Summary
Background
Fucoidan, a sulfated polysaccharide extracted from brown algae, possesses potent antiinflammatory effects.
Aims
To examine the effect of fucoidan treatment on inflammation‐mediated dopaminergic neuronal damage and its potential mechanisms.
Methods
Microglial activation and injury of dopaminergic neurons were induced by intranigral injection of lipopolysaccharide (LPS), and the effects of fucoidan treatment on animal behavior, microglial activation and survival ratio of dopaminergic neurons were investigated. We further observed the efficacy of fucoidan on tumor necrosis factor‐alpha (TNF‐α) and the production of reactive oxygen species (ROS) in LPS‐activated primary microglia.
Results
Fucoidan significantly improved the behavioral manifestation, prevented the loss of dopaminergic neurons and inhibited the deleterious activation of microglia in the substantia nigra pars compacta of LPS‐treated rats. Further in vitro experiments indicated that the excessive production of TNF‐α and ROS in LPS‐induced primary microglia were significantly inhibited by fucoidan administration.
Conclusion
This is the first study to demonstrate that fucoidan possesses neuroprotective effects on injured dopaminergic neurons in a LPS‐induced animal model of Parkinson's disease. The mechanisms underlying these effects may include its potent down‐regulation of intracellular ROS and subsequent proinflammatory cytokine release in LPS‐activated microglia.
Keywords: Fucoidan, Microglia, Parkinson's disease, Reactive oxygen species, Tumor necrosis factor‐alpha
Introduction
Parkinson's disease (PD) is a progressive neurological disorder characterized by the loss of dopamine in the striatum, which occurs mainly because of the death of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Although the mechanism underlying neuronal degeneration remains unknown, the progressive nature of PD is characterized by chronic neuroinflammation, which induces dopaminergic neurodegeneration within the SNpc 1. Microglial activation, a hallmark of brain inflammation, involves a change in their morphology and release of various cytotoxic mediators, such as nitric oxide (NO), tumor necrosis factor‐alpha (TNF‐α), interleukin‐1β (IL‐1β), prostaglandin E2 (PGE2), and reactive oxygen species (ROS). Overproduction of these mediators is toxic to neurons and results in a self‐propagating cycle of neuronal death 2.
Brown alga is a common food all over the world, especially in Asia. It has been described in Traditional Chinese Medicines for over 1000 years. Fucoidan (sulfated fucans) represents a class of fucose‐enriched sulfated polysaccharides that are found in the extracellular matrix of brown algae. Fucoidan exerts many different biological actions, including antiinflammatory and antioxidative effects 3. Recent evidence suggests that fucoidan possess potent neuroprotective effects. Jhamandas et al. 4 showed that fucoidan protects against amyloid β (Aβ)‐induced neurotoxicity in basal forebrain neuronal cultures. We previously showed that fucoidan has neuroprotective effects on dopaminergic neurons in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) animal model of PD 5. Many recent reports have indicated that fucoidan regulates release of proinflammatory cytokines from microglial cell lines. Do et al. 6 observed that fucoidan suppressed NO production and inducible nitric oxide synthase (iNOS) expression in TNF‐α‐ and interferon (IFN)‐γ‐stimulated C6 glioma cells. Park et al. 7 also reported that fucoidan treatment significantly inhibited excessive production of NO and PGE2 and attenuated expression of iNOS, cyclooxygenase (COX)‐2, monocyte chemoattractant protein‐1 (MCP‐1), IL‐1β, and TNF‐α in lipopolysaccharid (LPS)‐stimulated BV2 microglia. The aim of the present study was to investigate whether fucoidan protects dopaminergic neurons from inflammation‐mediated damage in a classic PD inflammatory rat model induced by an intranigral injection of LPS and to study the possible mechanisms involved in primary cultured microglia activated by LPS in vitro.
Methods
Source of Fucoidan
Fucoidan was provided by Professor Quan‐Bin Zhang (Institute of Oceanology, Chinese Academy of Sciences) and was prepared as previously described 8. Neutral monosaccharide analysis of fucoidan showed that fucose is the main component with a molar ratio to galactose of 1.0:0.24. The average molecular weight of fucoidan was determined to be 7000 Da by high‐performance steric exclusion chromatography analysis 9. The backbone of the main fraction of fucoidan is shown in the Data S1. Prior to use in the experiments described below, fucoidan was dissolved in physiological saline to the desired concentrations.
Animals and Treatment
Sixty adult male Sprague–Dawley rats weighing 250–300 g were supplied by the Vital River Laboratory Animal Technology Co. Ltd (Beijing, China) and housed with a standard 12‐h on/off light cycle with food and water ad libitum in their home cages. Rats were randomly divided into four groups: a sham‐operated group, an LPS‐injected group after vehicle treatment, and LPS‐injected groups receiving intraperitoneal injections with 7.5 and 15 mg/kg fucoidan for 3 days. LPS (5 mg/mL, 2.0 μL) was injected into the right SNpc following a previously described protocol 10. After LPS injection, rats were continuously treated with fucoidan for 21 days, and the total experimental period persisted for 24 days. All animal experimental procedures were performed in strict accordance with protocols that were approved by the Committee on Animal Care and Usage (Capital Medical University), and all efforts were made to minimize animal suffering.
Evaluation of the Rotational Behavior of Rats
To examine the rotational behavior induced by apomorphine, rats were placed into cylinders that were attached to a rotameter (Columbus Instruments, Columbus, OH, USA) on the second day after the final fucoidan injection. The rats were allowed to adapt to the testing environment for 10 min and were injected hypodermically with 0.5 mg/kg apomorphine (Sigma‐Aldrich, St. Louis, MO, USA) dissolved in physiological saline. Measurement of rotational activity began 5 min after injection and lasted for 30 min under minimal external stimuli. The rotameter recorded the number of full clockwise and counter‐clockwise turns the animals performed during the testing period. Clockwise turns (ipsilateral to LPS injection) were counted as positive turns, and counter‐clockwise turns (contralateral to LPS injection) were counted as negative turns. The net number of turns performed during the entire 30‐min testing period was counted.
Tissue Collection and Processing
On the second day after the rotational behavior assay, 8–11 rats were randomly selected from each group for morphological studies. Rats were deeply anesthetized with chloral hydrate. Brains were removed and postfixed. Frozen sections were cut into 35‐μm‐thick sections and processed for immunohistochemistry as described below. All other rats were decapitated, and the bilateral ventral mesencephalon was dissected quickly and stored at −80°C; these were used for the determination of tyrosine hydroxylase (TH) levels by Western blot analysis.
Immunohistochemical Staining of TH and CD11b
Every sixth section of the SN (bregma −4.8 to −6.3 mm) was stained with primary antibody against neuronal TH (1:2000 dilution; Chemicon, Billerica, MA, USA). Adjacent sections were immunostained for detection of the microglial marker CD11b (1:400 dilution; Sigma‐Aldrich). After being perforated, the cell membranes with 0.3% Triton‐X 100 (this step was not required for CD11b staining) and blocked with 2% horse serum, sections were incubated with primary antibodies for 24 h at 4°C. Then, the antibody was detected using an ABC Elite kit (Vector laboratories, Burlingame, CA, USA) with 3,3′‐diaminobenzidine (DAB) and nickel enhancement. The number of TH‐positive neurons in the SN was counted using a microscope (Olympic, Osaka, Japan) and analyzed using an advanced image analysis system (MetaMorph, Universal Imaging Corp, Westchester, PA, USA). The survival rate of TH‐positive neurons in the SN was determined by counting the number of TH‐positive neurons on LPS‐injected side relative to the number of TH‐positive neurons on the noninjected side. The average optic density value in the SNpc of each CD11b‐stained section was determined using an image analysis system. All sections were coded and examined blindly.
Western Blot Analysis
Cellular proteins were extracted from the ventral mesencephalon using an extraction buffer (Beyotime Company, Jiangsu, China). Tissues were homogenized in this buffer using a Fisher model 100 sonic dismembrator and put on ice for 1 h. Soluble extracts were separated by centrifugation at 13,362 × g for 5 min at 4°C. Equal amounts of protein samples (20 μg) were mixed with loading buffer (Beyotime Company), boiled for 5 min, resolved on SDS‐polyacrylamide gels, and transferred to nitrocellulose filters (Millipore, Bedford, MA, USA) using a semidry blotting apparatus (Bio‐Rad Laboratories, Hercules, CA, USA). After blocking with a solution containing 5% nonfat milk, the filters were incubated with TH (1:1000; Boehringer‐Mannheim, Indianapolis, IN, USA) or GAPDH (1:10,000; Sigma‐Aldrich) antibodies. The filters were incubated with IRDye 800‐labelled secondary antibody (1:10,000; Rockland Immunochemicals, Gilbertsville, PA, USA). The signal was visualized using the Odyssey infrared imaging system according to the manufacturer's instructions (LI‐COR instrument, Lincoln, NE, USA). The density of each band was determined using image software (LI‐COR Biosciences, Lincoln, NE, USA).
Quantification of TNF‐α by ELISA and Semiquantitative RT‐PCR
Microglia were isolated and purified from whole brain of neonatal (1‐day‐old) Sprague–Dawley rats as we previously described 8. After being seeded onto 96‐well plates or six‐well plates and cultured for 24 h, microglial cells were treated as described in our previous publication 8. In brief, cells were preincubated with fucoidan (31.25, 62.5, and 125 μg/mL) for 10 min, and then 0.01 μg/mL LPS was added to the medium. After 6 h, TNF‐α secretion was measured using the Rat TNF‐α Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's guidelines. The 96‐well plates were read at 450 nm using a microplate reader (Bio‐Rad Company). TNF‐α mRNA levels were determined by semiquantitative RT‐PCR 6 h after LPS treatment. The following primer sequences were used on the basis of a published article 10: TNF‐α 5′‐ CAC GCT CTT CTG TCT ACT GA ‐3′ (sense) and 5′‐ GGA CTC CGT GAT GTC TAA GT ‐3′ (antisense); and GAPDH 5′‐TCC CTC AAG ATT GCT AGC AA‐3′ (sense) and 5′‐AGA TCC ACA ACG G AT ACA TT‐3′ (antisense).
Measurement of Intracellular ROS
The presence of intracellular ROS was measured using a nonfluorescent dye, 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA; Sigma‐Aldrich). Microglial cells seeded onto culture slides or 96‐well plates were preincubated with fucoidan (31.25, 62.5, and 125 μg/mL) for 10 min before 0.01 μg/mL LPS or normal medium was added. After 2 h, the cultures were washed with PBS and loaded with 10 μm of DCFH‐DA in serum‐free DMEM/F12 for 30 min at 37°C. After washing out excess probe, the cells on culture slides were observed by fluorescence microscopy. The fluorescence intensity of the 96‐well plates was measured on a fluorescent plate reader (Molecular Device, Sunnyvale, CA, USA) at 485 nm for excitation and 530 nm for emission.
Statistical Analysis
Data are expressed as means ± SD. The statistical significance of differences between the groups was determined using a one‐way analysis of variance (ANOVA) followed by the Tukey–Kramer test. A P value < 0.05 was considered to represent statistical significance.
Results
Fucoidan Improves Behavioral Deficits and Prevents TH‐Positive Neuronal Loss Induced by LPS Treatment
As shown in Figure 1A, the apomorphine‐induced rotational cycles of sham‐operated animals were only 1.8 ± 2.3 turns/30 min. LPS‐treated animals exhibited significantly more apomorphine‐induced rotational cycles (88.9 ± 38.3 turns) during the experimental period. However, rats treated with 7.5 and 15 mg/kg fucoidan, the optimal doses determined in our previous work 5, for 24 days, showed significant decreases in the numbers of apomorphine‐induced rotational cycles to about 29.4 ± 23.9 turns/30 min (P < 0.001) and 10.3 ± 11.1 turns/30 min (P < 0.001), respectively. Representative microphotographs of TH immunohistochemical staining in the SNpc are shown in Figure 1B. The numbers of TH‐positive neurons on the ipsilateral and contralateral sides to the injection were similar in sham‐operated animals. Rats that received vehicle treatment after an LPS intranigral injection showed a marked loss of TH‐positive neurons and their dendrites. However, fucoidan (7.5 and 15 mg/kg) treatment significantly recovered this loss of nigral TH immunoreactivity. The ratio of dopaminergic neuron survival on the LPS‐injected side to the noninjected side is shown in Figure 1C. An intranigral injection of LPS reduced the number of TH‐positive neurons to 0.5 ± 0.2 of the noninjected side. The survival of TH‐positive neurons in the SNpc was reserved to 0.8 ± 0.2 (P < 0.001) and 0.8 ± 0.1 (P < 0.001) of the left side in LPS‐injected rats treated with 7.5 and 15 mg/kg fucoidan, respectively. TH protein expression was also analyzed by immunoblotting (Figure 1D) and quantified using GAPDH as an internal protein level control. LPS treatment markedly reduced the levels of nigral TH protein on the injected side compared with that in the control group (P < 0.01). Normalized fluorometric results revealed that fucoidan treatment restored the TH levels in LPS‐treated rats (P < 0.05). These results suggested that fucoidan treatment not only protected TH‐positive neurons but also reserved TH expression in LPS‐treated animals.
Figure 1.
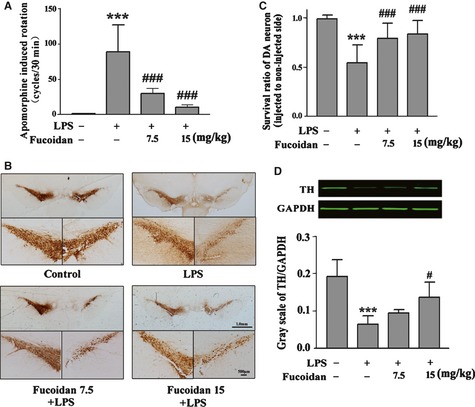
Fucoidan improves behavioral deficits and prevents dopaminergic neuronal damage induced by LPS treatment. (A) Rats were randomly grouped and pretreated with fucoidan (7.5 and 15 mg/kg) or vehicle 3 days before LPS injection and subsequently for 21 days after LPS injection (24 days in total). On day 25, rats were injected hypodermically with apomorphine (5 mg/kg) to induce rotational behavior. The total numbers of turns were counted using a computerized rotameter for 30 min, with the numbers regarded as an index of the degree of lesion. n = 12–15. (B) Photomicrographs of representative substantia nigra sections with staining of both the noninjected and injected sides using an antibody against tyrosine hydroxylase (TH). Scale bars represent 1.0 mm and 500 μm, respectively. (C). Survival ratio of TH‐positive neurons on the injected side to the noninjected side in the substantia nigra pars compacta (SNpc). n = 8–11. (D) Fucoidan treatment reserved the LPS‐induced decrease in TH protein expression in the SNpc on the injected sides, as detected by Western blot analysis. The ratio of gray scale of TH to GAPDH was calculated. n = 4. The data are shown as means ± SD. ***P < 0.001 compared with the control group, #P < 0.05 and ###P < 0.001 compared with the LPS group.
Fucoidan Inhibited LPS‐Induced Microglia Activation
Immunocytochemical staining of CD11b was used to evaluate microglial activation and morphological changes during neurodegenerative inflammation. As shown in Figure 2A, the cells in the SNpc of sham‐operated animals were ramified resting microglia with two or three fine processes. After LPS injection, activated microglia were readily identifiable throughout the SNpc by their thicker processes and more rounded cell bodies. However, pretreatment with fucoidan (7.5 and 15 mg/kg) before LPS injection significantly inhibited the activation of microglia, with more microglia exhibiting the ramified morphology than in the vehicle‐treated group. The average optical density value was determined to represent the CD11b expression level in the SNpc (Figure 2B). The CD11b content increased significantly in the LPS‐injected vehicle‐treated group (P < 0.001). After treatment with 7.5 and 15 mg/kg fucoidan for 24 days, the CD11b content in the SNpc was significantly reduced by 22% (P < 0.001) and 40% (P < 0.001), respectively.
Figure 2.
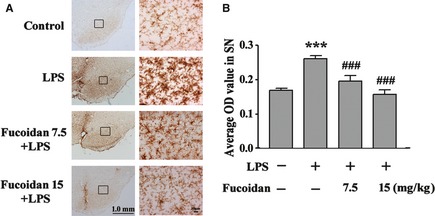
Fucoidan treatment inhibits microglial activation induced by LPS intranigral injection. (A) CD11b was detected by immunohistochemical staining to identify microglia in the substantia nigra pars compacta (SNpc). Scale bars represent 1.0 mm and 10 μm, respectively. (B) Effects of fucoidan treatment on the average optical density of CD11b in the SNpc. n = 4. Data are shown as the means ± SD. ***P < 0.001 compared with the control group, ###P < 0.001 compared with the LPS group.
Fucoidan Reduced LPS‐Induced TNF‐α Production
We further investigated the effects of fucoidan on LPS‐activated primary microglia. The cells were preincubated with 31.25, 62.5, and 125 μg/mL fucoidan for 10 min and then stimulated with LPS (0.01 μg/mL) for 6 h; TNF‐α levels were then determined using ELISA. As shown in Figure 3A, TNF‐α was barely detectable in the control group. Following LPS treatment, TNF‐α concentration increased to 2346 ± 210 pg/mL. However, the TNF‐α production induced by LPS was significantly suppressed by fucoidan. Specifically, the preincubation of cells with 125 μg/mL fucoidan decreased LPS‐induced TNF‐α production to 1639 ± 474 pg/mL (P < 0.05). Moreover, TNF‐α levels did not change significantly in microglia treated with the experimental concentrations of fucoidan compared with the control group (Figure 3B). Considering that 125 μg/mL fucoidan alone did not affect TNF‐α production and was not toxic (data not shown), these results suggested that the inhibition of LPS‐induced TNF‐α production in rat primary microglia cells by 125 μg/mL fucoidan was not related to any cytotoxic effects. Semiquantitative RT‐PCR was used to detect TNF‐α mRNA levels. As shown in Figure 3C, there was a low level of TNF‐α mRNA expression in microglia treated with normal medium or 125 μg/mL fucoidan alone. LPS treatment increased the expression of TNF‐α mRNA, and preincubation with fucoidan, especially at 125 μg/mL, inhibited mRNA expression by nearly 50% compared with LPS treatment alone.
Figure 3.
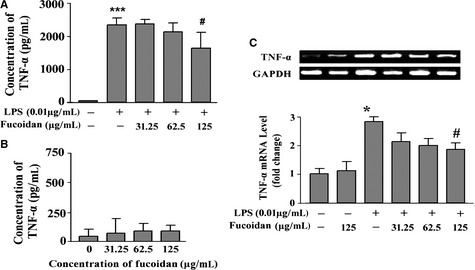
Fucoidan decreases tumor necrosis factor (TNF)‐α release and TNF‐α mRNA expression in LPS‐activated primary cultured microglia. (A) Fucoidan diminished LPS‐induced TNF‐α release in microglia. Microglia were incubated in a medium containing 31.25, 62.5, or 125 μg/mL fucoidan for 10 min and then activated by 0.01 μg/mL LPS for 6 h. n = 3. (B) Effect of fucoidan alone on TNF‐α production in microglia. Microglia were treated with the experimental doses of fucoidan. After 6 h, the concentration of TNF‐α released into the culture medium was detected using a commercial ELISA kit. n = 3. (C) Fucoidan inhibited the increase in TNF‐α mRNA expression in LPS‐activated microglia. The level of TNF‐α mRNA expression was determined by reverse transcription–polymerase chain reaction. The intensity of bands was quantified by densitometry and normalized against that for GAPDH (internal control). The expression of TNF‐α mRNA was further normalized against the level in the control group. n = 3. Data are shown as the means ± SD. *P < 0.05, ***P < 0.001 compared with the control group, #P < 0.05 compared with the LPS group.
Fucoidan Attenuated LPS‐Induced ROS Production
LPS increases the production of ROS, which are crucial mediators of inflammation. To test the effect of fucoidan on LPS‐induced intracellular ROS increase, microglial cultures were pretreated with fucoidan and exposed to 0.01 μg/mL LPS for 2 h. As shown in Figure 4A, the results of fluorescence microscopy showed that LPS induced a significant increase in the levels of intracellular ROS. In the presence of fucoidan (especially 125 μg/mL), the level of intracellular ROS was remarkably reduced, consistent with the results of the fluorescence intensity assay that measured fluorescence using a fluorescence plate reader (Figure 4B).
Figure 4.
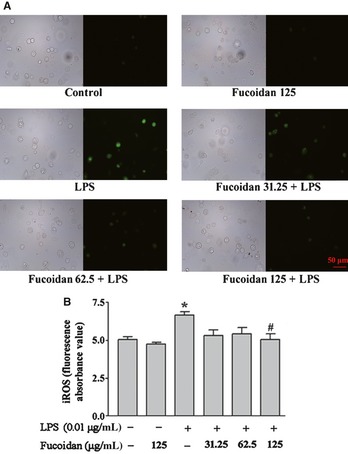
Fucoidan attenuates reactive oxygen species (ROS) production in LPS‐activated microglia. The cells were preincubated with fucoidan (31.25, 62.5, or 125 μg/mL) for 10 min before 0.01 μg/mL LPS or normal medium was added to the cells. After 2 h, the cells were exposed to serum‐free DMEM/F12 containing the fluorescent probe DCFH‐DA (10 μm), for 30 min at 37°C. (A) Representative images obtained by fluorescence microscopy of DCF‐sensitive intracellular ROS. Bar = 50 μm. (B) Fluorescence intensity was measured using a fluorescence plate reader. Data are shown as the means ± SD, n = 4. *P < 0.05 compared with the control group, #P < 0.05 compared with the LPS group.
Discussion
Our previous study had confirmed the neuroprotective effects of fucoidan in an MPTP animal model of PD, and the mechanisms might be related to the antioxidative effect of fucoidan 5. We had known that MPTP, a selective DA neurotoxin, directly damages DA neurons, resulting in oxidative mitochondrial damage, which leads to neuronal death 2. To further understand the mechanisms underlying the neuroprotective effects of fucoidan, we selected LPS, an endotoxin derived from the cell wall of Gram‐negative bacteria, to model neuroinflammation in PD, and investigated the effects of fucoidan on microglial inflammation and the subsequent dopaminergic neurodegeneration. Our data demonstrated that fucoidan protects dopaminergic neurons in an LPS‐induced animal model of PD.
In LPS‐injected rats, the degree of apomorphine‐elicited rotational behavior is related to LPS‐induced inflammatory reactions and neuronal damage in the nigrostriatal dopaminergic system. In this study, we found that fucoidan improved apomorphine‐induced rotational behavior and inhibited the loss of TH‐positive neurons in the SNpc in LPS‐treated rats, suggesting that fucoidan improved the behavioral deficit as a consequence of the protection of dopaminergic neurons.
Microglial cells play a critical role in forming a self‐propagating cycle that leads to sustained chronic neuroinflammation and drives the progressive neurodegeneration in PD. Our results showed that fucoidan inhibits the microglial activation induced by LPS administration, attenuates neuroinflammation, and protects dopaminergic neurons. Acute intranigral LPS injection produces a rapid activation of microglia and subsequent production of neurotoxic proinflammatory factors (e.g., ROS, TNF‐α, IL‐1β) within 24 h 11, which might begin the cascade of events that lead to the death of neighboring dopaminergic neurons 12. We further investigate the effects of fucoidan on microglial activation and proinflammatory factors' production in primary microglia. Among the numerous proinflammatory cytokines, TNF‐α is of particular importance, and the increased production of TNF‐α might be of marked relevance to dopaminergic neuronal death. In this study, we investigated the effects of fucoidan treatment on LPS‐activated primary microglia and found that fucoidan suppressed the increase in TNF‐α release and TNF‐α mRNA expression induced by LPS, which might have contributed to the neuroprotective effects of fucoidan in LPS‐treated animals.
Classic microglial activation results in the production of both extracellular and intracellular ROS. Intracellular ROS are crucial for both microglial proinflammatory function and survival 13. ROS production is thought to be the first step in cytokine production in microglia. The increase in the production of ROS is rapid, usually occurring within minutes, and is typically measured in microglia 10–30 min after the addition of LPS. After the microglial ROS response, cytokines (e.g., TNF‐α, and IL‐1 β), NO, and PGE2 are released and the levels of these peak after 6–12 h 14, 15. There is increasing evidence that intracellular ROS also function as second messengers regulating several downstream signaling molecules, including protein kinase C, mitogen‐activated protein kinases (MAPKs), and nuclear factor‐κB (NF‐κB), to amplify the proinflammatory function of microglia 2. Many recent reports found that fucoidan exhibits antiinflammatory properties via suppression of the phosphorylation of p38 and extracellular signal‐regulated kinase (ERK) in primary microglia 6, 8. Our present results indicate that fucoidan reduces the production of LPS‐induced ROS. This reduction in ROS production might be a crucial mediator of the neuroprotective effect of fucoidan toward LPS impairment, including the down‐regulation of MAPK pathways and the inhibitory effects on TNF‐α release and NO production.
Conclusion
In this study, we show in vivo that microglial activation induced by an intranigral injection of LPS has a degenerative effect on dopaminergic neurons. Fucoidan, a sulfated polysaccharide extracted from brown seaweeds, promoted the survival of these injured neurons through the inhibition of microglial activation, which consequently improved the LPS‐induced behavioral deficits. Further in vitro studies of the mechanisms underlying the neuroprotective effects of fucoidan indicated that fucoidan reduces intracellular ROS production and subsequent TNF‐α release in LPS‐activated primary microglia. Based on these findings, and its long history of safe use as a traditional herbal medicine, fucoidan may represent a therapeutic candidate for the treatment of neurodegenerative diseases such as PD.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Data S1. The backbone of the main component of fucoidan.
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB504100) and the National Nature Science Foundation of China Fund (81030062).
The first two authors contributed equally to this work.
References
- 1. Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm 2010;117: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloke ML, Hong JS. Microglia and inflammation‐mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 2005;76: 77–98. [DOI] [PubMed] [Google Scholar]
- 3. Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003;13: 29R–40R. [DOI] [PubMed] [Google Scholar]
- 4. Jhamandas JH, Wie MB, Harris K, MacTavish D, Kar S. Fucoidan inhibits cellular and neurotoxic effects of beta‐amyloid (A beta) in rat cholinergic basal forebrain neurons. Eur J Neurosci 2005;21: 2649–2659. [DOI] [PubMed] [Google Scholar]
- 5. Luo DZ, Zhang QB, Wang HM, et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro . Eur J Pharmacol 2009;617: 33–40. [DOI] [PubMed] [Google Scholar]
- 6. Do H, Pyo S, Sohn EH. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP‐1 and IRF‐1, and depends on up‐regulation of scavenger receptor B1 expression in TNF‐α‐ and IFN‐γ‐stimulated C6 glioma cells. J Nutr Biochem 2010;21: 671–679. [DOI] [PubMed] [Google Scholar]
- 7. Park HY, Han MH, Park C, et al. Anti‐inflammatory effects of fucoidan through inhibition of NF‐κB, MAPK and Akt activation in lipopolysaccharide‐induced BV2 microglia cells. Food Chem Toxicol 2011;49: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 8. Cui YQ, Zhang LJ, Zhang T, et al. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide‐activated primary microglia. Clin Exp Pharmacol Physiol 2010;37: 422–428. [DOI] [PubMed] [Google Scholar]
- 9. Zhang QB, Li Z, Xu ZH, Niu XZ, Zhang H. Effects of fucoidan on chronic renal failure in rat. Planta Med 2003;69: 537–541. [DOI] [PubMed] [Google Scholar]
- 10. Zhou HF, Niu DB, Xue B, et al. Triptolide inhibits TNF‐alpha, IL‐1beta and NO production in primary microglial cultures. NeuroReport 2003;14: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 11. Chung ES, Bok E, Chung YC, Baik HH, Jin BK. Cannabinoids prevent lipopolysaccharide‐induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res 2012;1451: 110–116. [DOI] [PubMed] [Google Scholar]
- 12. Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res 2002;69: 94–103. [DOI] [PubMed] [Google Scholar]
- 13. Block ML, Zecca L, Hong JS. Microglia‐mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8: 57–69. [DOI] [PubMed] [Google Scholar]
- 14. Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation‐mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem 2002;81: 1285–1297. [DOI] [PubMed] [Google Scholar]
- 15. Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect 2003;111: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. The backbone of the main component of fucoidan.


