SUMMARY
Background: Given the limited efficacy of current pharmacotherapy for major depressive disorder (MDD) and the historical decline in antidepressant development, there is increasing clinical urgency to develop more effective treatments. Objectives: To synthesize findings from clinical psychology and affective neuroscience related to the construct of emotional temperament; to examine the effects of antidepressants on the temperament dimensions of positive (PA) and negative affectivity (NA); and to propose a biobehavioral research paradigm for the treatment of MDD. Methods: We begin with an introduction to PA and NA, which emphasizes their construct development, historical context, and relevance to psychopathology. We then review studies of antidepressant effects on PA and NA, and explore two related hypotheses: (1) Cause‐correction: The antidepressant response may fundamentally occur through changes in emotional temperament, with subsequent spread to syndrome or symptom changes; (2) preferential effects: Antidepressants with different mechanisms of action may have preferential effects on PA or NA. Results: Preliminary findings appear to support the cause‐correction hypothesis; there is insufficient clinical evidence to support the preferential effects hypothesis. Conclusions: PA and NA are biologically based temperament dimensions, which modulate emotional, motivational, and behavioral responses to positive and negative incentives. They can be altered by antidepressants, and may independently contribute to depression improvement. In addition, the distinct biobehavioral features of PA and NA suggest that combined pharmacological and cognitive–behavioral treatments targeting these dimensions may have specific, and perhaps, synergistic antidepressant effects.
Keywords: Combined cognitive–behavioral and pharmacological treatment, Dopamine and the reward system, Emotional temperament, Major depressive disorder
Introduction
Early versions of The Diagnostic and Statistical Manual of Mental Disorders (DSM I and II) did not clearly distinguish between wellness and sickness [1], or emotional and psychotic disorders [2]. To address these problems, the authors of DSM‐III (1980–1987), and its current iteration DSM‐IV‐TR (2000‐), proposed diagnostic categories, which represent mental disorders as present or absent (i.e., categorical variables), using temporal, functional, and symptom‐based criteria. This has helped to create a reliable language for naming mental disorders [3]. However, the “splitting” of psychopathology into nearly 400 disorders may have obscured shared biological substrates and specific predictors of response.
In contrast, dimensional models seek to identify underlying factors, which influence phenotypic expression. Across emotional disorders, psychology research has shown that positive affectivity (PA) and negative affectivity (NA), defined as dimensions of emotional temperament, can help to account for the comorbid features of mood and anxiety disorders [4], and may provide a framework for transdiagnostic treatments [5]. However, PA and NA have only recently become a focus of pharmacological research (possibly related to the historical divide between psychology and psychiatry; the specialized nomenclature surrounding the constructs themselves; and their origin in quantitative methods [such as factor analysis and structural equation modeling; SEM]).
Given the limited efficacy of current pharmacotherapy for major depressive disorder [MDD; Refs. 6, 7, 8, 9] and the historical decline in the development of antidepressants [10], there is increasing clinical urgency to develop more effective treatments. In this article, we will synthesize findings from clinical psychology and affective neuroscience related to the construct of emotional temperament; examine the effects of antidepressants on PA and NA (Table 1); and propose a biobehavioral research paradigm for MDD.
Table 1.
Important Studiesa
| Authors | Design | Finding(s) | Significance |
|---|---|---|---|
| McCabe et al., 2009 [36] | Comparison of neural responses to reward‐related and aversive stimuli in unmedicated, recovered depressed subjects versus healthy controls. | Unmedicated, recovered depressed patients exhibited reduced response to reward, measured as hypoactivity in the ventral striatum, despite self‐reported levels of pleasure similar to healthy controls. | 1. Patients with a history of depression may have neural deficits in reward processing. 2. Deficits may also represent an endophenotype for depression and/or a target for treatment and prevention strategies. |
| Gotlib et al., 2010 [35] | Comparison of neural processing of reward and loss conditions in healthy children with strong genetic loading for depression (high risk) versus healthy children with no family history of depression (low risk). | High risk children showed decreased activity in putamen and left insula during anticipation of reward and increased activity in right insula compared to low risk controls. | 1. Abnormal neural processing of reward may be a susceptibility factor for depression. |
| Tang et al., 2009 [52] | Double‐blind, controlled trial of 240 MDD subjects randomized to 16 weeks of cognitive therapy, paroxetine, or 8 weeks of placebo. Placebo completers could then elect to enter into an additional open‐label trial of an SSRI. | 1. Linear regression analysis: all 3 groups showed improvement in depression over the first 8 weeks, but changes in trait extraversion/neuroticism on the NEO‐FI were 4–8 times greater with paroxetine than placebo. 2. Matching analysis: Paroxetine‐treated patients demonstrated 3.5 times greater change in PA and 6.8 times greater change in NA compared to placebo‐treated subjects; paroxetine‐treated patients had 1.9 times greater improvements in NA relative to PA. | 1. Supports the cause‐correction hypothesis. 2. Partially supports the preferential effects hypothesis. |
| Quilty et al., 2010 [54] | Data from naturalistic and randomized, controlled trials combined in a structural equation modeling analysis comparing MDD patients who received SSRIs with those receiving noradrenergic and dopaminergic reuptake blockers (NDMs) or reversible monoamine oxidase inhibitors (RIMAs). | 1. MDD subjects receiving SSRIs exhibited greater trait NA/neuroticism change than those receiving noradrenergic and dopaminergic reuptake blockers (NDMs) and reversible monoamine oxidase inhibitors (RIMAs). 2. Goodness‐of‐fit indices favored a mediation rather than complication model. | 1. SSRI induced reduction in NA may mediate depression symptom changes. 2. SEM may also be a valuable technique for modeling antidepressant effects through the dimension of PA. |
| Harmer et al., 2009 [55] | Double‐blind, controlled study of 31 depressed patients and 31 matched healthy controls randomized to a single dose of reboxetine (4 mg) or placebo. Subjects given a battery of emotional processing tasks before and 3 h after administration of treatment. | Depressed patients treated with reboxetine exhibited a reversal in emotional appraisal deficits, including recognition of positive facial expressions, response speed to positive self‐relevant personality adjectives, and memory for these positive adjectives compared to placebo. | 1. Emotional processing in depressed patients can be modified by the acute administration of an NRI antidepressant in the absence of symptom changes. 2. Reversal of these emotional processing deficits may provide a key substrate for synergistically combining pharmacological and psychological interventions. |
| Murphy et al., 2009 [56] | Double‐blind study of 26 healthy volunteers randomized to a single dose of citalopram (20 mg) or placebo. 3 h after administration subjects performed an fMRI block design task which measured neural response to backwardly masked and unmasked presentations of fearful, neutral, and happy facial expressions. | Subjects treated with citalopram demonstrated a significantly reduced amygdala response to fearful facial expressions compared to placebo. | 1. Emotional processing in healthy controls can be modified by the acute administration of an SSRI antidepressant. 2. Early modification of emotional appraisal may represent a functional mechanism for the delayed, clinical effects of SSRI antidepressants. |
| Knutson et al., 1998 [67] | Double‐blind, controlled trial of 51 healthy participants randomized to a fixed dose of paroxetine (20 mg/day) or placebo over a 4‐week period. | 1. Ratings of NA on the PANAS decreased significantly in the paroxetine group compared to placebo. The bulk of these changes occurred within the first week. 2. No significant changes in PA were detected in either the paroxetine or the placebo group. | 1. First randomized, controlled study to find that SSRIs may reduce NA in healthy participants. 2. Absence of SSRI‐induced improvement in PA is consistent with animal and human studies indicating SSRIs may not be effective for anergic symptoms in a subgroup of MDD patients |
| McCabe et al, 2010 [78] | Randomized, controlled study of 45 healthy participants randomized to 7 days of treatment with citalopram, reboxetine, or placebo. Neural responses to rewarding (sight and/or avor of chocolate) and aversive stimuli (sight of moldy strawberries and/or an unpleasant strawberry taste) assessed with fMRI. | 1. Citalopram reduced neural processing of rewarding stimuli in the ventral striatum and the ventral medial/orbitofrontal cortex and aversive stimuli in the lateral orbitofrontal cortex. 2. Reboxetine, increased neural responses to reward within the medial orbitofrontal cortex, and had weaker effects on neural processing of aversive stimuli. | 1. First study to demonstrate that SSRIs diminish the neural processing of both rewarding and aversive stimuli. 2. May also help to explain the often‐reported emotional flattening effect of SSRIs. |
| Taneja et al., 2007 [80] | Double‐blind, crossover trial of 12 healthy individuals randomized to modafinil (400 mg) versus placebo, with 4‐day washout period between phases. | 1. Ratings of PA on the PANAS increased significantly in the modafinil group compared to placebo. 2. Ratings of NA also increased in the modafinil group compared to placebo. | 1. First study to demonstrate that a pro‐dopaminergic agent may increase PA and NA in healthy individuals. 2. May help to understand potential for improved mood and worsened anxiety in patients with depression. |
| Tomarken et al., 2004 [88] | Double‐blind, controlled trial of 10 depressed patients and 9 matched healthy controls randomized to 300 mg/day of bupropion SR versus placebo. Subjects previously in the bupropion group had their dose increased to 400/day during a second 6‐week phase, and subjects previously in the placebo group were titrated to 300 mg/day. | 1. Bupropion produced significantly greater improvement in PA deficits (MASQ) compared to placebo. 2. Placebo had weaker effects on PA than on other symptom or dimensional measures. | 1. Suggests specific catecholaminergic effects on PA in MDD and supports previous studies relating dopaminergic dysfunction in depression to impairments in reward processing. 2. Dose/duration effect of buproprion corroborates other studies suggesting targeted treatment of low PA in depression should be sequenced in conjunction with, or after stabilization of high NA. 3. Given the weak placebo effects on PA deficits, dimensional ratings may provide a more generative metric for separating antidepressant and placebo effects. |
| Dichter et al., 2005 [92] | Double‐blind, controlled trial of 20 outpatients with MDD randomized to fixed doses of venlafaxine XR 225 mg/day, or paroxetine 30 mg/day, over 12 weeks. | 1. Both agents produced similar amounts of improvement in NA (MASQ) and depression severity. | 1. Did not support the hypothesis that antidepressants with different mechanisms have preferential effects on PA or NA. 2. Venlafaxine did not separate from paroxetine on ratings of PA. |
| Knutson et al., 2004 [102] | Within‐subject, double‐blind, placebo controlled study of 8 healthy volunteers to assess the effects of oral dextroamphetamine (AMPH) on neural processing of incentives. Subjects were scanned during a monetary incentive delay task, which separates anticipatory and consummatory incentive processing. | 1. Healthy subjects receiving AMPH demonstrated increased positive arousal for anticipating gain and avoiding loss, as measured by increased cue‐related excitement and changes in ventral striatum (VS) activity. 2. AMPH subjects displayed increased right NAcc activation during loss anticipation. | 1. These data are consistent with the “incentive salience” model of dopamine function (103), which posits that dopamine predominantly mediates “incentive” (how much work the organism will do in relation to the reward value assigned) and “salience” (how attractive a stimulus is to an organism). 2. Suggests a neural mechanism for how increased positive arousal may facilitate reframing of potential losses as potential gains. |
aIn order of reference.
AMPH, amphetamine; MASQ, Mood and Anxiety Symptom Questionnaire; MDD, major depressive disorder; NA, negative affect; NEO‐FFI, NEO Five‐Factor Inventory; PA, positive affect; PANAS, Positive and Negative Affect Schedule; SSRI, selective serotonin reuptake inhibitor.
Dimensions of Emotional Temperament
A Working Definition of Emotional Dimensions
PA and NA can be defined as an individual's propensity to feel positive (e.g., excitement, interest) and negative (e.g., fear, shame) emotions, respectively. Quantitatively, they are derived from factor analysis: a technique, which summarizes a large number of independent variables into a smaller number of latent, or unobserved variables, referred to as “factors” or “dimensions”[11]. Watson and Tellegen initially factor analyzed multiple mood descriptors (e.g., “delighted,”“frightened,”“jittery,”“sluggish”) taken from studies of emotions in healthy individuals. They identified 10 specific positive and negative “affects,” or emotional factors. They then factor analyzed the 10 emotional factors, and identified two “higher order factors”[i.e., factors of factors; Ref. 12], termed positive and negative “affectivity.”
Two principles of these dimensions are particularly relevant for psychiatrists. The first is convergence, meaning that emotions of the same valence strongly co‐vary [13], and cluster together, particularly in the high PA and NA octants (Figure 1). The second is discriminance, meaning that emotions of the opposite valence weakly co‐vary [13], and may change relatively independently. Just as convergence helps to understand why depressed mood, anger, and irritability often co‐occur [14, 15], discriminance evokes Kraepelin's independent dimensions of manic‐depression, and may help to explain why fluctuations and mixed mood states (e.g., excitement and nervousness; euphoria and anxiety) are present in everyday life and in emotional disorders (such as bipolar mixed episodes).
Figure 1.
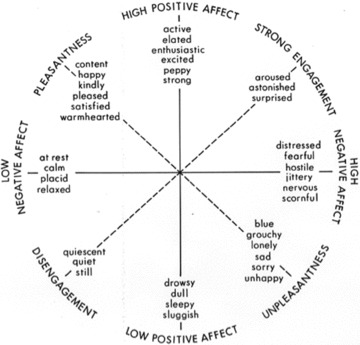
The two‐factor structure of affect.
Convergence and discriminance can also be related to patterns of symptom change [16, 17]. If a patient in remission from an episode of MDD were feeling “enthusiastic,” he would also be more likely to feel “alert,”“interested,” and “energetic” (convergence). However, recovery of function in NA–that is, a diminution of NA–would not be expected to correlate strongly with changes in PA (discriminance). This may underlie the finding that several common residual symptoms of depression, such as fatigue and lack of motivation, cluster together as deficits in PA [18]. Deficits in PA may be less responsive to first‐line treatment with serotonergic agents, and are likely underrepresented by clinician‐rated measures, such as the Hamilton Rating Scale for Depression (HAM‐D) and the Montgomery–Asberg Depression rating Scale (MADRS), which demonstrate bias toward the presence of general distress rather than the absence of PA and engagement [19].
Relevance to Psychopathology
The Integrative Hierarchical Model of Mood and Anxiety Disorders (IHHM)(20) correlates variations in emotional temperament with discrete DSM‐IV‐TR affective disorders, and characterizes PA and NA as predispositional factors, which may influence the development and treatment responsivity of MDD. Multivariate analyses of cross‐sectional and prospective cohorts have found that depressive and anxiety disorders are correlated with high levels of NA; panic disorder and specific phobias are distinguished by elevation of an additional factor, termed Autonomic Arousal (AA); and MDD and social phobia are specifically cor related with low PA [21, 22].
Directionality
It is important to remember that factor analysis models correlations, but does not provide information about causal relationships between variables. PA and NA can be measured as state or temperament variables, depending on the retrospective time‐course assessed (reviewed by Clark L.A., 2005 [23]). The direction of influence relating PA and NA to emotional disorders may involve at least four pathways: (1) predispositional—emotional temperament may influence the development of emotional disorders; (2) pathoplastic—emotional temperament may influence the course of emotional disorders; (3) complication—emotional temperament may be influenced, or reshaped, by the experience of emotional disorders; and (4) spectrum—emotional dimensions and emotional disorders may reflect a shared genetic diathesis [23, 24].
There is increasing evidence to support the predispositional hypothesis (Figure 2). Twin studies have found a high degree of overlap between the genetic factors thought to underlie high NA, measured as neuroticism, and anxiety and depressive disorders [25, 26]. Several of the most compelling fMRI studies have found that healthy daughters of mothers with recurrent MDD show attenuated neural responses during reward processing tasks, also characterized by decreased striatal activity, compared to daughters without known genetic loading [27]; and that unmedicated, recovered depressed patients exhibit abnormalities in the neural representation of reward (measured as decreased blood flow in the ventral striatum) despite reporting levels of pleasure similar to healthy controls [28]. Chemical depletion studies also suggest that catecholamines—dopamine in particular—are prominently involved in impaired reward processing and that low PA represents a good candidate endophenotype for MDD [29].
Figure 2.
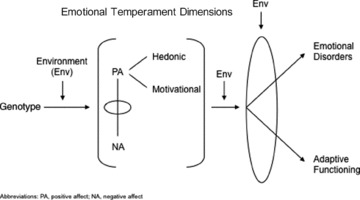
Predispositional Hypothesis.
Value of Dimensional Models for Classification of Emotional Disorders
The IHHM has been extensively reviewed in the psychology literature, and is closely aligned with the NIMH Research Domain Criteria (RDoC)—a dimensional framework for classifying mental disorders stemming from basic behavioral neuroscience [30]. The RDoC proposes a nosological matrix of “positive affect,”“negative affect,” and three other functional dimensions, which can then be analyzed by units of “genes, molecules, cells, circuits, behavior, and self‐reports.” The RDoC Project represents an important current approach to elucidating the pathophysiology of psychiatric disorders recognizing that genomic and neurobiological data have not fit neatly into DSM‐IV diagnostic categories and instead may be more relevant to broad domains that cut across several or more disorders and/or may relate to subgroups within heterogeneous disorders. Other potential benefits of the RDoC Project include: (1) Preservation of psychopathology and treatment data, which are currently lost by the exclusion of subthreshold disorders [31]; (2) Greater ability to account for high rates of comorbidity between anxiety and depressive disorders, which, as modeled by the IHHM, are phenotypically related by high levels of NA [21]; and (3) Greater likelihood of mapping emotional disorders onto genomic and neurobiological factors.
Studies of Antidepressant Effects on Positive and Negative Affectivity
Value of Dimensional Models for Therapeutic Studies in MDD
Current pharmacological treatments for MDD are considered to be suboptimal [7]: only approximately 50% of outpatients starting treatment with a serotonin reuptake inhibitor (SRI) will respond [32] and fewer will remit [9]. Perhaps more problematic, between 40% and 60% of responders will relapse within 1 year [6, 8].
In addition to the systemic limitations of large, multisite trials [33], signal detection has likely been limited by the phenotype problem, defined here as the inclusion of heterogeneous depressive samples in antidepressant trials. Following the RDoC approach, we propose this problem may be mitigated by shifting research paradigms away from depressive phenomenology and toward biologically based emotional dimensions. We examine two related and not necessarily mutually exclusive hypotheses:
-
1
Cause‐correction: The antidepressant response may fundamentally occur through changes in emotional temperament, with subsequent spread to syndrome or symptom changes;
-
2
Preferential effects: Antidepressants with different mechanisms of action may have preferential effects on PA or NA.
Measures
The dimensional scales used in the antidepressant trials reviewed below include: (1) the Positive and Negative Affect Schedule [PANAS; Figure 3; Ref. 34], (2) the Behavioral Inhibition and Activation System Scales [35], (3) the Revised Personality Inventory and Five‐Factor Inventory [NEO‐FI; Ref. 36], and (4) the Mood and Anxiety Symptom Questionnaire [MASQ; Ref. 17]. All four scales measure independently derived emotional constructs, but demonstrate high convergent and discriminant validity in nonclinical and MDD samples [13, 22, 31, 37]. It should also be noted that these constructs differ importantly from Cloninger's tridimensional theory of temperament (“harm avoidance,”“reward dependence,” and “novelty seeking”), which may be limited by measures, such as the Tridimensional Personality Questionnaire and the Temperament and Character Inventory, with unstable factor structures [23]. The specific properties and limitations of the PANAS, BIS‐BAS, NEO‐FI, and MASQ have been reviewed elsewhere [38].
Cause‐Correction
Empirical Data
Historically, changes in emotional temperament reported during treatment were often interpreted as “state effects”[meaning “effects” of rather than “causes” of depressive change; Refs. 39, 40, 41, 42]. However, there is growing support for the cause‐correction hypothesis[43], which posits that changes in emotional temperament may cause, or independently contribute to depression improvement. To test this hypothesis directly, Tang and colleagues reanalyzed data from a double‐blind MDD treatment study, which included the NEO‐FI [44]. Two hundred and forty MDD subjects were randomized to treatment with 16 weeks of cognitive therapy (CT), 16 weeks of paroxetine (flexibly dosed; mean 38.8 mg), or 8 weeks of matched placebo. Though all three groups showed substantial improvement in depressive symptoms over the first 8 weeks, changes in PA and NA, as measured by extraversion and neuroticism scores on the NEO‐PI, were 4–8‐times greater with paroxetine than placebo. Remarkably, when the authors controlled for changes in temperament, the efficacy advantage of paroxetine versus placebo was no longer significant.
The authors also compared 44 paroxetine responders with 44 placebo responders, each paired by equal amounts of depression improvement on the HAM‐D‐17. They found that paroxetine‐treated patients demonstrated 3.5 times greater change in extraversion and 6.8 times greater change in neuroticism compared to placebo‐treated subjects. Finally, the authors performed a within‐subject analysis of 31 patients who completed 8‐weeks of placebo‐treatment followed by crossover to another 8 weeks of selective serotonin reuptake inhibitor (SSRI)‐treatment. If the state‐effect hypothesis were correct, greater temperament changes would be expected to occur during periods of greater state changes. However, the authors found the reverse to be true: HAM‐D scores decreased by 6.4 points during the placebo phase and by 2.4 points during the SSRI phase; extraversion and neuroticism were relatively unchanged during the placebo phase, but improved significantly during the SSRI phase, suggesting independent and specific SSRI effects on emotional temperament dimensions.
The cause‐correction hypothesis, supported by the Tang et al. study above, has also been explored through SEM. Quilty, Meusel, and Bagby performed an SEM analysis, which combined data from a naturalistic, clinic‐based sample and a randomized, controlled trial [RCT; Ref. 45]. Subjects with MDD receiving SSRIs exhibited greater trait NA change, as measured by neuroticism ratings on the NEO‐PI, than those receiving noradrenergic and dopaminergic reuptake blockers (NDMs) or reversible monoamine oxidase inhibitors (RIMAs). Goodness‐of‐fit indices favored a mediation rather than complication model, meaning that SSRI‐induced reduction in NA may mediate depressive symptom changes. These data require replication in additional controlled treatment trials, and suggest that SEM may also be a valuable technique for modeling antidepressant effects through PA.
The cause‐correction hypothesis is also indirectly supported by recent affective neuroscience research exploring medication effects on emotional appraisal. Emotional appraisal can be defined as the process of automatically assigning emotional valence to environmental stimuli. Like emotional temperament, emotional appraisal appears to be pharmacologically malleable in depressed patients before symptom changes. A recent study by Harmer and her colleagues randomized 31 depressed patients and 31 matched healthy controls to a single dose of reboxetine (4 mg) or placebo. Subjects were given a battery of emotional processing tasks before and 3 h after administration of treatment. Although no mood or anxiety changes were reported in either group, depressed patients treated with reboxetine exhibited a reversal in positive emotional processing deficits (including recognition of positive facial expressions, response speed to positive self‐relevant personality adjectives, and memory for these positive adjectives) compared to placebo [46]. Harmer and her colleagues also found that early, antidepressant‐induced changes in emotional learning and appraisal occur in healthy individuals. In a study of healthy volunteers, they assessed the neural effects of treatment of a single dose of citalopram (20 mg) on emotional processing of facial expressions. Functional MRI performed 3 h after administration demonstrated a significantly reduced amygdala response to fearful facial expressions compared to placebo [47]. Similar reductions in limbic reactivity have been found in healthy individuals treated with citalopram over 7 days [48], and the emotional processing effects of antidepressants have also been partially replicated with the neurokinin‐1 (NK[1]) receptor antagonist, aprepitant [49].
Clinical Translations
The studies summarized above support the hypothesis that antidepressant‐induced changes in emotional temperament independently contribute to depression improvement. If these findings can be replicated, they would support a rationale for targeting emotional temperament in the treatment of patients with MDD, and perhaps, would also promote “intelligent phase I” studies, involving single administration of novel treatments in the context of cognitive neuroscience paradigms. Early antidepressant‐induced changes in emotional processing may also provide a key substrate for synergistically combining pharmacological and psychological interventions [50].
Preferential Effects
The Phenotype Problem
Historically, we can trace three different research paradigms—subtyping by syndromes [51, 52], mechanisms [53], and symptom differences [54, 55]—for studying heterogeneous MDD patient samples and treatment effects. Despite decades of research, depressive subtypes have not proven particularly helpful for guiding choice of antidepressant. For example, although a number of studies suggest that tricyclic antidepressants are more effective than SSRIs for inpatients with endogenous or melancholic depression [51, 52], and are inferior to monoamine oxidase inhibitors (MAOIs) for patients with atypical depression [56], these findings have relatively little relevance to contemporary clinical decisions given the very limited use of these agents [57]. To examine the related hypothesis—that antidepressants with different mechanisms of action may have preferential effects on PA or NA–we review the studies below.
Studies in Healthy Subjects
Knutson and colleagues conducted the first double‐blind, RCT to investigate the hypothesis that SSRIs may have different effects on NA and PA in healthy individuals [58]. They randomized 51 participants to a fixed dose of paroxetine (20 mg/day) or placebo over 4 weeks, and found that NA decreased significantly in the paroxetine group compared to placebo; no significant changes in PA were detected in either group.
Several findings from this study presaged temperament dimensions as potentially novel, biologically based treatment targets in MDD. The early dampening effects of serotonergic antidepressants on NA and the related construct, negative emotional appraisal, have now been established in clinical [46, 59] and nonclinical samples [48, 60]. The absence of a direct effect on PA is consistent with evidence from animal studies of opponency between serotonergic (2B and 2C receptors) and dopaminergic subsystems [61, 62, 63] and with clinical trials indicating SRIs may not be effective for anergic symptoms in a subgroup of MDD patients [64, 65, 66, 67, 68].
Although the serotonin system is complex and likely contains multiple, dynamic subsystems (e.g., 5HT1 vs. 5HT2), another study by Harmer and colleagues suggests that serotonergic antidepressants may “constrain” emotional responses across both NA and PA and that noradrenergic/dopaminergic agents may specifically enhance PA [69]. The study randomized 45 healthy participants to 7 days of treatment with citalopram, reboxetine, or placebo, and used functional magnetic resonance imaging to assess neural responses to rewarding (sight and/or avor of chocolate) and aversive stimuli (sight of moldy strawberries and/or an unpleasant strawberry taste). The SSRI, citalopram, reduced neural processing of rewarding stimuli in the ventral striatum and the ventral medial/orbitofrontal cortex and aversive stimuli in the lateral orbitofrontal cortex. Consistent with previous studies linking catecholaminergic effects to PA, the norepinephrine reuptake inhibitor (NRI), reboxetine, increased neural responses to reward within the medial orbitofrontal cortex, and had weaker effects on neural processing of aversive stimuli. This was the first study to show that SSRIs diminish the neural processing of both rewarding and aversive stimuli, and may also help to explain the often‐reported emotional flattening effect of SSRIs [67, 70].
Studies of dopaminergic agents have generated particularly strong and selective effects on PA. Historically, stimulants have often been prescribed to soldiers to combat fatigue and to enhance attention [24]. The dopaminergic anti‐narcolepsy agent, modafinil, has also been shown to increase PA in a randomized, double‐blind crossover trial of healthy individuals [71]. Amphetamine‐induced increased release of dopamine in the ventral striatum is associated with increased PA [72], and radioligand‐based positron‐emission tomographic (PET) studies have found increased activity in dopamine‐rich regions of the ventral striatum in response to rewarding stimuli [73, 74, 75]. Functional imaging suggest that anticipatory reward may localize to dopaminergic areas in the nucleus accumbens, ventral tegmental area, and orbital–frontal cerebral cortex, and that processing of consummatory rewards predominates in the orbital–frontal cortex, medial–prefrontal cortex, and putamen [76].
Open‐Label and Case‐Control Studies of Patients with Major Depression
Only a small number of open‐label and case‐control studies have investigated the hypothesis of preferential effects in depressed patients. Two case‐control studies suggested that SRIs may be particularly effective for depressive symptoms (anxiety, rumination, and compulsions) related to NA [64, 77]. The norepinephrine dopamine reuptake inhibitor (NDRI), bupropion, also appeared to reverse symptoms (fatigue, anergia, and poor concentration) related to low PA. However, both studies were limited by small sample sizes, absence of psychometrically validated dimensional measures, and retrospective designs. In contrast, two open‐label trials comparing depressed patients treated with SSRIs or serotonin‐norepinephrine reuptake inhibitors (SNRIs) showed nonspecific improvements of NA and PA in both groups [40, 78].
Effects of Bupropion on Low PA in Subjects with MDD
The first double‐blind, randomized trial to investigate antidepressant effects on low PA in MDD was conducted by Tomarken and colleagues [79]. The study randomized 19 subjects with MDD to treatment with 300 mg/day of bupropion SR versus placebo. Subjects previously in the bupropion group had their dose increased to 400/day during a second 6‐week phase, and subjects previously in the placebo group were titrated to 300 mg/day of bupropion. Bupropion produced greater improvement in PA deficits compared to placebo, and the rate of change increased at higher doses and with greater duration of time. In addition, placebo had weaker effects on PA than on other symptom or dimensional measures.
Despite several limitations (including a small sample and the absence of a drug comparator), the study's finding that bupropion had prominent and specific effects on low PA is consistent with previous studies relating dopaminergic dysfunction in depression to impairments in reward processing [80]. The authors described a duration and/or dose–response relationship between bupropion and PA. Given studies showing increased correlation between NA and PA with more severe depression [81, 82], it is also possible that high initial levels of NA inhibited the activation of PA. This dynamic affect model has not been tested in pharmacologic studies, but if correct, would suggest treatments targeting low PA in depression should be sequenced in conjunction with, or after stabilization of high NA.
Effects of Venlafaxine versus Paroxetine on PA and NA in Subjects with MDD
The first randomized, controlled antidepressant trial to investigate preferential effects on PA and NA was conducted by Dichter and colleagues [83]. They hypothesized that a combined serotonergic and noradrenergic antidepressant would produce greater increases in PA and similar decreases in NA compared to a predominantly serotonergic antidepressant. The study randomized 20 outpatients with MDD to fixed doses of venlafaxine XR 225 mg/day, or paroxetine 30 mg/day, over 12 weeks. As predicted both agents produced similar amounts of improvement in NA and depression severity (as measured by the HAM‐D‐17); however, venlafaxine failed to separate from paroxetine on MASQ ratings of PA deficits. The authors discussed several possible explanations for this result, including issues of power; the absence of a placebo comparator; the final common pathway hypothesis (“drugs with an NA or 5‐HT mechanism of action might act through a final common pathway resulting in similar response in the core symptoms of depression [84]”); and the weak but potentially confounding noradrenergic properties of paroxetine [85]. It is also possible that insensitivity measure bias affected the results, because the HAM‐D‐17 is predominantly composed of items related to NA.
Changes in Trait Measures of PA and NA within MDD Subjects Treated with Paroxetine versus CBT
The largest study to compare treatment changes across emotional dimensions was discussed above in the cause‐correction hypothesis section of this review [43]. Tang, DeRubeis, Hollon, et al. re‐analyzed data from a double‐blind study of 240 MDD subjects, randomized to 16 weeks of CT, 16 weeks of paroxetine (flexibly dosed; mean 38.8 mg), or 8 weeks of matched placebo [44]. They found that both paroxetine and CT were more effective than placebo as measured by improvement on the HAM‐D, but paroxetine produced greater changes on extraversion and neuroticism, with effect sizes of 0.63 (extraversion) and 0.57 (neuroticism). In addition, although CT separated from placebo in reducing neuroticism, this advantage was no longer significant after controlling for changes in depression. In the matching analysis—a purer comparison of dimensional and symptom change—paroxetine‐treated patients had 1.9 times greater improvements in neuroticism relative to extraversion, providing partial support for the preferential effects hypothesis.
Clinical Translations
Given the paucity of RCTs, there is currently insufficient evidence to support the preferential effects hypothesis. However, there are strong neurobiological and nonclinical data indicating that stimulants can serve as a powerful fulcrum for the motivational drives and approach behaviors impaired in depression.
Biobehavioral Features
In healthy individuals, PA and NA have distinct daily patterns. PA demonstrates a diurnal variation, with lower levels in the morning, rising and peaking as interpersonal and goal‐related activities increase throughout the day, and then ebbing during the evening, toward a nadir in sleep [13]. The distribution of NA over 24 hours can be plotted as a predominantly flat curve, with occasional, sharp spikes that seem to be stimulated by perceived environmental threats. Electroencephalography (EEG) measures of healthy subjects exhibit lateralization of electrical activity in the left prefrontal cortex for approach behaviors (PA sensitivity) and in the right prefrontal cortex for avoidance behaviors [NA sensitivity; Refs. 86, 87]. This has been extended to MDD with four studies showing a relative decrease in left frontal activity in depressed patients compared to controls [88, 89, 90].
Radioligand‐based positron‐emission tomographic (PET), functional magnetic resonance imaging (fMRI), and pharmacological studies have also linked PA and NA to two distinct motivational systems—the behavioral activation system (BAS) and the behavioral inhibition system (BIS)—for modulating approach and avoidance behaviors in response to positive and negative incentives [91, 92]. Evolutionarily conserved across species, these systems may have a unique emotional core in humans.
Clinical Translations
As discussed in the cause‐correction hypothesis section, both emotional temperament and emotional autoappraisal can be altered by medications, and antidepressant induced effects may be mobilized by early psychological interventions. The convergence of behavioral, pharmacological, and neurobiological findings supports a biobehavioral treatment paradigm for MDD. Proof‐of‐concept studies to assess for synergy between combined pharmacological and cognitive–behavioral treatments could be of considerable importance for advancing MDD treatment research.
Disclosures
David P. Soskin and Jenna R. Carl have no disclosures.
Dr. Jonathan Alpert has received research support from: Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals; Aspect Medical Systems, Astra‐Zeneca, Bristol‐Myers Squibb Company, Cephalon, Cyberonics, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth‐Ayerst Laboratories. Dr. Jonathan Alpert has participated on advisory boards for or consulted to: Eli Lilly & Company, Pamlab LLC, and Pharmavite LLC. Dr. Jonathan Alpert has received speakers’ honoraria from: Eli Lilly & Company, Xian‐Janssen, Organon, MGH Psychiatry Academy,Reed Medical Education and Primedia, and the American Psychiatric Association and has received editorial fees from Belvoir Publishing.
Dr. Maurizio Fava has received research support from: Abbot Laboratasuories; Alkermes, Inc.; Aspect Medical Systems; AstraZeneca; BioResearch; BrainCells Inc.; Bristol‐Myers Squibb; CeNeRx BioPharma; Cephalon; Clinical Trials Solutions, LLC; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Icon Clinical Research; i3 Innovus/Ingenix; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmavite® LLC; Photothera; Roche Pharmaceuticals; RCT Logic, LLC; Sanofi‐Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Synthelabo; Wyeth‐Ayerst Laboratories.
Advisory/Consulting
Abbott Laboratories; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Bayer AG; Best Practice Project Management, Inc.; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol‐Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Clinical Trials Solutions, LLC; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre‐Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Otsuka Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; RCT Logic, LLC; Sanofi‐Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering‐Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.
Speaking/Publishing
Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol‐Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth‐Ayerst Laboratories.
Equity Holdings
Compellis.
Royalty/patent, other income
Patent for Sequential Parallel Comparison Design (SPCD) and patent application for a combination of azapirones and bupropion in Major Depressive Disorder (MDD), copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation‐Emergent Signs & Symptoms (DESS), and SAFER. Patent for research and licensing of SPCD with RCT Logic; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Ross Baldessarini, Dr. Alexander Bodkin, Dr. Ronald Pies, and Dr. Theodore Stern for their thoughtful contributions to this review.
References
- 1. Rosenhan DL. On being sane in insane places. Science 1973;179:250–258. [DOI] [PubMed] [Google Scholar]
- 2. Kendell RE, Cooper JE, Gourlay AJ, Copeland JR, Sharpe L, Gurland BJ. Diagnostic criteria of American and British psychiatrists. Arch Gen Psychiatry 1971;25:123–130. [DOI] [PubMed] [Google Scholar]
- 3. Wilson M. DSM‐III and the transformation of American psychiatry: A history. Am J Psychiatry 1993;150:399–410. [DOI] [PubMed] [Google Scholar]
- 4. Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM‐IV anxiety and mood disorders: Implications for assessment and treatment. Psychol Assess 2009;21:256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilamowska ZA, Thompson‐Hollands J, Fairholme CP, Ellard KK, Farchione TJ, Barlow DH. Conceptual background, development, and preliminary data from the unified protocol for transdiagnostic treatment of emotional disorders. Depress Anxiety 2010;27:882–890. [DOI] [PubMed] [Google Scholar]
- 6. Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: A two‐year prospective follow‐up study. Psychol Med 1995;25:1161–1170. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association . Practice guideline for the treatment of patients with major depression. 2nd ed American Psychiatric Association, Washington , 2000. [Google Scholar]
- 8. Rush AJ, Trivedi MH, Wisniewski SR, et al Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry 2006;163:1905–1917. [DOI] [PubMed] [Google Scholar]
- 9. Trivedi MH, Rush AJ, Wisniewski SR, et al Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163:28–40. [DOI] [PubMed] [Google Scholar]
- 10. Shorter E, Tyrer P. Separation of anxiety and depressive disorders: Blind alley in psychopharmacology and classification of disease. BMJ 2003;327:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz MH. Multivariable analysis. Cambridge University Press, New York , 1999. [Google Scholar]
- 12. Watson D, Tellegen A. Toward a consensual structure of mood. Psychol Bull 1985;98:219–235. [DOI] [PubMed] [Google Scholar]
- 13. Watson D. Mood and temperament. Guilford Press, New York , 2000. [Google Scholar]
- 14. Fava M, Anderson K, Rosenbaum JF. “Anger attacks”: Possible variants of panic and major depressive disorders. Am J Psychiatry 1990;147:867–870. [DOI] [PubMed] [Google Scholar]
- 15. Fava M, Hwang I, Rush AJ, Sampson N, Walters EE, Kessler RC. The importance of irritability as a symptom of major depressive disorder: Results from the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995;104:3–14. [DOI] [PubMed] [Google Scholar]
- 17. Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol 1995;104:15–25. [DOI] [PubMed] [Google Scholar]
- 18. Nierenberg AA, Eidelman P, Wu Y, Joseph M. Depression: An update for the clinician. Focus 2005;3:3–12. [Google Scholar]
- 19. Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: Has the gold standard become a lead weight? Am J Psychiatry 2004;161:2163–2177. [DOI] [PubMed] [Google Scholar]
- 20. Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol 1998;49:377–412. [DOI] [PubMed] [Google Scholar]
- 21. Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM‐IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 1998;107:179–192. [DOI] [PubMed] [Google Scholar]
- 22. Brown TA. Temporal course and structural relationships among dimensions of temperament and DSM‐IV anxiety and mood disorder constructs. J Abnorm Psychol 2007;116:313–328. [DOI] [PubMed] [Google Scholar]
- 23. Clark LA. Temperament as a unifying basis for personality and psychopathology. J Abnorm Psychol 2005;114:505–521. [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen N. America's first amphetamine epidemic 1929–1971: A quantitative and qualitative retrospective with implications for the present. Am J Public Health 2008;98:974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS. Neuroticism, major depression and gender: A population‐based twin study. Psychol Med 2002;32:719–728. [DOI] [PubMed] [Google Scholar]
- 26. Hettema JM, Prescott CA, Kendler KS. Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am J Psychiatry 2004;161:1581–1587. [DOI] [PubMed] [Google Scholar]
- 27. Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 2010;67:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007;64:327–337. [DOI] [PubMed] [Google Scholar]
- 30. Insel T, Cuthbert B, Garvey M, et al Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry 2010;167:748–751. [DOI] [PubMed] [Google Scholar]
- 31. Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM‐IV anxiety and mood disorders: Implications for assessment and treatment. Psychol Assess 2009;21:256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agency for Health Care Policy and Research . Clinical practice guideline: Depression in primary care: treatment of major depression. U.S. Department of Health and Human Services, Rockville , 1993. [Google Scholar]
- 33. Gelenberg AJ, Thase ME, Meyer RE, et al The history and current state of antidepressant clinical trial design: A call to action for proof‐of‐concept studies. J Clin Psychiatry 2008;69:1513–1528. [DOI] [PubMed] [Google Scholar]
- 34. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 35. Carver CL, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Person Soc Psychol 1994;67:319–333. [Google Scholar]
- 36. Costa PT, Jr. , McCrae RR. Revised NEO personality inventory (NEO‐PI‐R) and NEO five‐factor inventory (NEO‐FFI) professional manual. Psychological Assessment Resources, Odessa , 1992. [Google Scholar]
- 37. Naragon‐Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. J Abnorm Psychol 2009;118:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sloan D, Kring A. Measuring changes in emotion during psychotherapy: Conceptual and methodological issues. Clin Psychol: Sci Prac 2007;14:307–322. [Google Scholar]
- 39. Bagby RM, Levitan RD, Kennedy SH, Levitt AJ, Joffe RT. Selective alteration of personality in response to noradrenergic and serotonergic antidepressant medication in depressed sample: Evidence of non‐specificity. Psychiatry Res 1999;86:211–216. [DOI] [PubMed] [Google Scholar]
- 40. Du L, Bakish D, Ravindran AV, Hrdina PD. Does fluoxetine influence major depression by modifying five‐factor personality traits? J Affect Disord 2002;71:235–241. [DOI] [PubMed] [Google Scholar]
- 41. Gracious KS. Do SSRIs affect personality traits. Br J Psychiatry 1999;175:287. [DOI] [PubMed] [Google Scholar]
- 42. Marchevsky D. Selective serotonin reuptake inhibitors and personality change. Br J Psychiatry 1999;175:589–590. [DOI] [PubMed] [Google Scholar]
- 43. Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B. Personality change during depression treatment: A placebo‐controlled trial. Arch Gen Psychiatry 2009;66:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeRubeis RJ, Hollon SD, Amsterdam JD, et al Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005;62:409–416. [DOI] [PubMed] [Google Scholar]
- 45. Quilty LC, Meusel LA, Bagby RM. Neuroticism as a mediator of treatment response to SSRIs in major depressive disorder. J Affect Disord 2008;111:67–73. [DOI] [PubMed] [Google Scholar]
- 46. Harmer CJ, O'Sullivan U, Favaron E, et al Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 2009;166:1178–1184. [DOI] [PubMed] [Google Scholar]
- 47. Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 2009;194:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006;59:816–820. [DOI] [PubMed] [Google Scholar]
- 49. Chandra P, Hafizi S, Massey‐Chase RM, Goodwin GM, Cowen PJ, Harmer CJ. NK1 receptor antagonism and emotional processing in healthy volunteers. J Psychopharmacol 2010;24:481–487. [DOI] [PubMed] [Google Scholar]
- 50. Danish University Antidepressant Group . Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 2009;195:102–108. [DOI] [PubMed] [Google Scholar]
- 51. Danish University Antidepressant Group . Paroxetine: A selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study. Danish University Antidepressant Group. J Affect Disord 1990;18:289–299. [DOI] [PubMed] [Google Scholar]
- 52. Danish University Antidepressant Group . Citalopram: Clinical effect profile in comparison with clomipramine. A controlled multicenter study. Psychopharmacology (Berl) 1986;90:131–138. [DOI] [PubMed] [Google Scholar]
- 53. Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry 2001;178:234–241. [DOI] [PubMed] [Google Scholar]
- 54. Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: A comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry 2006;60:1350–1355. [DOI] [PubMed] [Google Scholar]
- 55. Papakostas GI, Trivedi MH, Alpert JE, et al Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: A meta‐analysis of individual patient data from 10 double‐blind, randomized clinical trials. J Psychiatr Res 2008;42:134–140. [DOI] [PubMed] [Google Scholar]
- 56. Quitkin FM, Stewart JW, McGrath PJ, et al Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry Suppl 1993;21:30–34. [PubMed] [Google Scholar]
- 57. Stahl SM, Felker A. Monoamine oxidase inhibitors: A modern guide to an unrequited class of antidepressants. CNS Spectr 2008;13:855–870. [DOI] [PubMed] [Google Scholar]
- 58. Knutson B, Wolkowitz OM, Cole SW, et al Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 1998;155:373–379. [DOI] [PubMed] [Google Scholar]
- 59. Fu CH, Williams SC, Cleare AJ, et al Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004;61:877–889. [DOI] [PubMed] [Google Scholar]
- 60. Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ. Short‐term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int J Neuropsychopharmacol 2009;12:169–179. [DOI] [PubMed] [Google Scholar]
- 61. Di Mascio M, Di Giovanni G, Di Matteo V, Prisco S, Esposito E. Selective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental area. Brain Res Bull 1998;46:547–554. [DOI] [PubMed] [Google Scholar]
- 62. Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5‐HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci 2001;22:229–232. [DOI] [PubMed] [Google Scholar]
- 63. Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward‐learning signals in major depression. Brain 2008;131:2084–2093. [DOI] [PubMed] [Google Scholar]
- 64. Bodkin JA, Lasser RA, Wines JD Jr, Gardner DM, Baldessarini RJ. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry 1997;58:137–145. [DOI] [PubMed] [Google Scholar]
- 65. Cohn JB, Wilcox CS. Paroxetine in major depression: a double‐blind trial with imipramine and placebo. J Clin Psychiatry 1992;53(Suppl):52–56. [PubMed] [Google Scholar]
- 66. Hoehn‐Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol 1990;10:343–345. [PubMed] [Google Scholar]
- 67. Opbroek A, Delgado PL, Laukes C, et al Emotional blunting associated with SSRI‐induced sexual dysfunction. Do SSRIs inhibit emotional responses? Int J Neuropsychopharmacol 2002;5:147–151. [DOI] [PubMed] [Google Scholar]
- 68. Price J, Cole V, Goodwin GM. Emotional side‐effects of selective serotonin reuptake inhibitors: Qualitative study. Br J Psychiatry 2009;195:211–217. [DOI] [PubMed] [Google Scholar]
- 69. McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 2010;67:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Price J, Cole V, Goodwin GM. Emotional side‐effects of selective serotonin reuptake inhibitors: Qualitative study. Br J Psychiatry 2009;195:211–217. [DOI] [PubMed] [Google Scholar]
- 71. Taneja I, Haman K, Shelton RC, Robertson D. A randomized, double‐blind, crossover trial of modafinil on mood. J Clin Psychopharmacol 2007;27:76–79. [DOI] [PubMed] [Google Scholar]
- 72. Drevets WC, Gautier C, Price JC, et al Amphetamine‐induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 2001;49:81–96. [DOI] [PubMed] [Google Scholar]
- 73. Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A 2001;98:11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kunig G, Leenders KL, Martin‐Solch C, Missimer J, Magyar S, Schultz W. Reduced reward processing in the brains of Parkinsonian patients. Neuroreport 2000;11:3681–3687. [DOI] [PubMed] [Google Scholar]
- 75. Martin‐Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res 2001;139:278–286. [DOI] [PubMed] [Google Scholar]
- 76. Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Andrews W, Parker G, Barrett E. The SSRI antidepressants: exploring their “other” possible properties. J Affect Disord 1998;49:141–144. [DOI] [PubMed] [Google Scholar]
- 78. Bagby RM, Levitan RD, Kennedy SH, Levitt AJ, Joffe RT. Selective alteration of personality in response to noradrenergic and serotonergic antidepressant medication in depressed sample: Evidence of non‐specificity. Psychiatry Res 1999;86:211–216. [DOI] [PubMed] [Google Scholar]
- 79. Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC. Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord 2004;78:235–241. [DOI] [PubMed] [Google Scholar]
- 80. Papakostas GI. Dopaminergic‐based pharmacotherapies for depression. Eur Neuropsychopharmacol 2006;16:391–402. [DOI] [PubMed] [Google Scholar]
- 81. Diener E, Iran‐Nejad A. The relationship in experience between various types of affect. J Person Soc Psychol 1986;50:1031–1038. [Google Scholar]
- 82. Watson D. The vicissitudes of mood measurement: effects of varying descriptors, time frames, and response formats on measures of positive and negative affect. J Pers Soc Psychol 1988;55:128–141. [DOI] [PubMed] [Google Scholar]
- 83. Dichter GS, Tomarken AJ, Freid CM, Addington S, Shelton RC. Do venlafaxine XR and paroxetine equally influence negative and positive affect? J Affect Disord 2005;85:333–339. [DOI] [PubMed] [Google Scholar]
- 84. Nelson JC, Portera L, Leon AC. Are there differences in the symptoms that respond to a selective serotonin or norepinephrine reuptake inhibitor? Biol Psychiatry 2005;57:1535–1542. [DOI] [PubMed] [Google Scholar]
- 85. Gilmor ML, Owens MJ, Nemeroff CB. Inhibition of norepinephrine uptake in patients with major depression treated with paroxetine. Am J Psychiatry 2002;159:1702–1710. [DOI] [PubMed] [Google Scholar]
- 86. Harmon‐Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol 1997;106:159–163. [DOI] [PubMed] [Google Scholar]
- 87. Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and the behavioral inhibition systems. Psychol Sci 1997;8:204–210. [Google Scholar]
- 88. Gotlib IH, Ranganath Cl, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning: Neuropsychological perspectives on affective and anxiety disorders. Cogn Emot 1998;12:449–478. [Google Scholar]
- 89. Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol 1991;100:535–545. [DOI] [PubMed] [Google Scholar]
- 90. Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol 1994;103:460–466. [DOI] [PubMed] [Google Scholar]
- 91. Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annu Rev Psychol 1989;40:457–492. [DOI] [PubMed] [Google Scholar]
- 92. Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 1999;22:491–517. [DOI] [PubMed] [Google Scholar]


