Conflict of Interest
The authors declare no conflict of interest.
Cerebral ischemia caused by a blood supply deficiency triggers various pathophysiological changes. Many factors are involved in the development of ischemia injury. For example the longer ischemia time is more prone to ischemia injury, and the brain is very susceptible to energy‐depriving injuries due to its characteristics of low fuel reserves, high aerobic metabolism and low concentrations of oxygen radicals–scavenging enzymes. Rapid reperfusion is the most effective treatment for ischemia, minimizing both structural and functional injuries 1. Many signaling molecules have been postulated to contribute to ischemia, including both excitotoxicity and oxidative stress 2. Glutamate emerges as the major excitatory neurotransmitter stimulating energy production in the central nervous system (CNS) that is involved in many neurophysiological functions. A disruption of its homeostasis can damage neurons, which may eventually lead to neural cell death 3. This neuropathological process, designated excitotoxicity, can induce degeneration of neural cells following an excessive action on its specific ionotropic glutamate receptor, N‐methyl‐d‐aspartate (NMDA) 4. The activation of NMDA receptor (NMDAR) usually triggers intracellular transduction pathways associated with changes in [Ca2+]i levels, resulting in neural Ca2+ influx and a cascade of events involving free radical production, and mitochondrial dysfunction can mediate excitotoxicity, resulting in damage to the cell membrane, cytoskeleton, and DNA 5. Thus, modulating the glutamate pathway is a new concept and therapeutic option to attenuate cerebral ischemia 6.
Oxysophoridine (OSR) is an alkaloid extracted from Siphocampylus verticillatus. Its chemical structure is made of two piperidine rings. The report in the literature has shown that the hydroalcoholic extract of Siphocampylus verticillatus has significant and long‐lasting antinociception when assessed against both neurogenic and inflammatory models of nociception in mice 7. Previous work in our laboratory has demonstrated that OSR has an antinociceptive effect and such protection may be mediated by GABA and GABAA receptor up‐regulation after OSR pretreatment 8. In the present study, we tested the hypothesis that the protective effect of OSR was through regulating the glutamate pathway.
The hippocampal neurons are cultured using primary rat cells 6. Sprague‐Dawley rats were purchased from the Animal Center of Ningxia medical university, Ningxia, China. All procedures were approved by the Institutional Ethics Committee, and we analyzed the neuroprotective effect of OSR (Ningxia Institute of Materia Medica, Ningxia, China). The purity of OSR was determined to be 99% on the hippocampal neurons sustained oxygen–glucose deprivation/reperfusion (OGD/Rep) in vitro 9. The cells were randomly divided into six groups: control group; vehicle group (injury by OGD 2 h/Rep 24 h); positive nimodipine control group (12 μM NIM), and different OSR dosage group (5, 20, 80 μM OSR). All the data obtained in the experiments were expressed as mean ± standard deviation (SD). A one‐way analysis of variance (ANOVA) was used to compare the means of six groups. The Student Newman–Keuls' (SNK)/least significant difference (LSD) test was performed to compare all pair means. All statistical analyses were run with the SPSS 16.0 statistical software package (SPSS, Chicago, IL, USA). P values of <0.05 and 0.01 were considered statistically significant.
The injured neurons exposed to OGD for 2 h and OGD/Rep for 24 h were examined with MTT and LDH assay to evaluate the protective effects of OSR. The exposure of neurons to OGD/Rep led to a marked decrease in cell survival rate compared with the control group. In contrast, treatment with OSR (5, 20, 80 μM) and the NIM treatment can increase the cell survival rate compared with the vehicle group, and the OSR treatment presented remarkable dose dependency. LDH leakage increased compared with the control group after exposure to OGD/Rep. In contrast, in the OSR treatment and the NIM treatment, LDH leakage was significantly reduced compared with the vehicle group. As shown in Figure 1(A,B).
Figure 1.
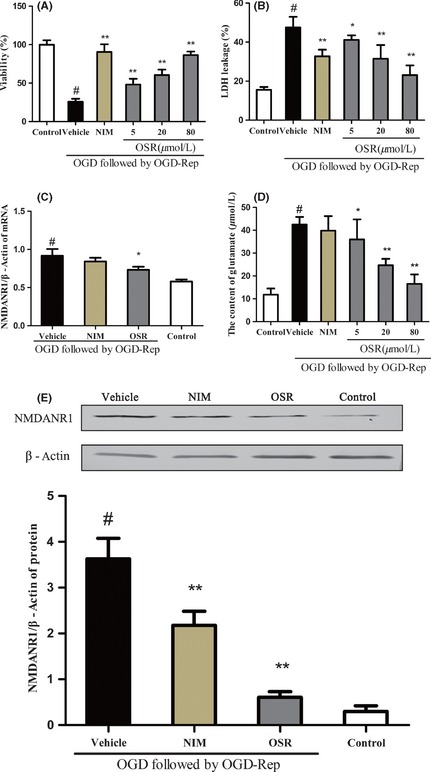
Effects of oxysophoridine on of cultured rat hippocampal neurons injured by oxygen–glucose deprivation (OGD). The viability (A), LDH leackage (B), NMDANR1 gene expression (C), glutamate content (D), and NMDANR1 protein expression (E). # P < 0.01 versus control group; **P < 0.01 versus vehicle group. Data were presented as the mean ± SD, n = 6.
To further confirm the effects OSR mediating the glutamate pathway, we analyzed the content of glutamate by chemical chromatometry, NMDARNR1 subunit gene expression by quantitative real‐time PCR and NMDARNR1 protein expression by Western blot (Figure 1C–E). We observed that the exposure of neurons to OGD/Rep increased the content of glutamate compared with the control group. In contrast, treatment with OSR (5, 20, 80 μM) and the NIM can decrease the content of glutamate compared with the vehicle group. Further, we observed that exposure to OGD/Rep resulted in increased levels of mRNA and protein expression from the NMDARNR1 subunit compared with the control group. However, the OSR (80 μM) and NIM groups exhibited decreased levels of mRNA and protein expression from the NMDARNR1 subunit compared with the vehicle group. These data showed that OSR exerted inhibitory effects against OGD‐/Rep‐induced excitotoxicity through overactivation of NMDAR.
Confocal microscopy was used to determine the intracellular calcium ion concentration ([Ca2+]i) and the level of mitochondrial membrane potential (MMP). The intracellular Ca2+ concentration at OGD 2 h/Rep 24 h showed significant increases compared with the control group. In contrast, NIM could significantly decrease the intracellular Ca2+ concentration in comparison with the vehicle group. Furthermore, the OSR treatment could also significantly decrease the intracellular Ca2+ concentration in a dose‐dependent manner in comparison with the vehicle group. This result indicated that OSR can inhibit the NMDA‐induced increase in [Ca2+]i (Figure 2). The fluorescent probe JC‐1 was used to measure the loss of MMP (△Ψm) in hippocampal cultures exposed to OGD/Rep 10. MMP was assessed in neurons to measure the changes in △Ψm with a fluorescent dye JC‐1 that presents red fluorescence when △Ψm is high, and green fluorescence when △Ψm is low. This result was depicted as Figure 2B. After exposure to OGD for 2 h followed by OGD/Rep for 24 h alone, MMP significantly decreased compared with the control group. However, the OSR treatment and the NIM treatment significantly raised the level of △Ψm. These data clearly demonstrated that OSR can maintain △Ψm at OGD 2 h/Rep 24 h.
Figure 2.
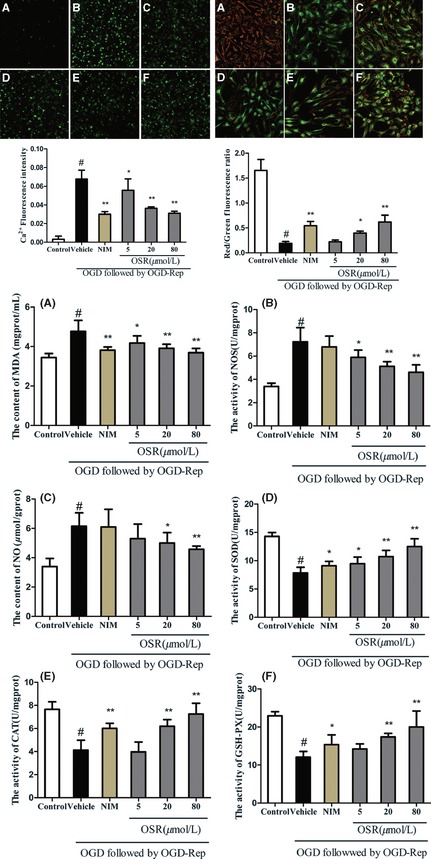
Effects of oxysophoridine (OSR) on oxygen–glucose deprivation (OGD)‐induced rat hippocampal neurons injury. The alteration of [Ca2+]i (left) and mitochondrial membrane potential (MMP) (right) as well as malondialdehyde (a), nitric oxide synthase (b), nitric oxide (c), superoxide dismutase (d), catalase (e), glutathione peroxidase (f) levels (middle) in rat hippocampal neurons. (A) normal cells; (B) rat hippocampal neurons exposed to OGD for 2 h followed by oxygen–glucose deprivation/reperfusion (OGD/Rep) for 24 h; (C) rat hippocampal neurons were pre‐incubated with 12 μM NIM before reperfusion induced damage. (D–F) Rat hippocampal neurons were pre‐incubated with 5, 20, 80 μM OSR, respectively, before reperfusion induced damage. # P < 0.01 versus control group; *P < 0.05, **P < 0.01 versus vehicle group. Data were presented as the mean ± SD, n = 6.
In the present study, malondialdehyde (MDA), nitric oxide synthase (NOS), nitric oxide (NO), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH‐Px) were measured by chemical colorimetry 11, and we found that MDA, NOS, and NO levels increased significantly as a result of free radical generation induced by OGD/Rep. Moreover, OGD‐/Rep‐induced neuronic injury resulted in a markedly decreased SOD, CAT, and GSH‐Px activity. These findings are in agreement with previous reports. In contrast, the OSR treatment obviously reduced the level of MDA, the content of NO and the activity of NOS, and increased SOD, CAT, and GSH‐Px at OGD 2 h/Rep 24 h (Figure 2).
In summary, we report a novel finding concerning the protective effect of OSR on OGD/Rep neuronal injury in vitro. Such protective effects are mediated by its action on anti‐excitotoxicity, suppression of the intracellular Ca2+ elevation, and inhibition of oxidative stress impairment. Although more precise mechanistic studies are necessary to fully clarify the neuroprotection of OSR, these findings can encourage further studies. The present findings provide a preliminary pharmacological basis for the therapeutic use of OSR for cerebral ischemia.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Grant No. 309605060) and the Natural Science Foundation of Ningxia (Grant No. NZ11212).
The first two authors contributed equally to this work.
References
- 1. Frantseva MV, Carlen PL, Perez VJ. Dynamics of intracellular calcium and free radical production during ischemia in pyramidal neurons. Free Radic Biol Med 2001;31:1216–1227. [DOI] [PubMed] [Google Scholar]
- 2. Lu RY, Luo DF, Xiao SH, et al. Kallikrein gene transfer induces angiogenesis and further improves regional cerebral blood flow in the early period after cerebral ischemia /reperfusion in rats. CNS Neurosci Ther 2012;18:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogins J, Crawford DC, Zorumski CF, Mennerick S. Excitotoxicity triggered by neurobasal culture medium. PLoS One 2011;6:e25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campos F, Perez‐Mato M, Agulla J, et al. Glutamate excitoxicity is the key molecular mechanism which is influenced by body temperature during the acute phase of brain. Stroke 2012;7:e44191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bulley S, Shen W. Reciprocal regulation between taurine and glutamate response via Ca2+‐dependent pathways in retinal third‐order neurons. J Biomed Sci 2010;17:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Q, Huang XJ, He W, et al. Neuroprotective potential of Fasudil Mesylate in brain ischemia‐reperfusion injury of rats. Cell Mol Neurobiol 2009;29:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trentin AP, Santos AR, Miguel OG, Yunes RA, Calixto JB. Mechanisms involved in the antinociceptive effect in mice of the hydroalcoholic extract of Siphocampylus verticillatus . J Pharm Pharmacol 1997;49:567–572. [DOI] [PubMed] [Google Scholar]
- 8. Yang G, Gao J, Jia J, Yan L, Yu J, Jiang Y. Oxysophoridine through intrathecal injection induces antinociception and increases the expression of the GABAAα1 receptor in the spinal cord of mice. Planta Med 2012;78:874–880. [DOI] [PubMed] [Google Scholar]
- 9. Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 2012;18:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang P, Feng L, Oldham E, Keating M, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000;407:390–395. [DOI] [PubMed] [Google Scholar]
- 11. Crack P, Taylor J. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005;38:1433–1444. [DOI] [PubMed] [Google Scholar]


