Abstract
SUMMARY Background and Purpose: The glial water channel aquaporin‐4 (AQP4) has been shown to be involved in a wide range of brain disorders. Although its important role in stroke has already been documented, the underlying mechanism was not clarified yet. Therefore, this study was designed to investigate the impacts of AQP4 deletion in ischemia/reperfusion (I/R). Methods and Results: Herein we found a higher mortality and more severe neurological deficits in AQP4 knockout (AQP4−/−) mice after transient middle cerebral artery occlusion while no difference was observed in water content variation during I/R between two genotypes except a higher basal water content developed in AQP4−/− mouse brain, implying the same increment of water content over a higher basal level may provoke an even more elevated intracranial pressure, which might be an important cause of increased mortality in AQP4−/− mice. Moreover, AQP4 knockout aggravated I/R injury with enlarged infarct size and a more serious loss of CA1 neurons accompanied by a striking hypertrophy of astrocytes, suggesting an involvement of AQP4 in astrocytic dysfunction. Conclusions: Our findings provide direct evidence that AQP4 plays a crucial role in the pathogenesis of I/R injury, which may confer a new option for stroke treatment.
Keywords: Aquaporin‐4, Astrocyte, Ischemia/reperfusion, Knockout, Stroke
Introduction
Aquaporin‐4 (AQP4), the predominant isoform in the central nervous system (CNS), is an attractive target for drug discovery with the features distinct from other aquaporins [1, 2]. AQP4 is expressed in glia, most strongly at the borders between major water compartments and brain parenchyma [3, 4], suggesting its involvement in the maintenance of cerebral homeostasis and may be the key element in modulating astrocytic function. To date, accumulating data have shown that AQP4 depletion or downregulation caused astrocytic dysfunction such as reduced membrane water permeability [5], impaired cell growth or migration [6], attenuated potassium buffering [7], and altered cytoskeleton rearrangement [8]. Phenotype analysis of AQP4 deficient mice also indicates that AQP4 knockout (AQP4−/−) altered basal levels of amino‐acids neurotransmitters [9], down‐regulated glutamate uptake and glutamate transporter‐1 expression in astrocytes [10], disrupted integrity of the blood‐brain barrier (BBB) [11], strongly inhibited formation of glial cell‐derived neurotrophic factor in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐treated mice [12], and an impairment of neurotrophin‐dependent synaptic plasticity [13]. So far, AQP4 has gained sufficient attention in a wide range of brain disorders including edema, traumatic injury, tumor, infection, and epilepsy, whereas little is known about its contribution to acute ischemic stroke.
Ranked as the second leading cause of death behind ischemic heart disease [14], about 80% of strokes are caused by focal cerebral artery occlusion [15] and brain injury is thought to result from a cascade of events from energy depletion to cell death. Many efforts are directed at the management of acute ischemic stroke, but few strategies have proved to be effective [16]. Numerous pharmaceutical agents targeting one or more cell death pathways such as N‐methyl‐d‐aspartate antagonists, radical scavengers, antiinflammatory agents, calcium antagonists, caspases inhibitors, and neurotrophic factors, showed no improvement in patient prognosis [16]. These highlight the urgent need of new therapeutic options for ischemic stroke.
It has been revealed that glia or endotheliocytes were suffered in acute ischemic stroke as well [17], suggesting that the impairment of neurovascular unit may probably be involved in ischemia/reperfusion (I/R) process [18]. As critical determinants in neurovascular unit [19], astrocytes participate in actual information processing and neuronal control of vascular tone by releasing gliotransmitters or vasoactive molecules [20]. These events critically contribute to the anoxic/ischemic process [20]. More recently, emerging data suggest that these cell–cell signaling mechanisms may also mediate parallel processes of neurovascular remodeling during stroke recovery [21] and may be responsible for I/R injury. Although it has been well documented that AQP4 regulates astrocytic function in physiological and pathological conditions, it remains unclear whether AQP4 expressed on astrocytes participates in I/R process. Thus, AQP4−/− mice were used in this study so as to demonstrate the impact of AQP4 in ischemic stroke. The results showed that AQP4 deficiency aggravated I/R injury, which might be due to astrocytic dysfunction.
Materials and Methods
Generation of AQP4−/− Mice
AQP4‐deficient mice were generated as previously described [9]. Mice were kept under environmentally controlled conditions (ambient temperature, 22.0 ± 1.0°C; humidity, 40%) on a 12 h light–dark cycle with food and water ad libitum. All experiments were approved by Institutional Animal Care and Use Committee of Nanjing Medical University. All efforts were made to minimize animal suffering and to reduce the number of animals used for the experiments.
Transient Middle Cerebral Artery Occlusion
Mice aged 3 months (male, body weight 25–30 g) were subjected to sham surgery or transient middle cerebral artery occlusion (tMCAO) with a modified intralumenal filament technique as described previously [22, 23, 24, 25, 26]. In brief, animals were anesthetized with 0.75% chloral hydrate (150 mg/kg, i.p.), and a 6–0 monofilament with a heat‐blunted tip was gently introduced into the right common carotid artery and then advanced via the internal carotid artery to occlude the origin of the MCA. Reperfusion was accomplished by withdrawing the occluding filament 5, 15, or 40 min after induction of ischemia. All animals were operated on by the same operator to reduce infarct variability; and operation time per animal did not exceed 15 min. The rectal temperature was maintained at 37.0 ± 0.5°C during surgery and tMCAO via a servo‐controlled heating blanket. Animals were then returned to their cages and closely monitored until they recovered from anesthesia. Sham‐operated animals underwent the same procedure except for tMCAO.
Immunohistochemistry
For immunohistochemical analysis, mice were perfused with saline followed by 4% paraformaldehyde and immunohistochemistry was performed on 40‐μm free‐floating sections using anti‐AQP4 rabbit monoclonal antibody (1:500; Chemicon, Temecula, CA, USA; http://www.millipore.com), anti‐Neuronal Nuclei (anti‐NeuN) mouse monoclonal antibody (1:250; Santa Cruz, CA, USA; http://www.scbt.com), or anti‐glial fibrillary acidic protein (anti‐GFAP) rabbit monoclonal antibody (1:400; ABCam, Cambridge, MA, USA; http://www.abcam.com). Sections were then incubated with corresponding secondary antibodies and immunoreactivity was visualized with 0.05% 3,3′‐diaminobenzidine as chromagen. Specimens were observed under a confocal microscope (Nikon, Japan) for visualization and photography. Negative controls received the same treatment with the primary antibodies omitted and showed no specific staining.
Western Blotting
Total cellular proteins of the ipsilateral cortical tissue were extracted with homogenization buffer. Equivalent amounts of extracted proteins (20 μg) were resolved on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotted to polyvinylidene difluoride membrane (Amersham Biosciences). After blocking the background staining with 5% skimmed milk in phosphate buffered saline, the membranes were incubated in a rabbit anti‐AQP4 antibody (1:800; Chemicon). Antibody against β‐actin (1:1000; ABCam) was used as an internal control for the concentration of protein loaded. Immunoreactive proteins were detected using horseradish peroxidase‐conjugated secondary antibodies and an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA) according to the manufacturer's instructions. The membranes were scanned and analyzed in an Omega 16ic Chemiluminescence Imaging System (Ultra‐Lum, Claremont, CA, USA).
Neurological Deficits
At hour 24 or 72 after recovery from anesthesia, neurological function was assessed by two blinded investigators. Neurological deficits of the experimental animals were graded on a scale of 0–5 as described [27]. The criteria were as follows: grade 0 = no observable neurological deficits, grade 1 = failed to extend left forepaw, grade 2 = circled to the left, grade 3 = fell to the left, grade 4 = could not walk spontaneously, and grade 5 = dead. Neurological deficits were evaluated at various reperfusion periods as needed by the individual experiment.
Infarct Volume
Mice were decapitated 24 h after ischemia, the brains were removed and transferred to ice‐cold saline, and four 2‐mm consecutive coronal slices beginning from the anterior pole were cut with a brain slicer. Slices were then stained with 2% 2,3,5‐triphenyltetrazolium chloride (TTC) (Wako, Osaka, Japan) at 37°C for 30 min and fixed in 10% formalin neutral buffer solution (pH 7.4) for 1 h. The extent of injury was quantified in four slices using an image analyzing system (NIH image, public domain software developed at the United States National Institutes of Health).
Brain Water Content
In AQP4+/+ and AQP4−/− mice, cerebral water content was estimated in the contralateral and ipsilateral cortical tissue at day 1 or 3 of reperfusion following tMCAO. Following a quick dissection, the wet weight of the tissues was measured. The tissues were then placed at 105°C in an oven for 1 day and reweighed. The brain water content was calculated by the formula: [(wet weight – dry weight)/wet weight]× 100%[28].
Statistics
All values are expressed as Mean ± SEM. Differences between means were analyzed using Student's t‐test for two groups and one‐way ANOVA followed by Tukey–Kramer multiple comparisons posttest for multiple groups. P < 0.05 was defined as significant.
Results
I/R Alters the Expression Patterns of AQP4 Protein
Immunohistochemistry and western blotting analysis were used to investigate the AQP4 expression patterns in wild‐type (WT) mice induced by I/R. Western blotting analysis showed that 40‐min ischemia followed by 24‐h reperfusion induced a significant reduction (Figure 1A) in AQP4 expression in the ipsilateral cortex. However, 40‐min ischemia followed by 72‐h reperfusion resulted in a striking increase in AQP4 expression (Figure 1B) in the ipsilateral cortex. Consistent with these observations, the results from immunohistochemistry analysis showed that AQP4 expression was downregulated at 24‐h reperfusion but upregulated at 72‐h reperfusion after ischemia. These results suggest that AQP4 was involved in the ischemic injury.
Figure 1.
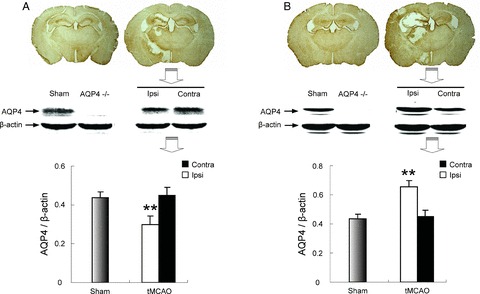
AQP4 protein levels as measured by immunohistochemistry or western blotting followed by 24‐h perfusion and 72‐h perfusion after 40‐min ischemia. (A) 40‐min ischemia/24‐h reperfusion; (B) 40‐min ischemia/72‐h reperfusion. **P < 0.01 ipsilateral versus contralateral hemisphere.
AQP4 Knockout Increases Mouse Mortality and Exacerbates Neurological Deficits Induced by I/R
Followed by 72‐h reperfusion after 5‐, 15‐, and 40‐min ischemia, there was 10%, 10%, and 24% mortality for WT mice, respectively. Compared with wild mice, I/R remarkably increased mortality and accelerated onset of death in AQP4−/− mice. At hour 72 after 5‐, 15‐, and 40‐min ischemia, the mortality rate of AQP4−/− mice increased to 45%, 77%, and 88%, respectively (Figure 2A–C).
Figure 2.
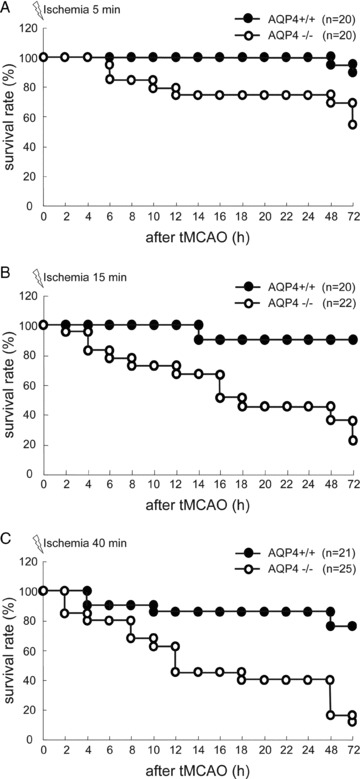
Cumulative survival rate of mice after tMCAO. Monitoring of survival rate was terminated at 72 h after tMCAO. (A) 5‐min ischemia; (B) 15‐min ischemia; (C) 40‐min ischemia.
As shown in Figure 3A, B, at 24‐h reperfusion and 72‐h reperfusion after ischemia, neurological deficits of 40‐min ischemia groups were more severe than those of 5‐min ischemia groups in AQP4+/+ mice (76% survival rate vs. 90% survival rate). Compared with WT mice, I/R aggravated neurological deficits in AQP4−/− mice. The survival rate was reduced to 12% in AQP4−/− mice after 40‐min ischemia followed by 72‐h reperfusion, and none of the surviving mice walked spontaneously (score 4). However, there is no difference in neurological deficits between 5‐min ischemia groups and 40‐min ischemia groups.
Figure 3.

Determination of neurological deficits of AQP4+/+ and AQP4−/− mice at 24 h (A) and 72 h (B) after tMCAO. The scoring scale ranged from 0 to 5. *P < 0.05 AQP4−/− versus AQP4+/+ mice.
AQP4 Knockout Increases I/R‐Induced Cerebral Infarction in Mice
TTC staining was used to examine the damaged areas in mouse brain induced by 40‐min ischemia and 24‐h reperfusion (Figure 4A). Consistent with the results of neurological deficits, I/R significantly enlarged infarct volume in AQP4−/− mice compared with that in WT mice (Figure 4B), indicating an exacerbated brain damage occurred in AQP4 deficient mice.
Figure 4.
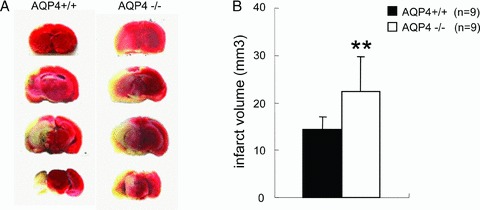
TTC‐stained coronal brain sections are from representative animals of two genotype mice. Infarcts are observed (pale region) involving the cerebral cortex and underlying striatum, representative of the MCA perfusion region. **P < 0.01 AQP4−/− versus AQP4+/+ mice.
AQP4 Knockout Exacerbates I/R‐Induced CA1 Neurons Loss and Astrocytic Proliferation in Mouse Brain
NeuN and GFAP immunohistochemistry were used to identify neuron and astrocyte. After 40‐min ischemia followed by 24‐h reperfusion, there was a mild loss of CA1 neurons in ipsilateral hippocampus of WT mice. However, neuronal injury was evident in AQP4−/− genotype mice with more severe loss of neurons in CA1 region of hippocampus (Figure 5A). Staining density of GFAP adjacent to the injured tissue was increased in both genotype mice, indicating that I/R activated astrocytes. Notably, a more striking hypertrophy of soma or a pronounced swelling of processes was found in AQP4−/− astrocytes (Figure 5B).
Figure 5.
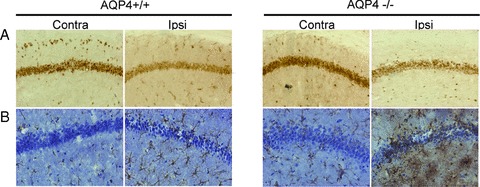
Immunohistochemical analysis of NeuN (A), and GFAP (B) in both genotype mouse brain after I/R.
AQP4 Knockout Alters Brain Water Content of Mice after Ischemia
Brain water content (BWC) was determined by wet‐to‐dry weight ratio. The basal level of BWC in AQP4−/− mice was higher than that in AQP4+/+ mice. 40‐minute ischemia followed by 24‐ or 72‐h reperfusion significantly increased BWC of ischemic (ipsilateral) hemisphere in both genotype mice (Figure 6A, B). However, no difference in BWC variation was observed between WT mice and AQP4−/− mice. These results indicated that edema might not be the immediate cause of higher mortality in AQP4−/− mice.
Figure 6.
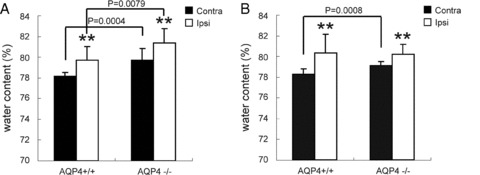
Brain water content of ipsilateral (ischemic) and contralateral hemispheres was measured by wet‐to‐dry weight ratio at 24‐h reperfusion and 72‐h reperfusion after tMCAO. **P < 0.01 ipsilateral versus contralateral hemisphere.
Discussion
Among the several aquaporin water channels expressed in the CNS, AQP4 is considered as an appealing therapeutic target for brain disorders such as brain tumor, traumatic injury, hydrocephalus and neuroinflammation [29, 30, 31, 32, 33]. Notwithstanding direct evidence of AQP4 expanding functions obtained from AQP4 deficient mice in various pathophysiological conditions, no information has been acquired for AQP4‐related pathophysiological functions in I/R injury.
In this study, we demonstrated a time‐course change of AQP4 in ipsilateral hemisphere of tMCAO mice consistent with previous observation [30], suggesting an involvement of AQP4 in I/R injury. AQP4 knockout increased the mortality of mice, accelerated the onset of death, aggravated the outcomes of neurological impairments, and enlarged the size of infarction. On the basis of experiments previously conducted with AQP4−/− mice [34, 35, 36], it was concluded that the role of AQP4 is dual in edema formation and resolution depending on the pathologic model studied. But the exact mechanism is not yet fully understood. As Papadopoulos recently stated [37], AQP4 controls water fluxes into and out of the brain parenchyma. In cytotoxic edema, AQP4 deletion slows the rate of water entry into brain, whereas in vasogenic edema, AQP4 deletion reduces the rate of water outflow from brain parenchyma. Water intoxication or early permanent ischemia (without a reperfusion in primary lesion) is a typical case of cytotoxic edema. The predominant causative agents are permeability changes and adenosine‐5′‐triphosphate depletion. It is why AQP4 deletion protects mice from water intoxication or permanent middle cerebral artery occlusion (not I/R injury as our model) [27]. While in the late phase of I/R produced by tMCAO, a case of vasogenic edema [36], additional mechanisms such as excitotoxicity, oxidative stress, inflammation, apoptosis, astrocyte dysfunction, neurogenesis inhibition, and BBB disruption are involved. With these agents, AQP4 deletion aggravates vasogenic (fluid leak) brain edema produced by tumor or cortical freeze [36], which may also contribute to our findings. In our model, we believe that the early downregulation (24 h) of AQP4 may contribute to the relief of cytotoxic edema formation whereas the following upregulation (72 h) may sustain water removal from brain tissue. Although the protective role of AQP4 induction against postischemic edema formation has been confirmed [38], a surprising result is that there was no difference in water content variation during I/R between both genotypes, except a higher basal level developed in AQP4−/− mice, indicating that the same increment of water content over a higher basal level may provoke an even more elevated intracranial pressure, which might be an important cause of increased mortality in AQP4−/− mice.
Immunohistochemistry analysis revealed that AQP4 deficient mice exhibited a predominant swelling of astrocytes. For decades, glial cells were regarded simply as “padding cells” and were overshadowed by the importance of neurons in the CNS. However, emerging evidence has provided new insights into astrocytic functions such as control of synapse formation and function [39, 40], regulation of cerebrovascular tone [41] and adult neurogenesis [42, 43]. These observations have greatly enhanced our understanding of the complexity of astrocytic multifunction. With the endfeet extending toward blood vessels on one side and enclosing synapses on the other, astrocytes are ideally situated to control metabolic supply, blood flow, ionic homeostasis, and neurotransmitter levels in brain. As a crucial element of neurovascular unit, the loss of key astrocytic functions may aggravate the neuronal injury and potentially influence the outcome of ischemic insult [20, 44]. In this study, our findings suggested that I/R‐induced glial dysfunctions might also contribute to more serious damage in AQP4−/− mice.
Accumulating data have shown that AQP4 depletion or downregulation caused astrocytic dysfunction [6, 8, 10, 12, 33]. Consistently, this work showed that AQP4 knockout exacerbated the loss of CA1 neurons associated with a striking swelling of astrocytes. Disturbance of astrocyte function may trigger a serial of exacerbated brain damages. The evidence from our laboratory has revealed that AQP4 knockout down‐regulated glutamate uptake by astrocytes [10]. Activation of glutamate receptors triggered by excessive glutamate during the course of ischemia was indicated to increase the rate of hypoosmotic tissue swelling in acute rat hippocampal slices [45]. When glutamate acts on astrocytes, two stages are presented: cell swelling initiated early (4–6 h) and biochemical signs of cell death triggered later (16–18 h), and almost all cells died after 24–30 h of glutamate exposure [46]. Therefore, excess water could be promptly eliminated from AQP4+/+ astrocytes with function rescued in the early stage, whereas intracellular water could not be eliminated from AQP4−/− astrocytes that subsequently resulted in more serious edema and astrocytic malfunction. Thus, downregulation of glutamate uptake by astrocytes and excess accumulated glutamate‐induced astrocytic dysfunction in AQP4−/− mice might be responsible for the aggravated I/R injury.
As a “molecular bridge” of astrocytes, AQP4 is generally accepted to be a key factor for edema elimination in I/R. With the disrupted BBB in AQP4−/− mice [11], water derived from the vasculature increased and edema ablation delayed due to the loss of AQP4. Recently, Hirt et al. provided evidence that the early induction of AQP4 may contribute to the limited formation of brain edema [38]. A study of Fu et al. also indicated that AQP4 knockdown may delay water clearance from the swollen astrocyte during reoxygenation [47], suggesting the key role of astrocytic AQP4 in edema elimination. Consistent with these findings, we herein demonstrated a more remarkable hypertrophy of astrocytic soma and pronounced swelling of processes in AQP4−/− genotype, which contributed to a worse I/R outcome in AQP4−/− mice.
In conclusion, we here provide direct evidence that AQP4 plays a crucial role in IR injury. AQP4 knockout elicited a higher mortality in mice and aggravated the outcomes of neurological impairments, which may due to a higher intracranial pressure. An enlarged infarct size and a more serious loss of CA1 neurons accompanied by a striking hypertrophy of astrocytes were observed in AQP4−/− mice, implying an involvement of AQP4 in astrocytic dysfunction. Taken together, our findings suggest that AQP4 participates in the pathogenesis of stroke, which may confer a new option for stroke treatment.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This study was supported by the grants from the National Key Basic Research Program of China (No.2009CB521906) and the National Natural Science Foundation of China (No.30973517).
References
- 1. Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 1995;108:2993–3002. [DOI] [PubMed] [Google Scholar]
- 2. King LS, Kozono D, Agre P. From structure to disease: The evolving tale of aquaporin biology. Nat Rev Mol Cell Bio 2004;5:687–698. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen S, Nagelhus EA, Amiry‐Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High‐resolution immunogold cytochemistry of aquaporin‐4 in rat brain. J Neurosci 1997;17:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin‐4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A 1998;95:11981–11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunnarson E, Axehult G, Baturina G, Zelenin S, Zelenina M, Aperia A. Lead induces increased water permeability in astrocytes expressing aquaporin 4. Neuroscience 2005;136:105–114. [DOI] [PubMed] [Google Scholar]
- 6. Auguste KI, Jin S, Uchida K, et al Greatly impaired migration of implanted aquaporin‐4‐deficient astroglial cells in mouse brain toward a site of injury. FASEB J 2007;21:108–116. [DOI] [PubMed] [Google Scholar]
- 7. Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long‐wavelength K+‐sensing fluorescent indicator. Nat Methods 2005;2:825–827. [DOI] [PubMed] [Google Scholar]
- 8. Nicchia GP, Srinivas M, Li W, Brosnan CF, Frigeri A, Spray DC. New possible roles for aquaporin‐4 in astrocytes: cell cytoskeleton and functional relationship with connexin43. FASEB J 2005;19:1674–1676. [DOI] [PubMed] [Google Scholar]
- 9. Fan Y, Zhang J, Sun XL, et al Sex‐ and region‐specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin‐4 knockout mice. J Neurosci Res 2005;82:458–464. [DOI] [PubMed] [Google Scholar]
- 10. Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G. Aquaporin‐4 deficiency down‐regulates glutamate uptake and GLT‐1 expression in astrocytes. Mol Cell Neurosci 2007;34:34–39. [DOI] [PubMed] [Google Scholar]
- 11. Zhou J, Kong H, Hua X, Xiao M, Ding J, Hu G. Altered blood‐brain barrier integrity in adult aquaporin‐4 knockout mice. Neuroreport 2008;19:1–5. [DOI] [PubMed] [Google Scholar]
- 12. Fan Y, Kong H, Shi X, et al Hypersensitivity of aquaporin 4‐deficient mice to 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyrindine and astrocytic modulation. Neurobiol Aging 2008;29:1226–1236. [DOI] [PubMed] [Google Scholar]
- 13. Skucas VA, Mathews IB, Yang J, et al Impairment of select forms of spatial memory and neurotrophin‐dependent synaptic plasticity by deletion of glial aquaporin‐4. J Neurosci 2011;31:6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 15. Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007;87:179–197. [DOI] [PubMed] [Google Scholar]
- 16. Röther J. Neuroprotection does not work! Stroke 2008;39:523–524. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita T, Kamiya T, Deguchi K, et al Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab 2009;29:715–725. [DOI] [PubMed] [Google Scholar]
- 18. Young AR, Ali C, Duretête A, Vivien D. Neuroprotection and stroke: Time for a compromise. J Neurochem 2007;103:1302–1309. [DOI] [PubMed] [Google Scholar]
- 19. Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 2007;54:34–66. [DOI] [PubMed] [Google Scholar]
- 20. Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia 2005;50:287–298. [DOI] [PubMed] [Google Scholar]
- 21. Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol 2011;26:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn Stroke J 1986;8:1–8. [Google Scholar]
- 23. Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 2007;115:2323–2330. [DOI] [PubMed] [Google Scholar]
- 24. Varga‐Szabo D, Braun A, Kleinschnitz C, et al The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med 2008;205:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A 2009;106:5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs 2011;13:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manley GT, Fujimura M, Ma T, et al Aquaporin‐4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 2000;6:159–163. [DOI] [PubMed] [Google Scholar]
- 28. Huang ZX, Kang ZM, Gu GJ, et al Therapeutic effects of hyperbaric oxygen in a rat model of endothelin‐1‐induced focal cerebral ischemia. Brain Res 2007;1153:204–213. [DOI] [PubMed] [Google Scholar]
- 29. Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin‐4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res 2005;82:499–506. [DOI] [PubMed] [Google Scholar]
- 30. Frydenlund DS, Bhardwaj A, Otsuka T, et al Temporary loss of perivascular aquaporin‐4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci U S A 2006;103:13532–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roemer SF, Parisi JE, Lennon VA, et al Pattern‐specific loss of aquaporin‐4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007;130:1194–1205. [DOI] [PubMed] [Google Scholar]
- 32. Miyamoto K, Nagaosa N, Motoyama M, Kataoka K, Kusunoki S. Upregulation of water channel aquaporin‐4 in experimental autoimmune encephalomyeritis. J Neurol Sci 2009;276:103–107. [DOI] [PubMed] [Google Scholar]
- 33. Li L, Zhang H, Varrin‐Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin‐4 in autoimmune neuroinflammation. FASEB J 2011;25:1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Badaut J, Brunet JF, Regli L. Aquaporins in the brain: From aqueduct to “multi‐duct”. Metab Brain Dis 2007;22:251–263. [DOI] [PubMed] [Google Scholar]
- 35. Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: Role of aquaporins. Trends Neurosci 2008;31:37–43. [DOI] [PubMed] [Google Scholar]
- 36. Papadopoulos MC, Verkman AS. Aquaporin‐4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem 2004;280:13906–13912. [DOI] [PubMed] [Google Scholar]
- 37. Papadopoulos MC, Verkman AS. Aquaporin‐4 and brain edema. Pediatr Nephrol 2007;22:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirt L, Ternon B, Price M, Mastour N, Brunet JF, Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab 2009;29:423–433. [DOI] [PubMed] [Google Scholar]
- 39. Christopherson KS, Ullian EM, Stokes CC, et al Thrombospondins are astrocyte‐secreted proteins that promote CNS synaptogenesis. Cell 2005;120:421–433. [DOI] [PubMed] [Google Scholar]
- 40. Allen NJ, Barres BA. Neuroscience: Glia—More than just brain glue. Nature 2009;457:675–677. [DOI] [PubMed] [Google Scholar]
- 41. Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol 2006;100:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: Cellular niches for adult neurogenesis. Curr Opin Neurobiol 2005;15:514–520. [DOI] [PubMed] [Google Scholar]
- 43. Pillai R, Scintu F, Scorciapino L, et al Human astrocytes can be induced to differentiate into cells with neuronal phenotype. Exp Cell Res 2006;312:2336–2346. [DOI] [PubMed] [Google Scholar]
- 44. Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke 2009;40:S8–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gunnarson E, Zelenina M, Axehult G, et al Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia 2008;56:587–596. [DOI] [PubMed] [Google Scholar]
- 46. Chen CJ, Liao SL, Kuo JS. Gliotoxic action of glutamate on cultured astrocytes. J Neurochem 2000;75:1557–1565. [DOI] [PubMed] [Google Scholar]
- 47. Fu X, Li Q, Feng Z, Mu D. The roles of aquaporin‐4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 2007;55:935–941. [DOI] [PubMed] [Google Scholar]


