Summary
Aims
Decreased baroreflex sensitivity is associated with poor outcome in many cardiovascular diseases including stroke, but the molecular mechanism underlying this relationship is unclear. This work was designed to test the hypothesis that acetylcholine (ACh) and α7 nicotinic ACh receptor (α7nAChR) mediate the protection of arterial baroreflex against stroke.
Methods
Sinoaortic denervation (SAD) was used to impair the function of arterial baroreflex, and anticholinesterase agents were used to activate the cholinergic system and increase endogenous ACh. Middle cerebral artery occlusion (MCAO) was performed in the α7nAChR knockout (KO) mice and Sprague–Dawley rats.
Results
We found decreased expression of vesicular ACh transporter (VAChT) and α7nAChR in rat brain after SAD. In rats subjected to MCAO, neostigmine significantly reduced the infarct size. The protective effects of neostigmine were abolished by selective nAChR antagonist vecuronium but not by mAChR antagonist anisodamine. In addition, the effect of neostigmine disappeared in α7nAChR KO mice. In cultured neurons, ACh inhibited cell death induced by H 2 O 2. In cultured microglial cells, ACh decreased the release of proinflammatory cytokines induced by lipopolysaccharide. These in vitro effects were blocked by selective α7nAChR antagonists.
Conclusion
Taken together, these findings indicate that the ACh‐α7nAChR involved in the protective effects of arterial baroreflex against ischemic stroke.
Keywords: Acetylcholine, Arterial baroreflex, Stroke, α7 Nicotinic acetylcholine receptor
Introduction
Stroke is an age‐related disease and the second most common cause of death 1, 2. It is also a major cause of disability worldwide and has a profound negative impact on the individuals it affects 3, 4. Arterial baroreflex is one of the most important mechanisms regulating cardiovascular activities 5, 6, 7. Impaired baroreflex sensitivity (BRS) is evident with increasing age 8 and has been repeatedly shown to be present in acute stroke 9, 10, 11. Robinson and colleagues reported that post‐stroke patients with impaired BRS values (<5.0 ms/mmHg) had a significantly poorer prognosis than patients with relatively higher BRS (>5.0 ms/mmHg) 12. Recently, we demonstrated that BRS is an important factor in determining the survival time in stroke‐prone spontaneously hypertensive rats (SHR‐SPs) 7. Rescuing BRS by ketanserin postponed stroke in SHR‐SPs 7. We also showed that impairing BRS using different approaches all leads to increased susceptibility to middle cerebral artery occlusion (MCAO) 13. These findings suggest a potential therapeutic strategy by the restoration of BRS or activation of the down‐stream pathway of arterial baroreflex in the prevention and treatment for ischemic stroke. Regrettably, molecular basis of such an important biological and medical phenomenon is lacking. The current study investigated the possible roles of the conserved cholinergic system.
Cholinergic system, including acetylcholine (ACh), vesicular ACh transporter (VAChT), choline acetyl transferase (ChAT), ACh receptors, high‐affinity choline uptake, esterase, has been demonstrated in mammalian neuronal or non‐neuronal cells 14. ACh modulates responses of neurons as well as non‐neuronal cells to internal or external stimuli via two types of receptors: muscarinic ACh receptor (mAChR) and nicotinic ACh receptor (nAChR) 14. Recent studies have implicated α7 nicotinic ACh receptor (α7nAChR), a sub‐type of nAChR, in many important biological events, including anti‐inflammation and anti‐apoptosis 15, 16, 17, 18.
In this study, we conducted a series of experiments to examine the potential role of cholinergic system on the protective effect of arterial baroreflex against stroke using several rodent models of stroke. Sinoaortic denervation (SAD) was used to impair the function of arterial baroreflex, and anticholinesterase agents were used to activate the cholinergic system and increase endogenous ACh. The α7nAChR knockout (KO) mice were used to examine its potential role and the down‐stream pathways of arterial baroreflex on stroke.
Materials and Methods
Animals
Male Sprague–Dawley (SD) rats and ICR mice were purchased from Sino‐British SIPPR/BK Laboratory Animals (Shanghai, China). Male SHR‐SPs were provided by the Animal Center of the Second Military Medical University. The α7nAChR KO mice were purchased from Jackson Laboratory (Bar Harbor, MA, USA) (B6.129S7‐Chrna7tm1 Bay, Stock Number: 003232). All animals were used in accordance with the guidelines of Second Military Medical University for Animal Care.
Sinoaortic Denervation
Sinoaortic denervation was carried out as described previously 13.
Middle Cerebral Artery Occlusion
The animals were anesthetized with chloral hydrate (300 mg/kg for both rats and mice). The surgery was performed as described previously 19.
Blood Pressure Recording and BRS Measurement in Conscious Animals
Systolic blood pressure (SBP), diastolic blood pressure and heart period were continuously recorded in conscious, freely moving rats 7, 13. BRS was measured using a pharmacological method 7, 13.
Neuron Culture and Apoptosis Assay
Primary rat and mouse neuronal cells were obtained from the cerebral cortex of neonatal animals within 6 h after birth. One day after isolation, the cultures were replenished with Neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2% B27 (Invitrogen). Glial growth was suppressed by the addition of uridine (10 μM). Staining for MAP‐2 (neuron marker; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and glial fibrillary acidic protein (GFAP, an astrocyte marker; Cell Signaling Technology, Inc., Danvers, MA, USA) revealed cultured cells contained >90% neurons. After 7 days in vitro, cultured cells were exposed to H2O2 (100 μM; Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) for 24 h prior to apoptosis assay. In some experiments, cells were co‐stained with Annexin V‐FITC and propidium iodide (PI) (Keygen Biotech, Nanjing, China) prior to flow cytometry (BD FACSCalibur™ Flow Cytometer, BD Biosciences, San Jose, CA, USA). Fluorescence‐activated cell sorting (FACS) analysis was used to detect 10,000 cells for each experiment.
Cell survival was examined by manually counting the cells double stained with dihydrochloride (DAPI; Beyotime, Jiangsu, China) and in situ cell death detection kit (Roche, Mannheim, Germany). The cell nuclei were counterstained with DAPI (1 mg/mL). The dead cells were labeled green with the kit. Images were acquired under a fluorescent microscope (IX‐71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color‐cooled camera (DP72; Olympus). The ratio of cells labeled green versus blue is used to define apoptotic rate 20. We also assessed cell death by manually counting the cells stained with 1 μg/mL Hoechst 33342 (Sigma‐Aldrich, St. Louis, MO, USA) under the fluorescent microscope (IX‐71).
Primary Microgliocytes Culture
Primary rat or mouse microgliocytes were obtained from the cerebral cortex of neonatal animals within 6 h after birth. The cultures were replenished with Dulbecco minimum essential medium (DMEM; Gibco, Grand Island, NY, USA) adding 10% fetal bovine serum (FBS; Gibco) and 10% equine serum (Gibco). Ten days after a confluent monolayer of microgliocytes obtained, cells were collected with agitation (200 rpm) and centrifuged for 5 min (240 g). Staining for OX‐42 (a microgliocyte marker; Serotec, AbD Serotec, Raleigh, NC, USA) revealed cultured cells contained >95% microgliocytes. Cells were seeded at 5 × 105 and 1 × 105/well in 24‐ and 96‐well plates and used for treatment the following day. The lipopolysaccharide (0.5 μg/mL; Sigma‐Aldrich) is used to stimulate the release of proinflammatory cytokine.
Immunochemistry and Immunofluorescence for VAChT
The experiment was performed as described previously 21. Tissue sections (20 μm) or cultured neurons were fixed in 4% paraformaldehyde, blocked by 8% normal donkey serum, and incubated in a VAChT antibody (ab62140, 1:1000; Abcam, Cambridge, MA, USA). Cy3‐labeled donkey secondary anti‐goat IgG (H + L) (A0502; Beyotime Biotechnology, Haimen, Jiangsu, China) was used for immunochemical and immunofluorescent imaging. Images were acquired using a fluorescent confocal microscopy (Leica TCS‐SP5, Leica, Wetzlar, Germany). The data were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunoblotting
The experiment was performed as described previously 22. Tissue/cells lysates were boiled in 4× loading buffer, subjected to the SDS‐PAGE, and transferred onto the pure nitrocellulose blotting membranes. The membranes were incubated with one of the following antibodies: caspase 8 rabbit mAb (Cell Signaling), cleaved caspase 8 antibody (Cell Signaling), Bcl‐xl rabbit mAb (Cell Signaling), Bax antibody (Cell Signaling), Bcl‐2 rabbit mAb (Cell Signaling), Bad rabbit mAb (Cell Signaling), purified rabbit anti‐caspase 12 (BD Biosciences.), goat polyclonal to VAChT (Abcam), and α7nAChR antibody (Sigma‐Aldrich) prior to incubation with IRDye800CW‐conjugated secondary antibody. The image was captured by the Odyssey infrared imaging system (Li‐Cor Bioscience, Lincoln, NE, USA). The data were analyzed using ImageJ software (NIH). The immunoblotting experiments were performed on samples from three different animals. The average from the 3 was used to indicate the value for each animal subject.
Analysis of Proinflammatory Cytokines in Blood
The levels of tumor necrosis factor alpha (TNFα), interleukin‐6 (IL‐6), and interleukin‐1 (IL‐1) in the serum were measured on the detection system (Infinite M200; Tecan Austria GmbH, Grödig, Austria) with ELISA kits (R&D Systems, Minneapolis, MN, USA).
Statistical Analysis
Data are expressed as the mean ± SD. Data were analyzed with Student's t‐test or one‐way analysis of variance (ANOVA) followed by LSD t‐test for pair‐wise comparison. P < 0.05 was considered statistically significant.
Results
SAD Impaired the Function of Arterial Baroreflex, Aggravated the Ischemic Cerebral Injury
One month after SAD, SD rats were subjected to MCAO under anesthesia. SAD significantly decreased the BRS values (0.24 ± 0.21 vs. 0.95 ± 0.07 ms/mmHg in sham operated rats, P < 0.01), but did not affect SBP (124 ± 11.0 vs. 122 ± 14.4 mmHg, P > 0.05, Figure 1A). The infarct size was significantly larger in SAD rats than in sham operated rats (49.0 ± 6.9% vs. 28.0 ± 5.1%). SAD significantly reduced the neurological function (neurological score 3.5 ± 0.5 vs. 2.0 ± 0.5 in sham operation group, P < 0.05, Figure 1B).
Figure 1.
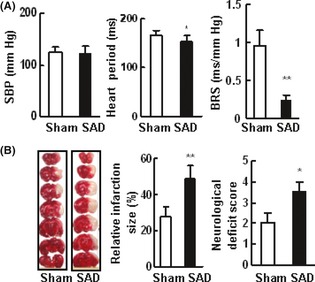
Sinoaortic denervation (SAD) impaired the arterial baroreflex and aggravated the cerebary injury. Male SD rats (250–300 g) received SAD or sham operation. (A) Blood pressure, heart period, and baroreflex sensitivity (BRS) values were measured in conscious, free‐moving rats. (B) SAD increased the infarction size of SD rats. The panel shows representative 2‐3‐5‐triphenyltetrazolium‐chloride (TTC)‐stained of seven corresponding coronal brain sections of sham operation and SAD groups rats on day 1 after MCAO (n = 10–11 in each group of A and B). MCAO, middle cerebral artery occlusion.
SAD Decreased Vagal Outflow
Baroreflex sensitivity values were measured in 25 SD rats. The vagal tone correlated positively with BRS in SD rats (r = 0.75, P < 0.001; Figure 2A). SAD significantly decreased the spontaneous discharge of the neurons in the nucleus ambiguous (NA; from 15.0 ± 4.00 spikes/second in control rats to 6.47 ± 2.23 spikes/second in SAD rats, Figure 2C,D). The tachycardia induced by atropine was markedly attenuated by SAD (13.0 ± 4.60 vs. 30.0 ± 9.50 beats/min in sham operated rats, 57% reduction, Figure 2E).
Figure 2.
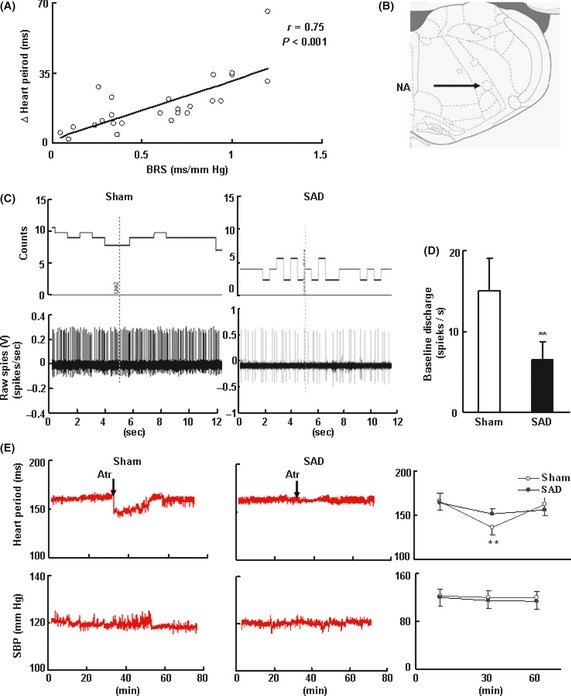
Cholinergic tone is diminished in rats with Sinoaortic denervation (SAD). (A) In male SD rats (350–400 g) with intact but varying degrees of arterial baroreflex function. The cholinergic tone was expressed as the changes of heart period induced by atropine sulfate (0.03 mg/kg, iv) in conscious, freely moving rats, n = 25. The relationships between the peripheral cholinergic tone and the baroreflex sensitivity (BRS) are assessed by univariate regression analysis. The Pearson r‐values were calculated. (B, C, D) SAD decreased the rate of spontaneous discharge. Single‐unit extracellular recording was made in nucleus ambiguous (NA) on both sides. The site of extracellular recording in NA is shown in B. Data are expressed as mean ± SD.*P < 0.05, **P < 0.01. Student's t‐test. n = 5–7. Neurons for each rats, n = 5 in each group. (E) SAD decreased the change of heart period induced by atropine sulfate. The systolic blood pressure (SBP) had no change. Data are expressed as mean ± SD.*P < 0.05, **P < 0.01. Paired Student's t‐test. n = 9–11 in each group.
SAD Decreased the Expression of VAChT and α7nAChR in the Brain
Vesicular ACh transporter protein level, as determined by immunohistochemistry and Western blot in the cerebrocortex, was significantly decreased by SAD (Figure 3A). The expression of α7nAChR in the cerebrocortex was also significantly decreased by SAD (Figure 3B).
Figure 3.
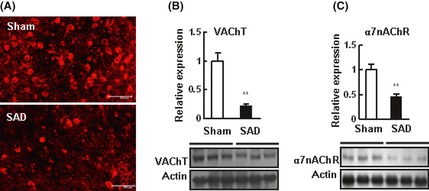
Sinoaortic denervation (SAD) decreased the expression of vesicular acetylcholine transporter (VAChT) and α7 nicotinic acetylcholine receptor (α7nAChR) in the brain of SD rats. (A) Representative images of VAChT in the cerebrocortex under a con‐focal fluorescent microscope. Scale bar, 50 μm. (B, C) Representative Western blots of VAChT and α7nAChR in the cerebrocortex, using beta‐actin as an internal control. Cerebrocortex homogenate was incubated with the VAChT or α7nAChR antibody, n = 3 in each group. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01. Student's t‐test.
Increasing Endogenous ACh by Neostigmine Decreased the Ischemic Cerebral Injury, Decreased the Apoptosis and Reduced the Release of Proinflammatory Cytokines
Neostigmine (40 μg/kg; Shanghai Xinyijinzhu Pharmaceutical Co., Ltd, Shanghai, China) significantly reduced the infarct size (22.2 ± 7.59% vs. 34.2 ± 9.86% in the vehicle control group, P < 0.05; Figure 4A). Neostigmine also significantly decreased the expression of cleaved caspase 8 and the ratio of cleaved caspase 8 to total caspase 8 in the ischemic penumbra of SD rats subjected to MCAO (Figure 5A), but did not change the expression of caspase 12. Neostigmine significantly decreased the expression of Bad and Bax. Bcl‐2, Bcl‐xl, and the ratio of Bcl‐xl to Bax were significantly increased. Serum levels of TNFα, IL‐6, but not IL‐1α or IL‐1β in the serum, were significantly decreased (Figure 5B).
Figure 4.
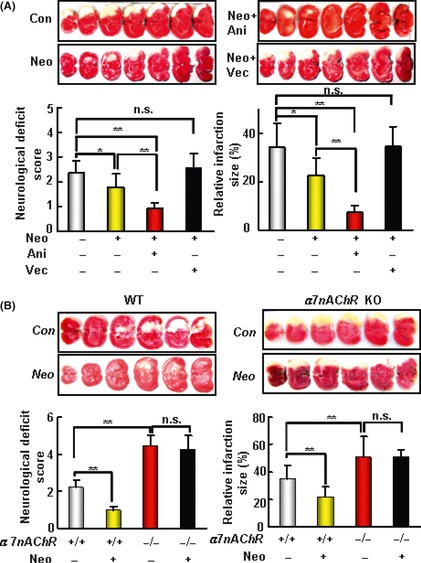
The protective effect of neostigmine (Neo) on acute ischemic cerebral injury induced by middle cerebral artery occlusion (MCAO) depends on nAChR‐α7nAChR. (A) The cerebral protective effect of Neo depends on nAChR. The upper panel shows representative 2‐3‐5‐triphenyltetrazolium‐chloride (TTC)‐stained of seven corresponding coronal brain sections of vehicle control (Con), Neo (40 μg/kg), Neo + anisodamine (Ani, 20 mg/kg), and Neo + vecuronium (Vec, 400 μg/kg) treatment group rats on day 1 after MCAO. (B) The cerebral protective effect of Neo depends on the subunit of nAChR, α7nAChR. The upper panel shows representative TTC‐stained of seven corresponding coronal brain sections of α7nAChR knockout (KO) and WT mice on day 1 after MCAO. Neo (80μg/kg) significantly decreased the infarct size and improved the neurological score in α7nAChR WT mice but not in KO mice. Data are expressed as mean ± SD. Data were analyzed by one‐way analysis of variance (ANOVA) followed by LSD t‐test.*P < 0.05, **P < 0.01. n = 9–12 in each group of A; n = 6–7 in each group of B.
Figure 5.

Neostigmine (Neo) decreased the apoptosis and reduced the release of proinflammatory cytokine induced by middle cerebral artery occlusion (MCAO) in SD rats. (A) The effects of Neo on the expressions of caspase 8, cleaved caspase 8, caspase 12, Bcl‐2, Bad, Bcl‐xl and Bax in the ischemic penumbra induced by MCAO. (B) The levels of IL‐1, IL‐6, and TNFα in serum were examined with ELISA. Data are expressed as mean ± SD.*P < 0.05, **P < 0.01. Student's t‐test, n = 9–11 in each group (both in A and B).
Neostigmine Decreased the Ischemic Cerebral Injury Through the nAChR
Neostigmine significantly reduced the infarct size. This effect was completely abolished by the nAChR antagonist vecuronium (400 μg/kg; Zhejiang Xianju Pharmaceutical Co., Ltd, China), but not by the mAChR antagonist anisodamine (anisodamine hydrochloride, 20 mg/kg; Shanghai 1st Biochemistry Pharmaceutical Co., Ltd, Shanghai, China). Data are showed in Figure 4A.
The Protective Effect of Neostigmine on Ischemic Cerebral Injury Depends on α7nAChR
The α7nAChR KO mice (3 months old) were subjected to MCAO then administrated by neostigmine or the vehicle. The infarct size was significantly larger, by approximately 65%, in α7nAChR KO mice than in the WT controls (50.8 ± 15% vs. 30.8 ± 10%, Figure 4B). The α7nAChR KO mice also had significantly worse overall neurological function (neurological score 4.5 ± 0.5 vs. 2.2 ± 0.4 in WT controls). Neostigmine (80 μg/kg, ip) significantly reduced the infarct size (21.4 ± 7.6% vs. 34.8 ± 10.0% in the vehicle control group, P < 0.05) in WT mice, but not in α7nAChR KO mice (50.9 ± 4.9% vs. 50.8 ± 15.2%, Figure 4B).
The Protective Effect of ACh was Mediated by α7nAChR in Primary Neurons
Pretreatment with ACh (0.5 h before adding H2O2) decreased neuronal death induced by H2O2. Methyllycaconitine (Sigma‐Aldrich), a selective α7nAChR blocker, abolished the protective effect of ACh (Figure 6A–C left). In cultured neurons obtained from α7nAChR KO mice, ACh did not attenuate H2O2 damage (Figure 6C,D).
Figure 6.
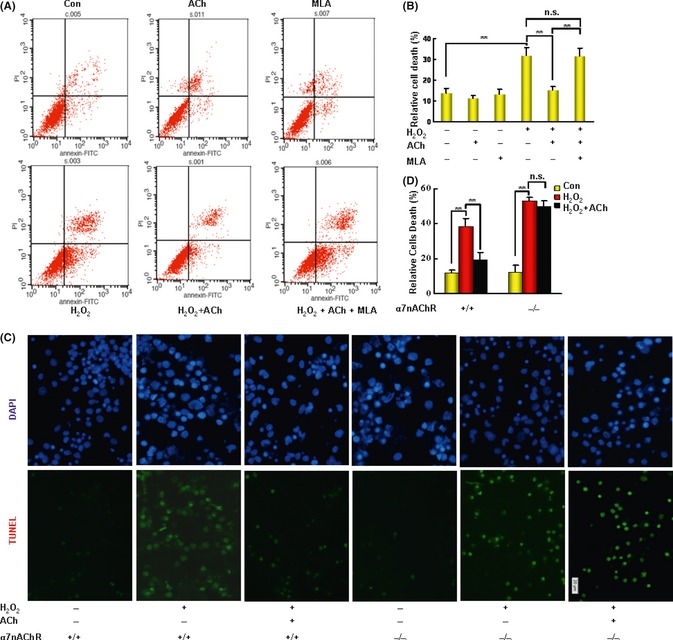
The neuroprotective effect of ACh depends on α7nAChR in cultured neurons. (A, B) Neocortical neurons were pretreated with ACh, with or without methyllycaconitine (MLA, the antagonist of α7nAChR), for 1 h prior to exposure to H 2 O 2 for 24 h. The cells were co‐stained with Annexin V‐FITC and PI (propidium iodide) and detected by flow cytometry. (C) Neocortical neurons from α7nAChR knockout (KO) or WT mice were pretreated with ACh for 1 h prior to exposure to H 2 O 2 for 24 h. Cells were labeled with TUNEL reaction mixture. The nuclei were counterstained with DAPI (dihydrochloride) (blue) for visualization. Cell survival was assessed by manually counting the cells. (n = 5 in each group). *P < 0.05, **P < 0.01. Data were analyzed with one‐way analysis of variance (ANOVA) followed by LSD t‐test. Scale bars: 20 μm. Data are presented as mean ± SD.
The Anti‐inflammatory Effect of ACh was Inhibited by the α7nAChR Blocker
In cultured primary microglial cells, ACh pretreatment decreased the production of IL‐1, IL‐6, and TNFα induced by lipopolysaccharide. This effect was abolished by methyllycaconitine (Figure 7).
Figure 7.
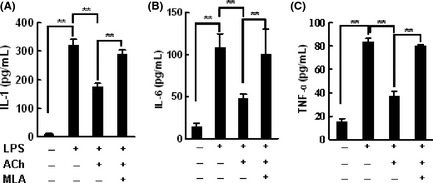
The anti‐inflammation effect of acetylcholine (ACh) was inhibited by methyllycaconitine. Microgliocytes were pretreated with ACh (0.1 mM) in the presence or absence of methyllycaconitine (MLA, 1 nM) for 2 h, then challenged with lipopolysaccharide (LPS, 0.5 μg/mL) for 24 h. Pro‐inflammatory cytokines in the supernatant were measured using ELISA. Experiments were repeated for three times. Data were analyzed with one‐way analysis of variance (ANOVA) followed by LSD t‐test. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Discussion
Baroreflex sensitivity is an important determinant of many cardiovascular diseases. Patients with a lower BRS exhibit shorter survival after myocardial infarction 5, heart failure 6 or stroke 12. We reported that, in rats, arterial baroreflex dysfunction promotes the development of atherosclerosis 23, determines the survival time in lipopolysaccharide‐induced lethal shock 24. Intact arterial baroreflex function is necessary to prevent aconitine‐induced ventricular arrhythmias 25. BRS is also an independent predictor for the incidence of stroke in hypertension 7. In that work, death after stroke was significantly delayed in rats with high BRS than those with low BRS. Restoration of BRS by ketanserin delayed stroke in SHR‐SPs 7. In the current study, we showed that impaired BRS increases the ischemic cerebral injury. The main parameters observed are the infarction area and neurological deficit score. It is well known that neuronal death or apoptosis and inflammation are very important in the pathogenesis of stroke. Therefore, apoptosis and inflammation were also used as valuing parameters in this work.
Arterial baroreflex consists of two arms with opposing action: the sympathetic and parasympathetic nervous systems 26. The sympathetic component is largely unaffected with increasing age or in cardiovascular diseases. In contrast, loss of parasympathetic activation is typically associated with old age as well as many cardiovascular diseases 27. Arterial baroreflex dysfunction could manifest as a decrease in reflex activity and also a decrease in tonic vagal activity. SAD decreased the spontaneous discharge of the neurons in the NA, the main nucleus driving the vagal nerve, of rats recorded under anesthesia. Tachycardia induced by atropine, reflecting the cardiac vagal tonic activity, was also significantly attenuated by SAD 28. Thus, vagal nerve may participate in the protective effects of arterial baroreflex against stroke.
Vesicular ACh transporter is responsible for recycling ACh from cytoplasm to vesicles and is critical for maintaining the activity of cholinergic terminals 29, 30. Decreased VAChT and BRS upon SAD in our study highlighted a close parallel relationship between the ACh and arterial baroreflex function. Functionally, increasing endogenous ACh with the anticholinesterase neostigmine significantly attenuated the ischemic cerebral injury after MCAO.
There are multiple nAChR subtypes in the brain 31, 32. Loss of neuronal nAChRs is associated with a number of central nervous system diseases 17. The nAChRs, particularly α7nAChR, are reported to be involved in neuronal survival and synaptic plasticity 33, 34. In the current study, MCAO produced much larger infarct size in α7nAChR KO mice than in WT controls. The protective effects of neostigmine on ischemic cerebral injury were nearly abolished by α7nAChR KO. Indeed, the protective effects of ACh in cultured neurons were also abolished by a α7nAChR antagonist or by α7nAChR KO.
It is well known that neostigmine does not increase cerebral blood flow in intact animals presumably because it is less permeable across the blood brain barrier 35, it likely does get into the brain during MCAO when the blood brain barrier is damaged. Indeed, neostigmine significantly decreased the infarct injury and increased antiapoptotic proteins and decreased proapoptotic protein in the ischemic penumbra. In cultured neurons, ACh decreased the cell apoptosis or death in an α7nAChR‐dependent manner. The α7nAChR activation also could reduce the release of proinflammatory cytokine 36, 37, which in turn plays a critical role in the outcome after stroke 38. In our experiments, neostigmine significantly decreased the proinflammatory cytokines levels. In culture microgliocytes, ACh decreased the production of proinflammatory cytokines in an α7nAChR‐dependent manner.
In conclusion, results from the current study indicated that ACh‐α7nAChR involved in the effects of arterial baroreflex on the ischemic cerebral injury. Activating the cholinergic system mimics the activation of arterial baroreflex against cerebral injury.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was funded by China Basic Research Program 2009CB521901 (D.F.S), and National Natural Science Foundation of China 30901809 (A.J.L), 30730106 (D.F.S.).
The first two authors contributed equally to this work.
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: Mechanisms and potential prevention strategies. CNS Neurosci Ther 2011;17:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology 2008;55:250–266. [DOI] [PubMed] [Google Scholar]
- 4. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 5. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 6. Mortara A, La Rovere MT, Pinna GD, et al. Arterial baroreflex modulation of heart enhancing baroreflex function is within the rostral ventrolateral medulla rate in chronic heart failure: Clinical and hemodynamic correlates and prognostic implications. Circulation 1997;96:3450–3458. [DOI] [PubMed] [Google Scholar]
- 7. Liu AJ, Ma XJ, Shen FM, Liu JG, Chen H, Su DF. Arterial baroreflex: A novel target for preventing stroke in rat hypertension. Stroke 2007;38:1916–1923. [DOI] [PubMed] [Google Scholar]
- 8. Gerritsen J, TenVoorde BJ, Dekker JM, Kostense PJ, Bouter LM, Heethaar RM. Baroreflex sensitivity in the elderly: Influence of age, breathing and spectral methods. Clin Sci (Lond) 2000;99:371–381. [PubMed] [Google Scholar]
- 9. Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2002;72:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eveson DJ, Robinson TG, Shah NS, Panerai RB, Paul SK, Potter JF. Abnormalities in cardiac baroreceptor sensitivity in acute ischaemic stroke patients are related to aortic stiffness. Clin Sci (Lond) 2005;108:441–447. [DOI] [PubMed] [Google Scholar]
- 11. Sykora M, Diedler J, Rupp A, Turcani P, Rocco A, Steiner T. Impaired baroreflex sensitivity predicts outcome of acute intracerebral hemorrhage. Crit Care Med 2008;36:3074–3079. [DOI] [PubMed] [Google Scholar]
- 12. Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long‐term outcome after acute ischemic stroke. Stroke 2003;34:705–712. [DOI] [PubMed] [Google Scholar]
- 13. Liu AJ, Ling G, Wu J, et al. Arterial baroreflex function is an important determinant on the acute cerebral ischemia in rats with middle cerebral artery occlusion. Life Sci 2008;83:388–393. [DOI] [PubMed] [Google Scholar]
- 14. Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: The non‐neuronal cholinergic system in humans. Br J Pharmacol 2008;154:1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384–388. [DOI] [PubMed] [Google Scholar]
- 16. Guarini S, Altavilla D, Cainazzo MM, et al. Efferent vagal fibre stimulation blunts nuclear factor‐kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation 2003;107:1189–1194. [DOI] [PubMed] [Google Scholar]
- 17. Shimohama S. Nicotinic receptor‐mediated neuroprotection in neurodegene‐ rative disease models. Biol Pharm Bull 2009;32:332–336. [DOI] [PubMed] [Google Scholar]
- 18. Tracey KJ. The inflammatory reflex. Nature 2002;420:853–859. [DOI] [PubMed] [Google Scholar]
- 19. Wang P, Tian WW, Song J, Guan YF, Miao CY. Deficiency of NG2+ cells contributes to the susceptibility of stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688–694. [DOI] [PubMed] [Google Scholar]
- 21. Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short‐term fasting induces profound neuronal autophagy. Autophagy 2010;6:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Li L, Chen S, et al. Rapamycin treatment augments motor neuron degeneration in SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 2011;7:412–425. [DOI] [PubMed] [Google Scholar]
- 23. Cai GJ, Miao CY, Xie HH, Lu LH, Su DF. Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis 2005;183:41–47. [DOI] [PubMed] [Google Scholar]
- 24. Shen FM, Guan YF, Xie HH, Su DF. Arterial baroreflex function determines the survival time in lipopolysaccharide‐induced shock in rats. Shock 2004;21:556–560. [DOI] [PubMed] [Google Scholar]
- 25. Shu H, Yi‐Ming W, Xu LP, Miao CY, Su DF. Increased susceptibility of ventricular arrhythmia to aconitine in anesthetized rats is attributed to the inhibition of baroreflex. Clin Exp Pharmacol Physiol 2004;31:249–253. [DOI] [PubMed] [Google Scholar]
- 26. Sykora M, Diedler J, Turcani P, Hacke W, Steiner T. Baroreflex: A new therapeutic target in human stroke? Stroke 2009;40:e678–e682. [DOI] [PubMed] [Google Scholar]
- 27. Ormezzano O, Cracowski JL, Quesada JL, Pierre H, Mallion JM, Baguet JP. Evaluation of the prognostic value of BARoreflex sensitivity in hypertensive patients: The EVABAR study. J Hypertens 2008;26:1373–1378. [DOI] [PubMed] [Google Scholar]
- 28. Yu JG, Song SW, Shu H, et al. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine‐α7‐nicotinic ACh receptor in rats. Eur Heart J 2011; doi: 10.1093/eurheartj/ehr299 [DOI] [PubMed] [Google Scholar]
- 29. Prado VF, Martins‐Silva C, de Castro BM, et al. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 2006;51:601–612. [DOI] [PubMed] [Google Scholar]
- 30. Guidine PA, Rezende GH, Queiroz CM, et al. Vesicular acetylcholine transporter knock‐down mice are more susceptible to pilocarpine induced status epilepticus. Neurosci Lett 2008;436:201–204. [DOI] [PubMed] [Google Scholar]
- 31. Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]‐ alpha‐bungarotoxin. J Neurosci 1985;5:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindstrom J, Anand R, Peng X, Gerzanich V, Wang F, Li Y. Neuronal nicotinic receptor subtypes. Ann N Y Acad Sci 1995;757:100–116. [DOI] [PubMed] [Google Scholar]
- 33. Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 2008;29:151–158. [DOI] [PubMed] [Google Scholar]
- 34. Parada E, Egea J, Romero A, del Barrio L, García AG, López MG. Poststress treatment with PNU282987 can rescue SH‐SY5Y cells undergoing apoptosis via α7 nicotinic receptors linked to a Jak2/Akt/HO‐1 signaling pathway. Free Radic Biol Med 2010;49:1815–1821. [DOI] [PubMed] [Google Scholar]
- 35. Scremin OU, Sonnenschein RR, Rubinstein EH. Cholinergic cerebral vasodilatation in the rabbit: Absence of concomitant metabolic activation. J Cereb Blood Flow Metab 1982;2:241–247. [DOI] [PubMed] [Google Scholar]
- 36. Akaike A, Takada‐Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: Role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci 2010;40:211–216. [DOI] [PubMed] [Google Scholar]
- 37. Rosas‐Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 2009;265:663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: The Edinburgh Artery Study. Circulation 2007;115:2119–2127. [DOI] [PubMed] [Google Scholar]


