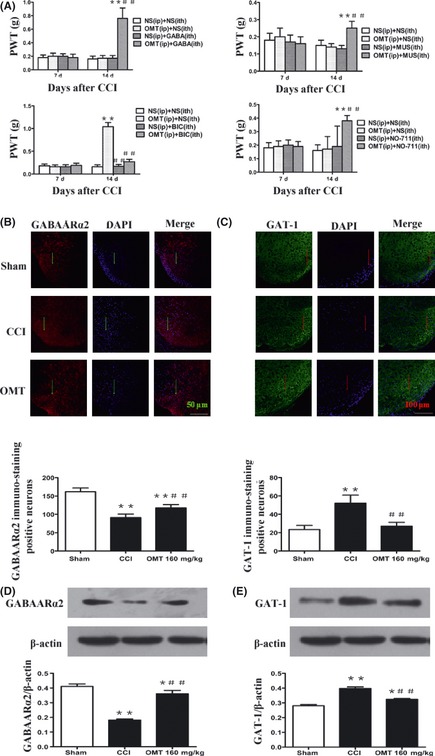
Effects of GABAAR agonist and antagonist on the antinociception of OMT (A). Data were presented as mean ± SD *P < 0.05, **P < 0.01 versus NS + NS group; # P < 0.05, ## P < 0.01 versus NS+ vehicles group. Representative images and qualitative presentation of immunostaining positive neurons of GABAAα2 (B),GAT‐1 (C), and immunoblot bands of GABAAα2 (D), GAT‐1 (E) in the spinal dorsal horn mice in each group. (a) sham group; (b) CCI group; (c) OMT‐treated group. Data were presented as mean ± SD *P < 0.05, **P < 0.01 versus sham group. # P < 0.05, ## P < 0.01 versus CCI group.
Behaviors such as allodynia and hyperalgesia are parameters that have been previously used to study the pharmacology and modulation of neuropathic pain 8. In this study, the antinociceptive effects of the OMT were assessed in mechanical allodynia, thermal hyperalgesia, and cold allodynia tests. Our results showed that the i.p. administration of OMT prevented the development of mechanical allodynia, thermal hyperalgesia, and cold allodynia induced by CCI. Neuropathic pain is a very complex process with multiple mechanisms, such as peripheral and central mechanisms 9. The pathophysiological properties that are responsible for neuropathic pain can be broadly categorized into five groups: ectopic impulse generation in damaged primary afferent fibers, fiber interactions, central sensitization, disinhibition (failure or reduction of normal inhibitory mechanisms), and plasticity (degenerative and regenerative changes associated with altered connectivity). However, the change of neurotransmitters (Glutamate or GABA) within the spinal dorsal horn plays an important role in the pathogenesis of chronic neuropathic pain 10. More recently, the study has shown that oxymatrine has the effect of sedative by increasing the content of GABA in mice 6. Therefore, we wondered whether oxymatrine could produce the antinociceptive effects on a chronic neuropathic pain model induced by chronic constrictive injury of the sciatic nerve by regulating the GABAergic nervous system. Our results show that the antinociceptive effects produced by i.p. administration of OMT (35 mg/kg) were potentiated by coadministered GABA, MUS, and NO‐711, while all alone in subthreshold doses did not show any effects in antinociception in the paw withdrawal threshold. The present data show that the antinociception of OMT (160 mg/kg) is selectively reversed by BIC. The present data show that i.p. OMT (160 mg/kg) can downregulate the expression of GAT‐1 and upregulate the expression of GABAARα2 on a chronic neuropathic pain model induced by chronic constrictive injury of the sciatic nerve. The results demonstrated that antinociceptive mechanism of OMT may be related to the GABAergic nervous system. But the mechanism was still not completely understood.
The first two authors contributed equally to this work.
References
- 1. Harden RH. Chronic neuropathic pain: mechanisms, diagnosis, and treatment. Neurologist 2005;11:111–22. [DOI] [PubMed] [Google Scholar]
- 2. Calo G, Rizzi A, Cifani C, et al. UFP‐112 a potent and long‐lasting agonist selective for the nociceptin/orphanin FQ receptor. CNS Neurosci Ther 2011;17:178–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87–107. [DOI] [PubMed] [Google Scholar]
- 4. Kurvers H, Daemen M, Slaaf D, et al. Partial peripheral neuropathy and denervation induced adrenoceptor supersensitivity. Functional studies in an experimental model. Acta Orthop Belg 1998;64:64–70. [PubMed] [Google Scholar]
- 5. Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol 2001;7:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou JJ, Yang G, Jin SJ, Tao LJ, Yu JQ, Jiang YX. Oxymatrine–carbenoxolone sodium inclusion compound induces antinociception and increases the expression of GABAAα1 receptors in mice. Eur J Pharmacol 2010;626:244–9. [DOI] [PubMed] [Google Scholar]
- 7. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 8. Dowdall T, Robinson I, Meert FT. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 2005;80:93–108. [DOI] [PubMed] [Google Scholar]
- 9. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Craparo EF, Bondì ML, Pitarresi G, Cavallaro G. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci Ther 2011;17:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


