SUMMARY
Introduction: The limitations of current Alzheimer's disease (AD) therapeutics have prompted investigation into innovative therapeutics focused on antiinflammatory, antioxidant, and neuroprotective agents including those from medicinal plants. Numerous plants have been tested for their potential for alleviating symptoms of AD. Aims: Zataria multiflora Boiss. (ZM) a member of Lamiaceae family has been used in Iranian traditional medicine for its beneficial effects on mental abilities. Therefore, the effect of its essential oil was evaluated in a rat model of AD. Methods: Amyloid β‐protein (Aβ) fragment 25–35 was injected bilaterally in the CA1 region of rats hippocampus and the effect of different doses of ZM essential oil (50, 100, or 200 μL/kg) on cognitive function was investigated in the Morris water maze. Acute toxicity of the essential oil was also studied. Results: The results showed increases in escape latency, traveled distance, heading angle, and decreases in target quadrant entries in Aβ‐received groups as compared to the control group. This impairment was reversed by ZM essential oil. The results of acute toxicity testing revealed that the calculated LD50 (1264.9 μL/kg) is much higher than the therapeutic dose (100 μL/kg). Conclusions: It seems that antioxidant, antiinflammatory, and anticholinesterase activities of ZM or its main constituents might contribute to its beneficial effects in this model. Our findings suggest that ZM may be a potentially valuable source of natural therapeutic agents for the treatment of AD. However, further investigations are necessary to establish its clinical efficacy and potential toxicity, before any recommendations concerning its use as a medication in the treatment of AD.
Keywords: Acute toxicity, Iranian traditional medicine, Lamiaceae, Morris water maze
Introduction
More than 25 million people in the world today are affected by dementia, most suffering from Alzheimer's disease (AD) [1]. The risk of AD grows exponentially with age, doubling approximately every 5 to 6 years. Doubling times of AD incidence rates are not statistically different among populations throughout the world [2]. In addition to its substantial economic impact, AD has devastating effects on the lives of affected individuals, their caregivers, and society. AD begins with gradual memory loss and progresses to personality change, behavioral disturbance, loss of executive function, and loss of the ability to perform basic activities of daily living, including eating, walking, dressing, and grooming. These impairments strain families and create challenges to the care and safety of the patient as well as threaten the health and well‐being of the caregiver [3].
The neuropathologic features of the disease are extracellular deposition of amyloid β‐protein (Aβ) surrounded by dead or dying neurons and inflammatory cells, intracellular formation of neurofibrillary tangles, basal forebrain cholinergic deficit, and extensive neuronal loss and synaptic changes in the entorhinal cortex and hippocampus portions of the brain related to memory [4]. Considerable evidence suggests increased Aβ levels correlate with declines in cognition and may be the initiator in a cascade of events that leads to cognitive decline observed in AD [5, 6]. Neuroinflammation [7] and oxidative stress [8] are also processes associated with the development and progression of the disease.
Although much progress has been made in understanding the pathogenesis of AD, the current therapeutic approaches are merely symptomatic, intended for the treatment of cognitive symptoms, such as disturbances in memory and perception [9, 10]. Two classes of medications have been approved by the US FDA for the treatment of AD: the acetylcholinesterase inhibitors (AChEIs) (tacrine, donepezil, rivastigmine, galantamine), mostly for mild‐to‐moderate AD, and the noncompetitive N‐methyl‐d‐aspartate (NMDA) receptor antagonist memantine for the moderate‐to‐severe stages of AD [9, 11]. While ameliorating symptoms, these drugs are not able to modify the course of disease progression, duration of their efficacy is limited, and also have been shown to produce some undesired effects such as gastrointestinal side effects of AChEIs, for example, nausea, diarrhea, vomiting, and weight loss. The side effects that may occur during the treatment with memantine are constipation, dizziness, headache, and confusion [12].
The limitations of current AD therapeutics have prompted investigation into innovative therapeutics over the last two decades. Research approaches are focusing on disease‐modifying and/or preventive agents [9]. Huge efforts are being made to identify drugs able to reduce Aβ accumulation through interfering with proteases regulating Aβ formation and drugs that increase its clearance. These drugs include compounds that stimulate alpha‐secretase, the enzyme responsible for nonamyloidogenic metabolism of amyloid precursor protein (APP), inhibitors of beta‐secretase, the enzyme that regulates the first step of amyloidogenic APP metabolism and inhibitors of gamma‐secretase, the pivotal enzyme that generates Aβ. However, all these therapeutic approaches await clinical trial completion and approval [13, 14]. At present, until a therapy is developed that can prevent or reverse the disease, the optimal goal for effective AD management is to develop a treatment regimen that will yield maximum benefits for individual patients across multiple domains, including cognition, daily functioning, and behavior [15]. Thus, therapeutic strategies have been focused on antiinflammatory, antioxidant, and neuroprotective agents including those from medicinal plants and health promoting foods that may protect against AD, possibly through scavenging of reactive oxygen species, cytokine downregulation, and strengthening the neurons antioxidant defense [16].
Numerous plants and their constituents have been used in traditional medicine to enhance cognitive function and to alleviate other symptoms of AD [17, 18, 19]. Recently, many medicinal plants have been tested for their potential for reducing symptoms of AD or for affecting the disease mechanism in animal and cellular models of AD and in clinical trials with AD subjects. Several species have shown in vitro or in vivo activities relevant to dementia (e.g., anticholinesterase, antiamyloid, antiinflammatory, antioxidant, neuroprotective, and memory enhancing). The most frequently reported are Ginkgo biloba, blueberry, cannabis, club moss, curcumin, garlic, ginseng, green tea, pomegranate, ginger, ginkgo, and rhubarb [18, 20, 21, 22, 23, 24].
In Iranian traditional medicine, beneficial effects of some plants consumed as food, spice, or natural remedies on mental abilities have been mentioned. Zataria multiflora Boiss. (ZM) a member of Lamiaceae family is one of these plants, which is widely distributed in Iran, Afghanistan, and Pakistan [25]. It has been used as a preservative or flavoring agent and also for its antiseptic, analgesic, and carminative properties [26]. This plant has been evaluated for its therapeutic effects in various studies. It has shown antibacterial [27, 28], antifungal [29, 30, 31], and antiparasitic [32] activities both in vivo and in vitro. Antinociceptive [33, 34] and antiinflammatory [33, 35] properties were also reported from the essential oil or extracts obtained from ZM. Furthermore, in different studies, ZM exhibited remarkable antioxidant activity [35, 36]. The composition of the essential oil of ZM was studied by gas liquid chromatography (GLC), column chromatography, nuclear magnetic resonance (NMR), and GLC/Mass spectrometry. The main constituents of the dry plant were carvacrol (61.3%) and thymol (25.1%), while the main constituents of the fresh plant were thymol (48.4%), carvacrol (12.6%), p‐cymene (13.5%), linalool (5.2%), and gamma‐terpinene (3.9%). The structures of the major components were confirmed by Infrared spectroscopy and NMR [37, 38]. In 2007, a study on Thymus vulgaris, another member of Lamiaceae family, showed anticholinesterase effect of thymol and carvacrol. It is interesting that the AChE inhibitory effect exerted by carvacrol was 10 times stronger than that exerted by its isomer thymol, although thymol and carvacrol have a very similar structure [39].
The above mentioned pharmacologic activities of ZM encouraged us to evaluate the ability of its essential oil against Aβ‐induced cognitive deficits in a rat model of AD.
Materials and Methods
Subjects
Male albino Wistar rats (200–250 g at the time of surgery) and male NMRI mice (25–30 g) (for LD50 determination) bred in the Pasteur Institute of Iran were used. They were housed four per cage in a constant temperature room (24 ± 1°C), under a 12‐h light/dark cycle (lights on at 07:00 h). All of the animals had free access to food and water throughout the experiment. The experimental procedures were carried out in accordance with international guidelines for care and use of laboratory animals and all efforts were made to minimize animal suffering and to reduce the number of animals used in the experiments.
Intrahippocampal Injection of Aβ (25–35)
Aβ Fragment 25–35 (Sigma, St. Louis, MO, USA) was dissolved in sterile deionized water (vehicle) at a concentration of 5 μg/μL (4.7 mM) and incubated at 37°C for 4 days to obtain the aggregated form. Animals were anesthetized with a combination of Ketamine (50 mg/kg, i.p.) and Xylazine (5 mg/kg, i.p.) and placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). One microlitre Aβ or vehicle (control group) were infused bilaterally into the CA1 region of the hippocampus (5 μg/side equivalent to 4.7 nmol/side) using a 1‐μL Hamilton microsyringe with the following coordinates according to the rat brain atlas of Paxinos and Watson [40]: AP −3.80 mm from bregma, ML ± 2.2 mm from midline, DV −2.7 mm from the skull surface. The incisor bar was lowered 3.3 mm below horizontal zero to achieve the flat skull position. The rate of injection was 0.5 μL/min. After completion of the infusion, the needle was left in place for 3 min to allow diffusion of the drug from the needle.
Plant Material and Essential Oil Preparation
Dried leaves of ZM were obtained from a local market. The plant was authenticated at the Department of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. A voucher specimen for this plant was deposited at the Herbarium of that department (No. 619). The essential oil was prepared by hydrodistillation for 4 h by using a Clevenger apparatus with 1–2% v/w yield. Essential oil was kept protected from light and stored at 4°C until use.
Essential Oil Administration
Seven days after surgery, rats were subjected to Morris water maze training. They were placed in groups of 10 (n = 10) and received different doses of ZM essential oil (50, 100, or 200 μL/kg), or equal volume (2 mL/kg) of the vehicle (5% v/v Tween 80, Merck, Germany). All injections were made i.p. 30 min prior to testing in Morris water maze each day. One Aβ‐injected group was tested without any injections.
Morris Water Maze Task
The water maze system and the procedure were described previously [41]. In short, animals received a block of four trials during five daily sessions. During the first 4 days, the platform, situated in the center of the southwest quadrant, was submerged 1.5 cm below the surface of water and therefore invisible, for testing spatial learning. The platform position remained stable over 4 days and acquisition of this task was assessed. On day 5, the platform was elevated above water level, covered with a piece of aluminum foil, and placed in the center of the southeast quadrant. This assessed motivation and sensorimotor coordination toward a visible platform. A trial was started by placing a rat into the pool, facing the wall of the tank. Each of four starting positions (north, east, south, and west) was used once in a series of four trials; their order was randomized. Each trial was terminated as soon as the rat had climbed onto the escape platform or when 90 seconds had elapsed. A rat was allowed to stay on the platform for 20 seconds. Then it was taken from the platform and the next trial was started. Rats that did not find the platform within 90 seconds, were put on the platform by the experimenter and were allowed to stay there for 20 seconds. After completion of the fourth trial, rats were gently dried with a towel, kept warm for an hour, and returned to their home cage. The path of each rat on each trial was automatically recorded by a computerized system and then analyzed by computing several parameters, for example, latency to find the platform, traveled distance, heading angle (the angle between a line intersecting rat's position after 50 cm and the start point and a line intersecting this point and the center of the platform), and percentage of quadrant entries as well as the swim speed. All tests were conducted between 08:00 and 10:00 h.
Acute Toxicity Test
Acute toxicity of ZM essential oil was investigated using a total of 13 mice according to the procedure described by Lorke in 1983 [42]. The study was carried out in two phases. In the initial phase, the range of doses producing toxic effects was determined. The animals were divided into three groups (n = 3) and received i.p. administration of 10, 100, or 1000 μL/kg of essential oil. They were observed for signs of toxicity and death within 24 h. In the second phase, four more mice were administered different doses of essential oil, which were chosen based on the results of the first phase and the treated animals were again monitored for 24 h. Then, the highest nonlethal dose and the lowest lethal dose were obtained and the LD50 was calculated as the geometric mean of these values [42].
Statistical Analysis
The data of first four training days with hidden platform were initially subjected to a one‐way analysis of variance (ANOVA) for repeated measures, followed by post hoc analysis, Tukey's honestly significant difference. Data of the fifth day with visible platform were analyzed by one‐way ANOVA. In all comparisons, P < 0.05 was used as the criterion for statistical significance.
Results
Morris Water Maze Task
There was significant difference in escape latencies as well as traveled distances among the groups during the 4 days with invisible platform (latency: F 5,54= 12.746, P < 0.0001; distance: F 5,54= 11.553, P < 0.0001). Post hoc analysis showed that Aβ significantly increased escape latency (P < 0.01) and traveled distance (P < 0.01) compared to the control group. Administration of ZM essential oil at doses of 100 (P < 0.001) or 200 μL/kg (P < 0.01) attenuated the effects of Aβ on both parameters. (Figures 1 and 2; open bars). Escape latencies and traveled distances on day 5 with visible platform were not significantly different compared to the control group (latency: F 5,54= 0.743, n.s.; distance: F 5,54= 1.076, n.s.) (1, 2; filled bars).
Figure 1.
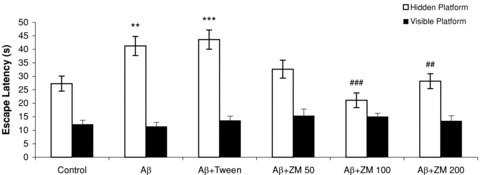
Mean escape latencies in seconds to find the platform in a water maze. On days 1–4, rats learned to reach an invisible platform (data were averaged to obtain a mean performance for each animal; open bars). On day 5, the platform was visible (filled bars). Six groups (n = 10) were tested: control; Aβ; Aβ+ Tween; Aβ+Zataria multiflora (ZM) essential oil (50, 100, or 200 μL/kg). Error bars indicate ± SEM. **P < 0.01, ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus Aβ.
Figure 2.
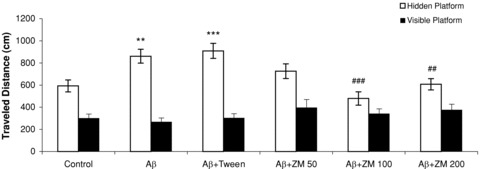
Mean traveled distances in centimeters to find the platform in a water maze. On days 1–4, rats learned to reach an invisible platform (data were averaged to obtain a mean performance for each animal; open bars). On day 5, the platform was visible (filled bars). Six groups (n = 10) were tested: control; Aβ; Aβ+ Tween; Aβ+Zataria multiflora (ZM) essential oil (50, 100, or 200 μL/kg). Error bars indicate ± SEM. **P < 0.01, ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus Aβ.
During days 1–4 a significant effect of groups was found in heading angles to locate the hidden platform among the groups (F 5,54= 6.861, P < 0.0001). Tukey test showed that the heading angle significantly differed for the Aβ group compared to the control group (P < 0.01). The effect of Aβ on heading angle was also overcome by ZM essential oil at doses of 50 (P < 0.05) or 100 μL/kg (P < 0.001). (Figure 3; open bars). There were no significant changes induced by Aβ or essential oil heading angles on day 5 with visible platform (F 5,54= 1.798, n.s.) (Figure 3; filled bars).
Figure 3.
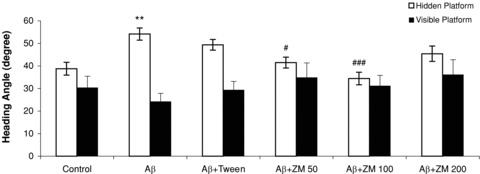
Mean heading angles in degrees for finding the platform in a water maze. On days 1–4, rats learned to reach an invisible platform (data were averaged to obtain a mean performance for each animal; open bars). On day 5, the platform was visible (filled bars). Six groups (n = 10) were tested: control; Aβ; Aβ+ Tween; Aβ+Zataria multiflora (ZM) essential oil (50, 100, or 200 μL/kg). Error bars indicate ± SEM. **P < 0.01 versus control; #P < 0.05, ###P < 0.001 versus Aβ.
There was also significant difference in percent of entries into the target (F 5,54= 10.515, P < 0.0001) and opposite (F 5,54= 8.020, P < 0.0001) quadrants. The number of entries of the groups, which had received Aβ into the target quadrant was significantly lower (P < 0.05) compared to the control group. Aβ significantly increased the number of entries into the opposite quadrant (P < 0.01). This effect was also reversed by administration of ZM essential oil at doses of 100 (P < 0.001) or 200 μL/kg (P < 0.01). (Figure 4).
Figure 4.
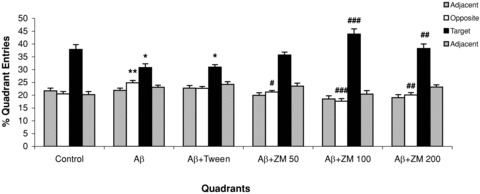
Mean percent of quadrant entries ± SEM during the 4 days of training in a water maze with the hidden platform (data were averaged to obtain a mean performance for each animal). Six groups (n = 10) were tested: control; Aβ; Aβ+ Tween; Aβ+Zataria multiflora (ZM) essential oil (50, 100, or 200 μL/kg). Error bars indicate ± SEM. *P < 0.05, **P < 0.01 versus control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus Aβ.
Swimming speeds did not significantly differ among the groups neither during the four days with hidden platform (F 5,54= 1.311, n.s.) nor on the fifth day with visible platform (F 5,54= 1.692, n.s.) (data not shown).
Acute Toxicity Test
The result of the acute toxicity studies is shown in Table 1. Doses of 600, 1000, 1600, or 2900 μL/kg were chosen based on the results of the first phase. The LD50 of the essential oil was found to be 1264.9 μL/kg.
Table 1.
Acute toxicity test of Zataria multiflora essential oil. LD50 = (1000 × 1600)1/2= 1264.9 μL/kg.
| Dose (μL/kg) | Number of death at 24 h after treatment per group |
|---|---|
| Phase I | |
| 10 | 0/3 |
| 100 | 0/3 |
| 1000 | 1/3 |
| Phase II | |
| 600 | 0/1 |
| 1000 | 0/1 |
| 1600 | 1/1 |
| 2900 | 1/1 |
Discussion
The Aβ‐related neuropathological features of the AD brain can be mimicked by intracerebral or intracerebroventricular infusion of Aβ peptides of different lengths in the rodent brain. Infusion of Aβ peptides can lead to AD‐related behavioral alterations learning and memory deficits, and disruption of cholinergic function [43]. The Aβ model, as a complementary alternative model to transgenic animals, provides insight into the cellular and molecular pathways induced by amyloid and AD‐related memory deficits. Data obtained from this model make it a particularly valuable tool for screening new therapeutic strategies and preclinical evaluation of drugs targeting Aβ[43, 44].
In this study, we assessed the effects of ZM essential oil on Aβ‐induced cognitive impairment in rats. Our results showed that Aβ administration in the CA1 region of rat hippocampus significantly impaired the ability of rats to locate the hidden platform. Because Aβ did not change the swimming speeds of the animals and ability of rats to find the visible platform, the observed effects are unlikely to be associated with changes in locomotion or sensorimotor coordination and can be attributed to spatial performance. The Aβ‐induced learning deficits were reversed by administration of ZM essential oil. On the other hand, the results of acute toxicity testing of ZM essential oil revealed that the calculated LD50 (1264.9 μL/kg) is much higher than the therapeutic dose (100 μL/kg), which appears to reflect its safety.
Evidence gathered over the last two decades suggests that the gradual accumulation of soluble and insoluble Aβ peptide species triggers a cascade of events that leads to the clinical manifestation of AD. Aβ accumulation has also been associated with the cholinergic dysfunction observed in AD [45]. The hippocampus is especially susceptible in AD and early degenerative symptoms include significant deficits in the performance of hippocampal‐dependent cognitive abilities such as spatial learning and memory. Recent experimental evidence suggests that Aβ disturbs NMDA receptor‐dependent long‐term potentiation induction in the CA1 and dentate gyrus both in vivo and in vitro. [46].
Neuroinflammation is considered to play a key role during the development of AD and has been associated with the formation of amyloid plaques and neurofibrillary tangles [7, 47]. Increased levels of inflammatory markers have been correlated with an advanced cognitive impairment [48]. Oxidative stress and impaired mitochondrial function are also processes that always accompany AD [8, 49]. Increased amounts of Aβ peptide was shown to induce elevated reactive oxygen species production [50, 51]. A recent study has demonstrated that mitochondria‐targeted antioxidants could prevent Aβ toxicity in AD neurons [52].
Although a number of drugs, including several AChEIs and an NMDA receptor antagonist, have been approved for use, they provide only modest symptomatic relief without any disease‐modifying activity [15, 53]. Furthermore, it should be considered that medication adherence in AD is not high and good adherence is essential for attempting to slow disease progression and improve or stabilize quality of life [9].
Recently, drug discovery strategies based on natural products and traditional medicines are reemerging as attractive options [54]. A variety of plants and their active constituents have been used in traditional medicine, for their reputed cognitive‐enhancing and anti‐Alzheimer's effects. They have shown pharmacological activities including enhancement of cholinergic function in the central nervous system, antiinflammatory, and antioxidant effects that may be relevant to the treatment of neurodegenerative disorders such as AD [17]. Some natural products have been evaluated for their ability to protect neuronal cells from direct Aβ insult. Ginkgo biloba extract and red wine‐derived polyphenols were shown to protect rat hippocampal cells against toxic effects of Aβ peptides and oxidative stress [21]. Curcuma longa (turmeric) has also been reported to protect PC12 and HUVEC cells from Aβ (1–42) insult. [55]. Zingiber officinale (ginger) extracts, Cinnamum cassia (Chinese cinnamon), Rheum coreanum (Korean rhubarb) [23], and Lycium barbarum (Wolfberry) [56] have demonstrated the same ability.
In this study, we observed that pretraining i.p. administration of ZM essential oil could reverse the learning deficits caused by Aβ. ZM, a member of Lamiaceae family, has been used in Iranian traditional medicine for its beneficial effects on cognitive function. It has demonstrated antiinflammatory [33, 35] and antioxidant activities [35, 36] in different studies. Numerous plants and plant constituents have demonstrated antioxidant or antiinflammatory properties and therefore are suggested to have favorable effects in AD [17, 48, 53, 57]. The observation of ZM ability in ameliorating Aβ‐induced cognitive impairment could be partially attributed to its antioxidant and antiinflammatory activities.
The main constituents of the dry plant essential oil have been reported to be carvacrol (61.3%) and thymol (25.1%) [37, 38]. Carvacrol and thymol both have shown AChEI effect, the activity of carvacrol being stronger than thymol [39]. AChEIs are the mainstays of current pharmacotherapy for AD [11]. A potential source of AChEIs is certainly provided by the plant sources in nature [19]. Most of the current AD medications with AChEI activity were originally isolated from plants. For example, physostigmine, galantamine, and huperzine A were obtained from Physostigma venenosum, Galanthus nivalis and Huperzia serrata, respectively [17, 19]. In addition, numerous plants have been investigated for their effects on AChE and have shown inhibitory activity. For example, extracts or essential oils of Ginkgo biloba [19], Salvia species (S. officinalis L., S. lavandulaefolia Vahl. and S. miltiorrhiza Bung.) [19, 58], Melissa officinalis, and Rosmarinus officinalis [17] have been reported to inhibit AChE. Because current therapeutic strategy in AD patients is to restore cholinergic function through inhibition of AChE and thereby facilitating cholinergic neurotransmission, it seems that the AChEI activity of carvacrol and thymol as the major constituents of ZM essential oil might contribute to its beneficial effects in this study.
Medicinal plants are the source of a large number of essential drugs and are the basis of herbal medicine, which is not only the primary source of health care for most of the world's population living in developing countries but also enjoys growing popularity in developed countries [59]. A range of herbal preparations, either single herbs or herbal mixtures, are potentially beneficial for the improvement of cognitive function in various age‐related dementias [18]. In AD animal and cellular models, herbal extracts appear to have fewer adverse effects than beneficial effects on cognitive function [20, 54]. It should be considered that medicinal and aromatic plants are intimately linked with human health and culture [60]. Because most of these compounds are part of routinely used traditional medicines, their tolerance and safety are relatively better known than synthetic chemicals [54].
In conclusion, this study provides preliminary positive evidence for the effectiveness and safety of ZM essential oil for Aβ toxicity management. Our results suggest that ZM may be a potentially valuable source of natural therapeutic agents for alleviating cognitive symptoms of AD. While AD cannot be cured, disease progression can be delayed and quality of life improved with beneficial complementary and alternative medicinal tools. Due to their small molecular size and lipophilicity, volatile constituents of essential oils are likely to readily cross the blood‐brain barrier [58] and exert their therapeutic actions effectively. Because ZM has been consumed as a spice and natural remedy for years, its toxicity is probably less than unknown substances. However, further investigations are necessary to establish its clinical efficacy and potential toxicity, before any recommendations concerning its use as an antidementia treatment. We are going to assess the protective ability of ZM essential oil and its main constituents in cellular models of AD and then study its potential therapeutic effects in clinical trials.
Author Contributions
Nahid Majlessi: Concept/design, writing the article.
Samira Choopani: Data collection, approval of article.
Mohammad Kamalinejad: Plant identification/preparation of essential oil, approval of article.
Zahra Azizi: Data analysis/interpretation, approval of article.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgment
This work was supported by a grant (No. 475) from the Pasteur Institute of Iran.
References
- 1. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 2009;11:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ziegler‐Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer's disease incidence rates. Alzheimers Dement 2008;4:316–323. [DOI] [PubMed] [Google Scholar]
- 3. Christensen DD, Lin P. Practical treatment strategies for patients with Alzheimer's disease. J Fam Pract 2007;56:S17–S23. [PubMed] [Google Scholar]
- 4. Law A, Gauthier S, Quirion R. Say NO to Alzheimer's disease: The putative links between nitric oxide and dementia of the Alzheimer's type. Brain Res Brain Res Rev 2001;35:73–96. [DOI] [PubMed] [Google Scholar]
- 5. Rodrigue KM, Kennedy KM, Park DC. Beta‐amyloid deposition and the aging brain. Neuropsychol Rev 2009;19:436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bharadwaj PR, Dubey AK, Masters CL, Martins RN, Macreadie IG. Abeta aggregation and possible implications in Alzheimer's disease pathogenesis. J Cell Mol Med 2009;13:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akiyama H, Barger S, Barnum S, et al Inflammation and Alzheimer's disease. Neurobiol Aging 2000;21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer's disease. J Alzheimers Dis 2009;16:741–761. [DOI] [PubMed] [Google Scholar]
- 9. Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer's disease. CNS Drugs 2009;23:293–307. [DOI] [PubMed] [Google Scholar]
- 10. Neugroschl J, Sano M. An update on treatment and prevention strategies for Alzheimer's disease. Curr Neurol Neurosci Rep 2009;9:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lleó A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer's disease. Annu Rev Med 2006;57:513–533. [DOI] [PubMed] [Google Scholar]
- 12. Mimica N, Presecki P. Side effects of approved antidementives. Psychiatr Danub 2009;21:108–113. [PubMed] [Google Scholar]
- 13. Panza F, Solfrizzi V, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, Capurso A, Imbimbo BP. Disease‐modifying approach to the treatment of Alzheimer's disease: From alpha‐secretase activators to gamma‐secretase inhibitors and modulators. Drugs Aging 2009;26:537–555. [DOI] [PubMed] [Google Scholar]
- 14. Spencer B, Rockenstein E, Crews L, Marr R, Masliah E. Novel strategies for Alzheimer's disease treatment. Expert Opin Biol Ther 2007;7:1853–1867. [DOI] [PubMed] [Google Scholar]
- 15. Farlow MR, Miller ML, Pejovic V. Treatment options in Alzheimer's disease: Maximizing benefit, managing expectations. Dement Geriatr Cogn Disord 2008;25:408–422. [DOI] [PubMed] [Google Scholar]
- 16. Steele M, Stuchbury G, Münch G. The molecular basis of the prevention of Alzheimer's disease through healthy nutrition. Exp Gerontol 2007;42:28–36. [DOI] [PubMed] [Google Scholar]
- 17. Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother Res 2003;17:1–18. [DOI] [PubMed] [Google Scholar]
- 18. May BH, Lit M, Xue CC, et al Herbal medicine for dementia: A systematic review. Phytother Res 2009;23:447–459. [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007;14:289–300. [DOI] [PubMed] [Google Scholar]
- 20. Anekonda TS, Reddy PH. Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Res Brain Res Rev 2005;50:361–376. [DOI] [PubMed] [Google Scholar]
- 21. Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging 2002;23:891–897. [DOI] [PubMed] [Google Scholar]
- 22. Kalaria RN, Maestre GE, Arizaga R, et al; World Federation of Neurology Dementia Research Group . Alzheimer's disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol 2008;7:812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DS, Kim JY, Han YS. Alzheimer's disease drug discovery from herbs: Neuroprotectivity from beta‐amyloid (1–42) insult. J Altern Complement Med 2007;13:333–340. [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J Neurochem 2010;112:1415–1430. [DOI] [PubMed] [Google Scholar]
- 25. Ali MS, Saleem M, Ali Z, Ahmad VU. Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry 2000;55:933–936. [DOI] [PubMed] [Google Scholar]
- 26. Zargari A. Medicinal plants, 4th ed Vol. 4 Tehran : Tehran University Press, 1990. (in Persian). [Google Scholar]
- 27. Mahboubi M, Bidgoli FG. Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine 2010;17:548–550. [DOI] [PubMed] [Google Scholar]
- 28. Simbar M, Azarbad Z, Mojab F, Majd HA. A comparative study of the therapeutic effects of the Zataria multiflora vaginal cream and metronidazole vaginal gel on bacterial vaginosis. Phytomedicine 2008;15:1025–1031. [DOI] [PubMed] [Google Scholar]
- 29. Amanlou M, Beitollahi JM, Abdollahzadeh S, Tohidast‐Ekrad Z. Miconazole gel compared with Zataria multiflora Boiss. gel in the treatment of denture stomatitis. Phytother Res 2006;20:966–969. [DOI] [PubMed] [Google Scholar]
- 30. Khosravi AR, Eslami AR, Shokri H, Kashanian M. Zataria multiflora cream for the treatment of acute vaginal candidiasis. Int J Gynaecol Obstet 2008;101:201–202. [DOI] [PubMed] [Google Scholar]
- 31. Mahmoudabadi AZ, Dabbagh MA, Fouladi Z. In vitro anti‐candida activity of Zataria multiflora Boiss. Evid Based Complement Alternat Med 2007;4:351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ziegler HL, Franzyk H, Sairafianpour M, et al Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: Structure‐activity relationships for betulinic acid analogues. Bioorg Med Chem 2004;12:119–127. [DOI] [PubMed] [Google Scholar]
- 33. Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti‐inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 2000;73:379–385. [DOI] [PubMed] [Google Scholar]
- 34. Jaffary F, Ghannadi A, Siahpoush A. Antinociceptive effects of hydroalcoholic extract and essential oil of Zataria multiflora . Fitoterapia 2004;75:217–220. [DOI] [PubMed] [Google Scholar]
- 35. Nakhai LA, Mohammadirad A, Yasa N, et al Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med 2007;4:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saei‐Dehkordi SS, Tajik H, Moradi M, Khalighi‐Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol 2010;48:1562–1567. [DOI] [PubMed] [Google Scholar]
- 37. Saleem M, Nazli R, Afza N, Sami A, Ali MS. Biological significance of essential oil of Zataria multiflora Boiss . Nat Prod Res 2004;18:493–497. [DOI] [PubMed] [Google Scholar]
- 38. Shafiee A, Javidnia K. Composition of essential oil of Zataria multiflora . Planta Med 1997;63:371–372. [DOI] [PubMed] [Google Scholar]
- 39. Jukic M, Politeo O, Maksimovic M, Milos M, Milos M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res 2007;21:259–261. [DOI] [PubMed] [Google Scholar]
- 40. Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed. Orlando, FL : Academic Press, 1986. [Google Scholar]
- 41. Majlessi N, Choopani S, Bozorgmehr T, Azizi Z. Involvement of hippocampal nitric oxide in spatial learning in the rat. Neurobiol Learn Mem 2008;90:413–419. [DOI] [PubMed] [Google Scholar]
- 42. Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983;54:275–287. [DOI] [PubMed] [Google Scholar]
- 43. Van Dam D, De Deyn PP. Drug discovery in dementia: The role of rodent models. Nat Rev Drug Discov 2006;5:956–970. [DOI] [PubMed] [Google Scholar]
- 44. Stéphan A, Phillips AG. A case for a non‐transgenic animal model of Alzheimer's disease. Genes Brain Behav 2005;4:157–172. [DOI] [PubMed] [Google Scholar]
- 45. Thathiah A, De Strooper B. G protein‐coupled receptors, cholinergic dysfunction, and Abeta toxicity in Alzheimer's disease. Sci Signal 2009;2:re8. [DOI] [PubMed] [Google Scholar]
- 46. Yamin G. NMDA receptor‐dependent signaling pathways that underlie amyloid beta‐protein disruption of LTP in the hippocampus. J Neurosci Res 2009;87:1729–1736. [DOI] [PubMed] [Google Scholar]
- 47. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McNaull BB, Todd S, McGuinness B, Passmore AP. Inflammation and anti‐inflammatory strategies for Alzheimer's disease–a mini‐review. Gerontology 2010;56:3–14. [DOI] [PubMed] [Google Scholar]
- 49. Shi Q, Gibson GE. Oxidative stress and transcriptional regulation in Alzheimer disease. Alzheimer Dis Assoc Disord 2007;21:276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: A common pathology. J Alzheimers Dis 2009;17:245–257. [DOI] [PubMed] [Google Scholar]
- 51. Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA. Subcellular and metabolic examination of amyloid‐beta peptides in Alzheimer disease pathogenesis: Evidence for Abeta (25–35). Exp Neurol 2010;221:26–37. [DOI] [PubMed] [Google Scholar]
- 52. Manczak M, Mao P, Calkins MJ, et al Mitochondria‐targeted antioxidants protect against amyloid‐beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis 2010;20:S609–S631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: Different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol 2009;9:434–444. [DOI] [PubMed] [Google Scholar]
- 54. Patwardhan B, Mashelkar RA. Traditional medicine‐inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov Today 2009;14:804–811. [DOI] [PubMed] [Google Scholar]
- 55. Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett 2001;303:57–61. [DOI] [PubMed] [Google Scholar]
- 56. Chang RC, So KF. Use of anti‐aging herbal medicine, Lycium barbarum, against aging‐associated diseases. What do we know so far? Cell Mol Neurobiol 2008;28:643–652. [DOI] [PubMed] [Google Scholar]
- 57. Pendry B, Busia K, Bell CM. Phytochemical evaluation of selected antioxidant‐containing medicinal plants for use in the preparation of a herbal formula—A preliminary study. Chem Biodivers 2005;2:917–922. [DOI] [PubMed] [Google Scholar]
- 58. Savelev SU, Okello EJ, Perry EK. Butyryl‐ and acetyl‐cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res 2004;18:315–324. [DOI] [PubMed] [Google Scholar]
- 59. Sucher NJ, Carles MC. Genome‐based approaches to the authentication of medicinal plants. Planta Med 2008;74:603–623. [DOI] [PubMed] [Google Scholar]
- 60. Gómez‐Galera S, Pelacho AM, Gené A, Capell T, Christou P. The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 2007;26:1689–1715. [DOI] [PubMed] [Google Scholar]


