Summary
Background and Purpose
Emerging evidence indicates that stimulating adult neurogenesis provides novel strategies for central nervous system diseases. Iptakalim (Ipt), a novel ATP‐sensitive potassium (K‐ATP) channel opener, has been demonstrated to play multipotential neuroprotective effects in vivo and in vitro. However, it remains unknown whether Ipt could regulate the adult neurogenesis.
Methods and Results
Based on the finding that adult neural stem cells (ANSCs) in hippocampus expressed Kir6.1/SUR1‐composed K‐ATP channel, Kir6.1 heterozygotic (Kir6.1+/−) mice were used to investigate whether and how Ipt regulates adult hippocampal neurogenesis. We showed that administration of Ipt (10 mg/kg) or fluoxetine (Flx, 10 mg/kg) for 4 weeks significantly increased newborn ANSCs in subgranular zone (SGZ) of Kir6.1+/+ mice but failed to affect those of Kir6.1+/− mice. Meanwhile, ANSCs in Kir6.1+/− mice exhibited decreased survival rate and impaired ability of differentiation into astrocytes. We further found that Kir6.1+/− mice showed lower level of brain‐derived neurotrophic factor (BDNF) in hippocampus compared with Kir6.1+/+ mice. Furthermore, Ipt increased the levels of BDNF and basic fibroblast growth factor (FGF‐2) throughout the hippocampus in Kir6.1+/+ mice but not in Kir6.1+/− mice. Moreover, Ipt and Flx enhanced the phosphorylation of Akt and CREB in the hippocampus of Kir6.1+/+ mice. Notably, these effects were completely abolished in Kir6.1+/− mice.
Conclusions
Our findings demonstrate that Ipt stimulates the adult hippocampal neurogenesis via activation of Akt and CREB signal following the opening of Kir6.1‐composed K‐ATP channels, which gives us an insight into the therapeutic implication of Ipt in the diseases with adult neurogenesis deficiency, such as major depression.
Keywords: Adult neurogenesis, Hippocampus, Iptakalim, Kir6.1‐composed K‐ATP channel
Introduction
Adult hippocampal neurogenesis is involved in the pathogenesis of various diseases including depression, schizophrenia, and stroke 1, 2. Developing dugs that enhance adult neurogenesis will be beneficial for the treatment of neuropsychological diseases. Adult neurogenesis has been clearly demonstrated in two regions under normal and pathological conditions: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus 3. The regulation of adult neurogenesis involves a number of factors including neurotransmitter systems, growth factors, transcription factors, stress, and others 1, 3, 4. These intrinsic and extrinsic factors interwove the microenvironment of SGZ and SVZ, which is known as the neurogenic niche. Glia, particularly astrocytes, and microglia play key roles in controlling adult neurogenesis within the niches 5. In vivo experiments demonstrated that astrcocytes exert an instructive role in fate determination of neural stem cells (NSCs) to neurons. The over‐activation of microglia is detrimental for neurogenesis, but new studies have demonstrated that unchallenged microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis 6. An emerging concept is that adult neural stem cells (ANSCs) are able to sense and respond to changes in energy homeostasis in brain 7. On the other hand, energy‐sensing molecules existed in the niche such as sirtuins, and hypoxia‐inducible factors (HIFs) have been found to play an important role in NSC biology 7. These studies intrigue the importance of the energy biosensors in regulating adult neurogenesis.
ATP‐sensitive potassium (K‐ATP) channels, the unique channels coupling energy metabolism to membrane excitability–dependent processes, are widely expressed in most metabolically active tissues throughout the body including brain regions 8 and are essential in distress resolution and energy balance 9. K‐ATP channel consists of two different subunits: the pore‐forming inward rectifier K+ channel member, Kir6.1 or Kir6.2, and the sulfonylurea receptor SUR1 or SUR2 that regulate sensitivity of the channels 10. Within the brain, Kir6.2 is predominantly expressed in neurons. It is generally agreed that Kir6.2 exerts critical roles on glucose sensing and adapting neuronal activity to metabolic demands 11. However, Kir6.1 is mainly expressed in astrocyte and microglia that can instruct adult neurogenesis 12, 13, 14. In addition, Kir6.1 had been found to regulate astrocyte and microglia function 14. Moreover, it has been proven that radial glia in development and specific subpopulations of astrocytes in adult mammals function as primary progenitors or NSCs 15, raising the potential functions of Kir6.1‐composed K‐ATP channels in ANSCs.
Iptakalim (Ipt) is a novel K‐ATP channel opener and can pass through the blood–brain barrier 16. Pharmacological, electrophysiological, biochemical studies, and receptor binding test demonstrate that Ipt can activate Kir6.1‐composed K‐ATP channels expressed in center and periphery tissue 17, 18. Our previous studies have demonstrated that Ipt exerts the neuroprotective effects in various animal models, such as stroke and Parkinson's disease (PD) 19, 20. Ipt exhibited an intrinsic neuroprotective effecet against astrocytic necrosis and apoptosis 21. In additon, Ipt had been found to protect astrocytes by reversing the dysfunction of connexin43 induced by rotenone 22. Even so, it remains unknown whether Ipt regulates adult neurogenesis. In the present study, therefore, Kir6.1 heterozygotic (Kir6.1+/−) mice were used to investigate the effect of Ipt on adult hippocampal neurogenesis so as to gain insight into the therapeutic implication of Ipt in the diseases with adult neurogenesis deficiency, such as depression and neurodegenerative disorders.
Materials and Methods
Animals and Reagents
Kir6.1+/− mice were made available via the NIH‐funded Mouse Mutant Regional Resource Center (MMRRC; http://www.mmrrc.org) for Material Transfer Agreement. These mice were subsequently mated to each other to generate homozygotes (Kir6.1−/− mice). Knockout of the gene‐encoding Kir6.1 was verified by PCR genotyping. C57BL/6 mice were used as wild‐type (Kir6.1+/+) controls. The Kir6.1+/+, Kir6.1−/−, and Kir6.1+/− mice were bred and maintained in the Animal Resource Centre of the Faculty of Medicine, Nanjing Medical University, with free access to standard chow and water in a room with an ambient temperature of 24 ± 1°C and a 12:12 h light/dark cycle. Age‐matched adult male mice (3‐month‐old) were used in the experiments. All experiments were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Iptakalim hydrochloride (Ipt), with a purity of 99.36%, was synthesized and provided by the Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences of China. Fluoxetine (Flx), with a purity of 99.9%, was obtained form Eli Lilly, Indianapolis, Indiana. Ipt and Flx were prepared in saline.
Neural Stem Cell Culture
Adult neural stem cells isolated from hippocampus of adult male mice were used to detect the subunits of K‐ATP channels. Isolation of mouse ANSCs was performed as described previously 23. In brief, tissues from the hippocampus of adult Kir6.1+/+ mice (2–3‐month‐old) were dissociated and cultured in the DMEM/F‐12 serum‐free medium with 2% B27, 20 ng/mL EGF, and 20 ng/mL bFGF. After culturing for 7 days, NSCs aggregated and formed spheroid‐like bodies, called neurosphere. At least three, independent cultures of neurospheres from three different adult mice were used for analysis.
Reverse Transcription‐Polymerase Chain Reaction
The mRNA of K‐ATP channels expressed on ANSCs were detected by reverse transcription‐polymerase chain reaction (RT‐PCR). Total RNA was extracted from neurospheres using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) followed by treatment with RNase‐free DNaseI (Invitrogen Life Technologies). Reverse transcription was performed using random hexamer primers. PCR primers were as follows: Kir6.1 forward: 5′‐CTGCAGGACATCTTCACCACCT‐3′, reverse: 5′‐GTTGATGATCAGACCCACGATG‐3′; Kir6.2 forward: 5′‐TAGGCCAAGCCAGTGTAGTG‐3′, reverse: 5′‐GTGGTGAACACATCCTGCAG‐3′. PCR cycles were as follows: Kir6.1 primers: 94°C, 4 min; 94°C, 30 second; 57°C, 30 second; 72°C, 30 second; 72°C, 5 min (27 cycles); Kir6.2 primers: 94°C, 4 min; 94°C, 30 second; 64°C, 30 second; 72°C, 30 second; 72°C, 5 min (33 cycles). Reaction products were then visualized on a 1.2% agarose gel containing 0.06 μg/mL ethidium bromide, and the resulting bands were confirmed using Molecular Imager FX (Bio‐RAD, Hercules, CA, USA).
Drug Treatment
We first examined whether Kir6.1‐composed K‐ATP channels were involved in regulating adult hippocampal neurogenesis. Individually housed Kir6.1+/+ and Kir6.1+/− or Kir6.1−/− C57 male mice (n = 5 for each groups) were allowed to acclimate for 1 week and then were intraperitoneally (i.p.) administered with Flx (10 mg/kg per day) dissolved in saline for 28 days. The concentration of Flx was consistent with previous research 24.
Next, we detected the potential regulatory capacity of Ipt on hippocampal neurogenesis and the possible molecular mechanism. As our previous studies have proven that treatment with Ipt at 10 mg/kg for 4 week exerted strong neuroprotective effect, Kir6.1+/+ and Kir6.1+/− mice (n = 14 for each groups) were administrated intraperitoneally (i.p.) with Ipt (10 mg/kg per day) or Flx (10 mg/kg per day) or vehicle (saline, 10 mL/kg) for 28 days.
Bromodeoxyuridine (BrdU) Labeling
Two hours after the last injection of Ipt and Flx, six mice of each group were given BrdU intraperitoneally (4 × 50 mg/10 mL/kg every 2 h). For analysis of cell proliferation (Figure 1A), 24 h after the last BrdU injection, six mice were transcardially perfused with a saline solution for 5 min and then followed with 4% paraformaldehyde dissolved in cold 0.01 mol/L phosphate buffer (PBS), and brains were processed for immunohistochemistry. For determination of ANSC phenotype and survival (Figure 1B), five mice were transcardially perfused 4 weeks after BrdU injection. Other mice were killed 24 h after the last drug or saline injection. The hippocampus were quickly isolated and frozen at −70°C.
Figure 1.
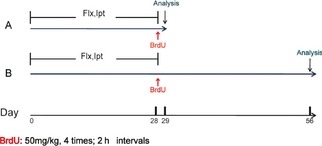
Schematic representation of the experimental procedure. (A) Fluoxetine and Iptakalim (10 mg/kg per day) were administered to mice once daily for 4 weeks. For the analysis of cell proliferation, mice received four times injection of bromodeoxyuridin (BrdU) (4 × 50 mg/kg, every 2 h) on the last day of the 4‐week drug treatment. Animals were killed 24 h after last BrdU administration. (B) To determine the survival and differentiation of newborn cells, mice were killed 4 weeks after the last BrdU administration.
Immunohistochemistry and Quantification of Staining
After perfusion, brains were dissected from the skull, postfixed overnight in buffered 4% paraformaldehyde at 4°C, stored in a 20% sucrose solution at 4°C for 3 days, and then changed into 30% sucrose solution until they sank. Serial sections of the brains were cut (30‐μm sections) through each entire hippocampus (from approximately −0.94 mm to −3.88 mm from bregma according to Paxinos and Franklin 25) using a freezing microtome. All sections were collected in six separate series. For immunohistochemistry and immunofluorescence, free‐floating sections were immunolabeled according to previous procedures 23. Briefly, after DNA denaturation and elimination of endogenous peroxidases (for immunofluorescence without this step), sections were placed in a blocking solution consisting of 5% bovine serum albumin (BSA) and 0.3% triton X‐100 in 0.01 mol/L PBS for 2 h. For BrdU immunohistochemistry, sections were incubated in anti‐mouse BrdU (1:2500; Millipore, Bedford, USA). For double‐labeling immunofluorescence, sections were incubated with anti‐rat BrdU (1:150; Millipore) and one of the following anti‐mouse NeuN (1:200; Millipore) or anti‐rabbit GFAP (1:2500; Abcam, Cambridge, UK) overnight at 4°C. After washes, secondary antibodies were applied for 1 h. Then, sections were washed with PBS, wet‐mounted, and later dried in the dark.
For visualization and photography, specimens were observed under a confocal microscope (Axiovert LSM510; Carl Zeiss Co., Jena, Germany). At least 100 BrdU‐labeled cells per animal were analyzed for double labeling.
Western Blotting Analysis
Protein lysates were prepared from the right lobes of hippocampus using Key Gene kit, and protein concentrations were quantified by BCA method (Biyuntian kit, Beyotime, Shanghai, China). A total of 40–100 μg of protein samples were separated on SDS‐PAGE gels and then transferred to PDVF membranes (Millipore). Blots were washed for 5 min in T‐BST and subsequently blocked in T‐BST (10% milk) for 1 h. Primary antibodies were then applied to blots overnight at 4°C, washed three times with T‐BST, and secondary antibodies applied for 1 h at room temperature. The primary antibodies were using rabbit anti‐CREB (1:1000), anti‐phospho‐CREB‐ser133 (1:1000; Cell Signaling Technology, Beverly, MA, USA); rabbit anti‐Akt(1:1000), anti‐phospho‐Akt‐ser473 (1:1000; Cell Signalling Technology); rabbit anti‐brain‐derived neurotrophic factor (BDNF) (1:200; Santa Cruz, Delaware, CA, USA); mouse anti‐FGF‐2(1:200; Santa Cruz). To determine the subunit composition of K‐ATP channels in ANSCs, samples of whole‐cell lysates of neurospheres were electrophoresed and transferred to nitrocellulose membranes. After blocking, membranes were incubated with different antibodies against Kir6.1, Kir6.2, SUR1, and SUR2 (1:100; Santa Cruz). The blots were incubated HRP‐conjugated secondary antibodies and signals detected by enhanced chemiluminescence (ECL) Western blotting detection reagents (Pierce, Rockford, IL, USA). The membranes were scanned and analyzed using an Omega 16ic Chemiluminescence Imaging System (Ultra‐Lum, Claremont, CA, USA).
Statistical Analysis
All values are reported as mean ± SEM. The significance of the difference between controls and samples treated with various drugs was determined by one‐way ANOVA test followed by the least significant difference (LSD) for post hoc comparisons using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The level of statistical significance is defined as P < 0.05 differences.
Results
Adult Neural Stem Cells Expressed Kir6.1/SUR1‐Composed K‐ATP Channels
Adult neural stem cells isolated from hippocampus of adult male mice were used to determine whether ANSCs expressed K‐ATP channels. The expression of K‐ATP channels were detected by RT‐PCR and Western blotting. As shown in Figure 2A, single‐band amplification product of Kir6.1 was observed in ANSCs by RT‐PCR detection. However, Kir6.2 was not detectable. Consistent with the results of RT‐PCR, Western blotting analysis confirmed that Kir6.1 and SUR1 were expressed in ANSCs. In contrast, Kir6.2 and SUR1A or SUR1B subunits could not be detected in ANSCs (Figure 2B).
Figure 2.
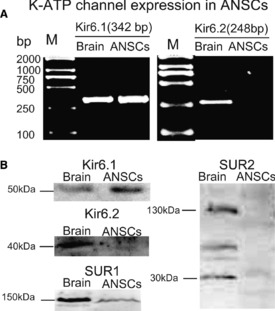
Adult neural stem cells (ANSCs) expressed Kir6.1/SUR1‐composed ATP‐sensitive potassium (K‐ATP) channels. (A) Reverse transcription‐polymerase chain reaction screening showed that neural stem cells expressed Kir6.1 but not Kir6.2 subunit of K‐ATP channels. (B) Kir6.1/SUR1 subunit was expressed in ANSCs. Western blotting was probed with antibodies to Kir6.1(51 kDa) and Kir6.2 (40 kDa) subunits as well as SUR1(150 kDa) and SUR2A (150 kDa) or SUR2B (30 kDa) subunits. At least three independent cultures of neurospheres from three different litters were used for analysis.
Administration of Iptakalim Enhanced the Proliferation of Adult Neural Stem Cells in Hippocampus
As ANSCs expressed Kir6.1, we examined whether Kir6.1‐composed K‐ATP channel would play a role in adult neurogenesis. The effect of Flx, which has been documented to enhance hippocampal neurogenesis in adult brain 26, on the proliferation of ANSCs in Kir6.1+/+, Kir6.1+/−, and Kir6.1−/− mice was first identified. Proliferating cells were labeled by BrdU injection after Flx and saline treatments. Under basal condition, levels of BrdU+ cells in the SGZ were similar among the Kir6.1+/+, Kir6.1+/−, and Kir6.1−/− mice. We further found that the number of proliferating cells in Kir6.1+/+ mice was significantly increased by 45% (P < 0.05) after Flx administration compared with those treated with saline (Figure 3A). However, Flx failed to induce an enhanced neurogenesis in either Kir6.1+/− or Kir6.1−/− mice (Figure 3A). These results indicated that Kir6.1 might be involved in the regulation of adult neurogenesis, and the properties of ANSCs were not significantly different between Kir6.1+/− and Kir6.1−/− mice. Considering Kir6.1−/− mice are prone to premature death and most die between 5 and 6 weeks after birth 27, we used the Kir6.1+/− mice for further studies.
Figure 3.
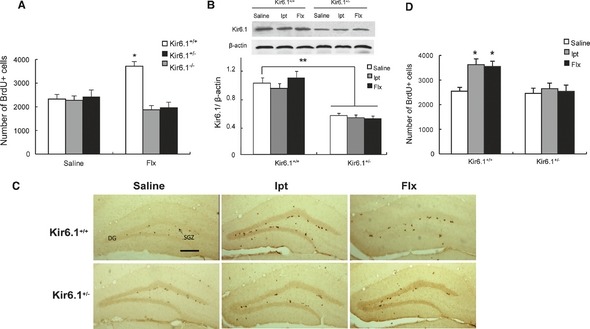
Flx or Ipt administration enhanced adult hippocampal neurogenesis. (A) Quantification of proliferating neural stem cells (NSCs) and neural progenitors in the Kir6.1+/+, Kir6.1+/−, and Kir6.1−/− mice injected with saline or Flx. The number of BrdU‐positive cells in the SGZ was calculated in the granular cell layer (GCL). (B) Expression of Kir6.1 in the hippocampus of Kir6.1+/+ and Kir6.1+/− mice. Western blottings of protein lysates from adult mouse hippocampus. (C) Coronal sections of adult WT and Kir6.1+/− mouse brains showed BrdU‐positive nuclei in the SGZ 24 h after BrdU injection. Scale bar = 200 μm. (D) Quantification of proliferating NSCs and neural progenitors in the WT and Kir6.1+/− mice injected with saline, Ipt, or Flx. n = 5 in A, n = 4 in B, n = 6 in D. Values represent mean ± SEM. *P < 0.05 as compared with Kir6.1+/+‐saline, **P < 0.01 as compared with Kir6.1+/+‐saline.
At the end of Ipt treatment, Western blotting analysis showed that Kir6.1 abundantly expressed in hippocampus of Kir6.1+/+ mice, but was 46% (P < 0.01) decreased in Kir6.1+/− mice (Figure 3B). Administration of Ipt or Flx for 4 weeks failed to affect Kir6.1 expression in the hippocampus of both Kir6.1+/+ and Kir6.1+/− mice. Similar to Flx, after Ipt treatment, Kir6.1+/+ mice tend to have more BrdU‐positive cells (P < 0.05) than Kir6.1+/− mice in the SGZ (Figure 3C, D). The results indicated that treatment with Ipt promoted the proliferation of ANSCs in SGZ via opening of Kir6.1‐composed K‐ATP channels.
Kir6.1+/− Mice Exhibited Defective Fate Determination of Adult Neural Stem Cells
The survival of the newborn ANSCs is an important issue in neurogenesis, and a majority of the newborn cells in SGZ undergo death by apoptosis early in their life 6. To determine whether Ipt could prolong the survival of NSCs, we cumulatively labeled the entire dividing NSC population in the SGZ with BrdU for 8 h, and BrdU‐positive cells in the DG were counted 1 month after the last BrdU injection. The mature cells were found throughout the DG (Figure 4A). Remarkably, under the basal condition, Kir6.1+/− mice displayed a significant reduction in the number of label‐retaining ANSCs compared with Kir6.1+/+ mice (P < 0.05, Figure 4A, B). Both Ipt and Flx treatments increased the survival BrdU+ cells in Kir6.1+/+ mice (P < 0.05, Figure 4B) but not in Kir6.1+/− mice. The survival rate of BrdU+ cells referred to the percentage of BrdU+ cells between 28 days and 24 h after the last BrdU administration. For the Kir6.1+/+ mice, neither Ipt nor Flx administration altered the survival rates compared with the saline. But the survival rates displayed a reduction in the Kir6.1+/− mice: (Kir6.1+/+‐Saline: 44.5%, Flx: 47.2%,Ipt:45.8%; Kir6.1+/−‐Saline: 36.1%, Flx: 35.5%, Ipt: 36.6%).
Figure 4.
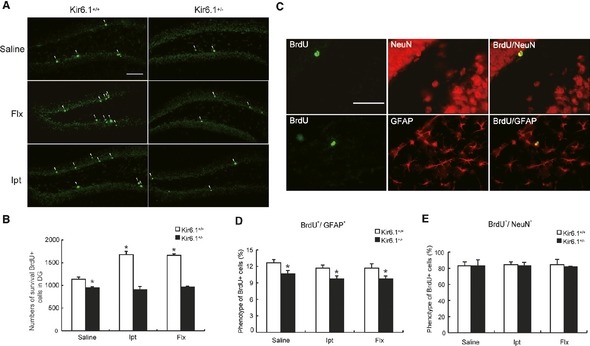
Survival and differentiation of BrdU + cells in the dentate gyrus. (A) Coronal sections of adult Kir6.1+/+ and Kir6.1+/− mice brains showed BrdU‐positive nuclei (bright green) in the SGZ 1 month after BrdU injection. White arrowhead: labeled survival neural stem cells (NSCs), Scale bar = 200 μm. (B) Quantification of survival NSCs in hippocampus. (C) Representative confocal microscopic images of BrdU‐positive nuclei (bright green) and neuronal nuclear antigen (NeuN; red) or glial fibrillary acidic protein (GFAP; red)‐labeled cells in the dentate granule cell layer. Scale bar = 100 μm. (D) The percentages of BrdU +/NeuN + did not differ significantly among various groups in both Kir6.1+/+ and Kir6.1+/− mice. (E) Kir6.1 haploinsufficiency significantly decreased the percentages of BrdU +/GFAP + cell in comparison with Kir6.1+/+ mice. n = 4 in each group. Values represent mean ± SEM. *P < 0.05 as compared with Kir6.1+/+‐saline group.
The survived ANSCs give rise to three types of progeny (neurons, astrocytes, and oligodendrocytes), and the proper balance between the three fates is pivotal for the functionality of the NSC pool 28. The differentiation of labeled cells was determined 4 weeks after drug or saline treatment by colocalization of neuronal and glial phenotypic markers in BrdU‐labeled cells. Therefore, the phenotype of survival BrdU+ cells was determined by BrdU+/NeuN+ or BrdU+/GFAP+ double immunofluorescent labeling (Figure 4C). Confocal microscopy, using z‐plane sections to confirm colocalization for each cell, revealed that the majority of BrdU‐positive cells were neuronal (~80%) and no significant difference in the percentage of BrdU+/NeuN+ was observed in various groups (Figure 4E). The level of BrdU+/GFAP+ colabeled cells in the DG of Kir6.1+/− mice was significantly lower than that of Kir6.1+/+ mice (P < 0.05, Figure 4D). Furthermore, there was no differences in the percentages of the BrdU+/GFAP+ cells after Ipt or Flx treatment. These data indicated that Ipt treatment could not affect the differentiation of ANSCs, but Kir6.1 haploinsufficiency resulted in a deficiency in the ability of ANSCs to generate astrocytes. The remaining cells that labeled with neither neuron nor astrocyte marker might represent quiescent undifferentiated cells 29. The results further suggested that the fate balance of ANSCs was disturbed in Kir6.1+/− mice.
Iptakalim Administration Increased the Expression of BDNF and FGF‐2 in Mouse Hippocampus through the Akt–CREB Signaling
To understand the basic mechanisms of Ipt in the stimulation of adult hippocampal neurogenesis, we focused on neurotrophic factors including BDNF and FGF‐2. As shown in Figure 5A, the level of BDNF expression in Kir6.1+/− mice hippocampus was significantly lower compared with Kir6.1+/+ mice (P < 0.05). Administration of Flx and Ipt significantly up‐regulated the protein levels in the hippocampus of the Kir6.1+/+ mice (P < 0.01). In contrast, in the Kir6.1+/− mice, Ipt treatment failed to the level of BDNF in hippocampus while the effect of Flx was partially suppressed (P < 0.05). As the 18‐kDa isoform of FGF‐2 regulates the NSCs' survival 30, we analyzed the 18‐kDa FGF‐2 by Western blotting. We found that the enhancement of FGF‐2 expression in the hippocampus of Kir6.1+/+ mice after Ipt and Flx administration (P < 0.05) was completely halted in Kir6.1+/− mice (Figure 5B). No significant difference was found between the two drug‐treated groups. These results indicated that Ipt might promote the BDNF and FGF‐2 production in the hippocampus via opening the Kir6.1‐composed K‐ATP channels.
Figure 5.
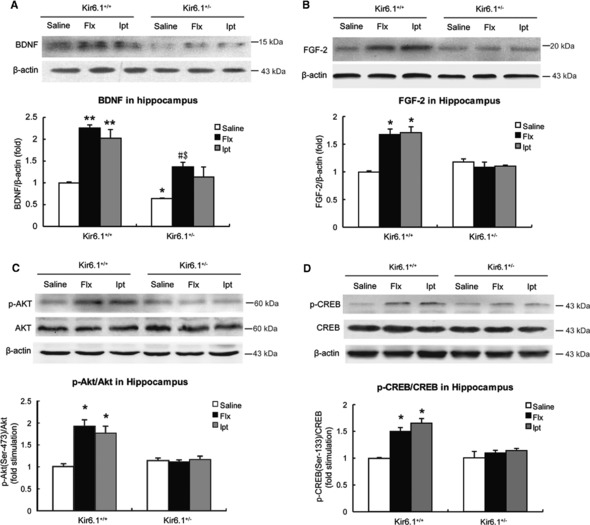
Ipt up‐regulated the expression of brain‐derived neurotrophic factor (BDNF) and FGF‐2 in adult hippocampus involving the Akt‐CREB signaling. (A, B) The expression of BDNF and FGF‐2 in the hippocampus of Kir6.1+/− mice compared with Kir6.1+/+ mice after 4‐week Ipt and Flx treatment. Representative Western blotting images of BDNF, FGF‐2, and β‐actin of control. (C, D) Tissue lysates were analyzed by Western blotting for activated Akt and CREB levels in the hippocampus of Kir6.1+/− mice compared with Kir6.1+/+ mice. The blot was probed with Ser473 phosphorylated Akt antibody and Ser133‐phosphorylated CREB antibody. n = 4 in each group. Values represent mean ± SEM. *P < 0.05 as compared with Kir6.1+/+‐saline; **P < 0.01 as compared with Kir6.1+/+‐saline; # P < 0.05 as compared with Kir6.1+/−‐saline group; $ P < 0.05 as compared with Kir6.1+/+‐Flx group.
To further investigate the possible intracellular molecular pathways underlying Ipt‐induced neurotrophic factor up‐regulation, we analyzed the activation of Akt and CREB, which are important for cell survival and plasticity. Phosphorylation of the hydrophobic C‐terminal domain (serine 473) is necessary for the full activation of Akt; therefore, we detected p‐Akt (Ser 473) in the present study. Similar to the effect of Flx, 4‐week administration of Ipt strongly increased the phosphorylation of Akt (P < 0.05, Figure 5C) in the hippocampus of Kir6.1+/+ mice, but not in the Kir6.1+/− mice. As transcriptional activity for CREB mainly depends on its phosphorylation in Ser133, the hippocampal phospho‐CREB (p‐CREB) was measured using an antibody directed against p‐CREB (Ser133). Parallel to the activation of Akt, p‐CREB was significantly increased by 63% in the hippocampus of Kir6.1+/+ mice after Ipt administration (P < 0.05, Figure 5D) while Kir6.1 haploinsufficiency abolished the effect of both Ipt and Flx. These results demonstrated that opening Kir6.1/K‐ATP channel by Ipt triggered the activation of CREB and Akt in adult hippocampus.
Discussion
In the present study, we showed that administration of Ipt for 4 weeks enhanced adult hippocampal neurogenesis via opening Kir6.1‐composed K‐ATP channels expressed in the ANSCs. Kir6.1+/− mice exhibited reduced response to Ipt in ANSC proliferation, survival, and differentiation. Our findings indicate that Ipt potentiates the adult hippocampal neurogenesis via activation of Akt and CREB signal following the opening of Kir6.1‐composed K‐ATP channels.
The functional diversity of K‐ATP channels in the brain is broad and important for brain function. Kir6.2 are expressed in most neurons while Kir6.1 are mainly expressed in the cells contributing to the NSC niche, such as astrocytes, endothelial cells, as well as microglia 31. Notably, our results identified that ANSCs expressed Kir6.1/SUR1‐composed K‐ATP channels. This finding raises the possibility that Kir6.1‐composed K‐ATP channel plays a critical role in adult neurogenesis.
Regulation of neurogenesis occurs at multiple stages, including cell proliferation, survival, and differentiation. It is well known that treatment with fluoxetine stimulates the proliferation of NSCs in adult hippocampus 32. In this study, we found that the enhancement of adult neurogenesis induced by fluoxetine was abolished in Kir6.1+/− mice. Similarly, Ipt, a blood–brain barrier–permeable K‐ATP channel opener, significantly increased the proliferation of ANSCs in the hippocampus of Kir6.1+/+ mice but not in Kir6.1+/− mice. As Kir6.1+/− mice exhibited less‐survived ANSCs, it may ultimately result in the depletion of adult NSC pool later in life. Furthermore, the number of GFAP+ cells in Kir6.1+/− mice decreased as compared with Kir6.1+/+ mice, but the ratio of labeled NeuN+ cells was similar in various groups. It implies that lack of Kir6.1 resulted in the defect of astrocyte differentiation. Obviously, Kir6.1‐forming K‐ATP channels are involved in the regulation of proliferation and fate determination of adult ANSCs. As chronic administration of Ipt and Flx failed to affect the protein level of Kir6.1, the effect of Ipt on ANSC proliferation may result from the activation of Kir6.1‐composed K‐ATP channel. K+ channels are known to regulate proliferation in many cell types including fibroblasts and lymphocytes 33. Activation of K+ channels occurs prior to DNA synthesis in hematopoietic cells as well as in neural cells 34. Harmeet M. et al. reported that K‐ATP channel openers (minoxidil, cromakalim, and pinacidil) increased mitogenically induced proliferation in primary rat hepatocytes 35. Furthermore, opening of Kir6.1 on capillary endothelial cells increases the synthesis of NO and the permeability of the capillary wall 36. The functions of astrocytes including gap junction, lactate metabolism, and glutamate uptake are modulated by the Kir6.1 14, 37. Thus, loss of Kir6.1 resulted in a deficiency of ANSCs to generate astrocytes. It suggests that the unique biological function of K‐ATP channels determines its importance and necessity for adult neurogenesis. Notably, chronic treatment of Flx failed to increase the proliferation of ANSCs in both Kir6.1−/− and Kir6.1+/− mice, which suggests that the lack of Kir6.1 is detrimental to the neurogenic niche formation. On the other hand, no direct evidence showed that Flx could affect the function of Kir6.1, but we recently demonstrated that both Flx and Ipt could inhibit inflammation (data not shown). These findings suggest that Flx may share a common downstream mechanism in regulating adult neurogenesis.
Current experiments demonstrated that Ipt exerted its function via regulating the neurotrophic factors including BDNF and FGF‐2. BDNF is shown to play a role in neurogenesis as heterozygous BDNF knockout mice (BDNF+/−) display reduced levels of adult hippocampal neurogenesis 38. FGF‐2 mediates a variety of cellular responses during embryonic development and in the adult organism, including the proliferation and survival of NSCs 39. It has been reported that administration of FGF‐2 enhanced rat hippocampal neurogenesis. On the other hand, lacking the FGF‐2 receptor displayed defects in hippocampal neurogenesis 40. We noted that Ipt treatment increased the expression of BDNF and FGF‐2 in the hippocampus of adult Kir6.1+/+ mice but not in Kir6.1+/− mice. The neurotrophic factors bind to the receptor tyrosine kinases 41 and induce the activation of the signaling molecules such as PI3K/Akt 42. Akt is capable of phosphorylating and activating CREB 43 that can induce the expression of classes of genes to regulate adult neurogenesis 44. We found that chronic Ipt administration led to a significant increase in the phosphorylation of Akt and CREB in adult hippocampus of Kir6.1+/+ mice but not in Kir6.1+/− mice, indicating that Ipt activates PI3k‐Akt and CREB pathways because of the opening of Kir6.1‐composed K‐ATP channels. So we surmise that Ipt opens the Kir6.1‐composed K‐ATP channels expressed in ANSCs, astrocytes, and microglia, which in turn couple cellular metabolism to membrane potential. As Akt appears to be common to signaling pathways that mediate the energy metabolism 45, opening of Kir6.1 results in stimulation of Akt. The transcriptional activity of CREB was enhanced by Akt signals and then induced the expression of NGFs such as FGF‐2 and BDNF. Meanwhile, NGFs binding with their ligands also induce the activation of Akt pathways 7, which can produce a positive feedback effect.
In conclusion, our study demonstrates that ANSCs express Kir6.1‐composed K‐ATP channels that play important role in the regulation of adult hippocampal neurogenesis. As a novel K‐ATP channel opener, Ipt potentiates the adult hippocampal neurogenesis via regulating BDNF and FGF‐2 expression and enhancing the Akt–CREB signaling. These findings give us an insight into the therapeutic implication of Ipt in the diseases with adult neurogenesis deficiency, such as depression and neurodegenerative disorders.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The work reported herein was supported by grants from the National Key Program of Basic Research of China (2009CB521906 and 2011CB504103) and the National Natural Science Foundation of China (81030060).
References
- 1. Balu DT, Lucki I. Adult hippocampal neurogenesis: Regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev 2009;33: 232–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011;476: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132: 645–660. [DOI] [PubMed] [Google Scholar]
- 4. Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 2009;25: 253–275. [DOI] [PubMed] [Google Scholar]
- 5. Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: Cellular niches for adult neurogenesis. Curr Opin Neurobiol 2005;15: 514–520. [DOI] [PubMed] [Google Scholar]
- 6. Sierra A, Encinas JM, Deudero JJ, et al. Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell 2010;7: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol 2011;93: 182–203. [DOI] [PubMed] [Google Scholar]
- 8. Dunn‐Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP‐sensitive K+ channel in rat brain. Brain Res 1998;814: 41–54. [DOI] [PubMed] [Google Scholar]
- 9. Olson TM, Terzic A. Human K(ATP) channelopathies: Diseases of metabolic homeostasis. Pflugers Arch 2010;460: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bryan J, Vila‐Carriles WH, Zhao G, Babenko AP, Aguilar‐Bryan L. Toward linking structure with function in ATP‐sensitive K+ channels. Diabetes 2004;53(Suppl 3): S104–S112. [DOI] [PubMed] [Google Scholar]
- 11. Thomzig A, Laube G, Pruss H, Veh RW. Pore‐forming subunits of K‐ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol 2005;484: 313–330. [DOI] [PubMed] [Google Scholar]
- 12. Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G. Iptakalim alleviates rotenone‐induced degeneration of dopaminergic neurons through inhibiting microglia‐mediated neuroinflammation. Neuropsychopharmacology 2007;32: 2570–2580. [DOI] [PubMed] [Google Scholar]
- 13. Walton NM, Sutter BM, Laywell ED, et al. Microglia instruct subventricular zone neurogenesis. Glia 2006;54: 815–825. [DOI] [PubMed] [Google Scholar]
- 14. Sun XL, Hu G. ATP‐sensitive potassium channels: A promising target for protecting neurovascular unit function in stroke. Clin Exp Pharmacol Physiol 2010;37: 243–252. [DOI] [PubMed] [Google Scholar]
- 15. Kriegstein A., Alvarez‐Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009;32: 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Zhang YL, Tang XC, Feng HS, Hu G. Targeting ischemic stroke with a novel opener of ATP‐sensitive potassium channels in the brain. Mol Pharmacol 2004;66: 1160–1168. [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Hu J, Chen YP, Takeo T, et al. Iptakalim modulates ATP‐sensitive K(+) channels in dopamine neurons from rat substantia nigra pars compacta. J Pharmacol Exp Ther 2006;319: 155–164. [DOI] [PubMed] [Google Scholar]
- 18. Gao S, Long CL, Wang RH, Wang H. K(ATP) activation prevents progression of cardiac hypertrophy to failure induced by pressure overload via protecting endothelial function. Cardiovasc Res 2009;83: 444–456. [DOI] [PubMed] [Google Scholar]
- 19. Zhang S, Ding JH, Zhou F, Wang ZY, Zhou XQ, Hu G. Iptakalim ameliorates MPP+‐induced astrocyte mitochondrial dysfunction by increasing mitochondrial complex activity besides opening mitoK(ATP) channels. J Neurosci Res 2009;87: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 20. Wang S, Hu LF, Yang Y, Ding JH, Hu G. Studies of ATP‐sensitive potassium channels on 6‐hydroxydopamine and haloperidol rat models of Parkinson's disease: Implications for treating Parkinson's disease? Neuropharmacology 2005;48: 984–992. [DOI] [PubMed] [Google Scholar]
- 21. Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G. ATP‐sensitive potassium channel opener iptakalim protects against MPP‐induced astrocytic apoptosis via mitochondria and mitogen‐activated protein kinase signal pathways. J Neurochem 2007;103: 569–579. [DOI] [PubMed] [Google Scholar]
- 22. Zhang S, Liang R, Zhou F, Huang X, Ding JH, Hu G. Reversal of rotenone‐induced dysfunction of astrocytic connexin43 by opening mitochondrial ATP‐sensitive potassium channels. Cell Mol Neurobiol 2011;31: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G. Requirement of AQP4 for antidepressive efficiency of fluoxetine: Implication in adult hippocampal neurogenesis. Neuropsychopharmacology 2009;34: 1263–1276, 224. [DOI] [PubMed] [Google Scholar]
- 24. Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. Blockade of CRF(1) or V(1b) receptors reverses stress‐induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 2004;9: 278–286. [DOI] [PubMed] [Google Scholar]
- 25. Franklin KAGP. The mouse brain in stereotaxic coordinates, Second edition San Diego: Academic Press, 1997. [Google Scholar]
- 26. Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 2006;103: 8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 2002;8: 466–472. [DOI] [PubMed] [Google Scholar]
- 28. Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 2009;5: 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20: 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eiselleova L, Matulka K, Kriz V, Kunova M, Schmidtova Z, Neradil J. A complex role for FGF‐2 in self‐renewal, survival, and adhesion of human embryonic stem cells. Stem Cells 2009;27: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng KE, Schwarzer S, Duchen MR, Tinker A. The intracellular localization and function of the ATP‐sensitive K+ channel subunit Kir6.1. J Membr Biol 2010;234: 137–147. [DOI] [PubMed] [Google Scholar]
- 32. David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I. Neurogenesis‐dependent and ‐independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009;62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deutsch C. K+ channels and mitogenesis. Prog Clin Biol Res 1990;334: 251–271. [PubMed] [Google Scholar]
- 34. Partiseti M, Korn H, Choquet D. Pattern of potassium channel expression in proliferating B lymphocytes depends upon the mode of activation. J Immunol 1993;151: 2462–2470. [PubMed] [Google Scholar]
- 35. Malhi H, Irani AN, Rajvanshi P, Suadicani SO, Spray DC, McDonald TV, Gupta S. KATP channels regulate mitogenically induced proliferation in primary rat hepatocytes and human liver cell lines. Implications for liver growth control and potential therapeutic targeting. J Biol Chem 2000;275: 26050–26057. [DOI] [PubMed] [Google Scholar]
- 36. Mederos y Schnitzler M, Derst C, Daut J, Preisig‐Muller R. ATP‐sensitive potassium channels in capillaries isolated from guinea‐pig heart. J Physiol 2000;2: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parsons MP, Hirasawa M. ATP‐sensitive potassium channel‐mediated lactate effect on orexin neurons: Implications for brain energetics during arousal. J Neurosci 2010;30: 8061–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee J, Duan W, Mattson MP. Evidence that brain‐derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 2002;82: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 39. Mudo G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF‐2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm 2009;116: 995–1005. [DOI] [PubMed] [Google Scholar]
- 40. Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor‐1 is required for long‐term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry 2007;62: 381–390. [DOI] [PubMed] [Google Scholar]
- 41. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103: 211–225. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by NGF and BDNF against neurotoxin‐exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3‐kinase and MAPK pathways. Neurochem Res 2009;34: 942–951. [DOI] [PubMed] [Google Scholar]
- 43. Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up‐regulates Bcl‐2 expression through cAMP‐response element‐binding protein. J Biol Chem 2000;275: 10761–10766. [DOI] [PubMed] [Google Scholar]
- 44. Kowalczyk A, Filipkowski RK, Rylski M, et al. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol 2004;167: 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 2002;13: 444–451. [DOI] [PubMed] [Google Scholar]


