SUMMARY
Introduction: Animal and human research suggests that the development of posttraumatic stress disorder (PTSD) may involve the overconsolidation of memories of a traumatic experience. Previous studies have attempted to use pharmaceutical agents, especially the β‐adrenergic blocker propranolol, to reduce this overconsolidation. Aims: In this randomized, placebo‐controlled study of the efficacy of propranolol in reducing the development of PTSD, we optimized dosages and conducted both psychophysiological and clinical assessments 1 and 3 months after the traumatic event. Forty‐one emergency department patients who had experienced a qualifying acute psychological trauma were randomized to receive up to 240 mg/day of propranolol or placebo for 19 days. At 4 and 12 weeks post‐trauma, PTSD symptoms were assessed. One week later, participants engaged in script‐driven imagery of their traumatic event while psychophysiological responses were measured. Results: Physiological reactivity during script‐driven traumatic imagery, severity of PTSD symptoms, and the rate of the PTSD diagnostic outcome were not significantly different between the two groups. However, post hoc subgroup analyses showed that in participants with high drug adherence, at the 5‐week posttrauma assessment, physiological reactivity was significantly lower during script‐driven imagery in the propranolol than in the placebo subjects. Conclusions: The physiological results provide some limited support for a model of PTSD in which a traumatic conditioned response is reduced by posttrauma propranolol. However, the clinical results from this study do not support the preventive use of propranolol in the acute aftermath of a traumatic event.
Keywords: Post‐traumatic stress disorder, Propranolol, PTSD prevention script‐driven imagery, Trauma
Introduction
Posttraumatic stress disorder (PTSD) is a disabling psychiatric disorder. A growing area of research interest is focused on the possibility of secondarily preventing the development of PTSD after the occurrence of a psychologically traumatic event. Some psychotherapeutic approaches, such as cognitive behavioral therapy administered weeks after a traumatic event, appear to confer a benefit [1]. In contrast, immediate psychological debriefing has not been found to be beneficial [2]. Administration of pharmacological agents, such as the β‐adrenergic receptor antagonist propranolol shortly following the trauma, is another approach that has demonstrated some promise.
It has been theorized that high levels of stress hormones, such as epinephrine, stimulated by the traumatic event act to overconsolidate memory and thereby support the development of the intrusive re‐experiencing symptoms found in PTSD [3]. Memory consolidation is a time‐dependent process that occurs following learning. This process is enhanced by noradrenergic stimulation and reduced by noradrenergic blockade. In one study, yohimbine, which stimulates norepinephrine release, was administered to participants after they viewed slides of an emotionally arousing story [4]. Higher plasma levels of the noradrenergic metabolite 3‐methoxy‐4‐hydroxyphenylglycol (MHPG) were found to correlate with better memory for the story. In another study, participants remembered an emotionally arousing story better than a neutral one, but propranolol blocked that enhancement [5]. Recent work has indicated that propranolol reduces the basolateral amygdala's response to emotion‐provoking stimuli [6].
Four studies have used propranolol in individuals who have experienced an acute medical trauma. In the first study [7], 41 patients who had experienced a traumatic event accompanied by physiological arousal, defined as a heart rate of 80 beats per minute (BPM) or higher, were recruited from the emergency department (ED) and randomized to receive double‐blind propranolol 40 mg daily or placebo for 10 days, beginning approximately 4 h after the trauma. Eleven propranolol patients and 20 placebo patients who completed the study were assessed for PTSD symptoms at 4 weeks using the Clinician‐Administered PTSD Scale (CAPS). They also completed a psychophysiological procedure, during which they engaged in script‐driven mental imagery of the traumatic event, while heart rate, skin conductance, and facial electromyography were measured. The mean 1‐month posttrauma CAPS score of the propranolol versus placebo completers was 27.6 versus 35.5, but this difference was not statistically significant. During script‐driven imagery performed at 3 months, none of the propranolol group but 43% of the placebo group showed an elevated physiologic response. This preliminary investigation suggested that propranolol administered shortly after an acute traumatic event may have preventive potential for PTSD.
In a nonrandomized study, Vaiva et al. [8] administered propranolol 40 mg daily to patients recruited in the emergency room; patients who declined propranolol made up the control group. The first dose was administered 2–20 h after the traumatic event (mean = 9.5, SD = 6) and continued for 7 days. Two months later, the 11 propranolol participants had significantly lower levels of PTSD symptoms than the 8 control participants on the Treatment Outcome PTSD scale.
Stein et al. [9] randomized 48 surgical trauma patients (out of 5602 who were initially screened) to receive within 48 h either double‐blind propranolol 60 mg a day, the anticonvulsant gabapentin, or placebo. Patients were interviewed by phone and assessed for acute stress disorder (ASD) and PTSD at 1 and 4 months, respectively. No group differences in the incidence of either disorder were found.
Incidence of ASD was obtained retrospectively as part of a hypermetabolic state study that had randomized pediatric burn patients to receive an average daily dose of 4 mg/kg propranolol or placebo, starting an average of 2 days postinjury and lasting 4 weeks. Results yielded no difference in ASD incidence between the groups [10]. Another retrospective study examined the prevalence of the PTSD outcome in burned military combatants who did or did not receive propranolol. Propranolol conferred no benefit. However, dose and timing were not considered [11].
In the above studies, initiation of propranolol administration varied widely, occurring as late as 20 h [8] or even 48 h [9] after the traumatic event. In addition, doses of propranolol were mostly in the range of 40–60 mg, which may be inadequate to attenuate trauma‐induced hyperarousal. In this study, we administered higher doses but allowed participants to skip doses if necessary in an attempt to minimize dropouts due to side effects. Another improvement in this study was the employment of a medication‐use recording device, to determine adherence. We hypothesized that at the outcome assessments participants who had previously been treated with propranolol would show (a) lower physiological responses during script‐driven imagery of their traumatic events, consistent with reduction of the traumatic conditioned response, and (b) fewer PTSD symptoms.
Method
Participants
Inclusion Criteria
Research participants were males and females ages 18–65 who were recruited from the ED at the Massachusetts General Hospital in Boston from September 2004 to May 2008. Participant candidates had to experience an event that met the DSM‐IV PTSD A.1 (stressor) and A.2 (response) criteria. Additional eligibility criteria initially included an ED admission heart rate of 80 BPM or greater, and occurrence of the traumatic event no earlier than 4 h prior to first dose of study medication. However, due to recruitment difficulties, we found it necessary to eliminate the minimal heart rate criterion as the study progressed, and to increase the permissible time since the occurrence of the traumatic event from 4 to 12 h, in order to obtain more participant candidates.
Exclusion Criteria
These included physical injury that would complicate participation, hospital stay longer than overnight (the great majority of participants were discharged from the ED the same day), head injury with loss of consciousness, a medical condition that contraindicated the administration of propranolol (e.g., asthma), use of medications with potentially dangerous interactions with propranolol, previous adverse reaction to a β‐blocker, blood alcohol concentration above 0.02% or presence of substances of abuse on saliva testing, pregnancy, traumatic event reflecting ongoing victimization, contraindicating psychiatric condition such as psychotic, bipolar, major depressive, or posttraumatic stress disorder from another event, suicidality or homicidality, unwillingness or inability to come to Boston for the research visits, or treating physician did not concur with enrollment in the study.
Ethical Approval and Informed Consent
Following a full explanation of the study's procedures, all participants gave written, informed consent on a form approved by the Partners Healthcare institutional review board (IRB).
Recruitment and Participation
These data are presented via a flow chart (Figure 1). Percentages in parentheses represent percentage of the original 2014 participants screened.
Figure 1.
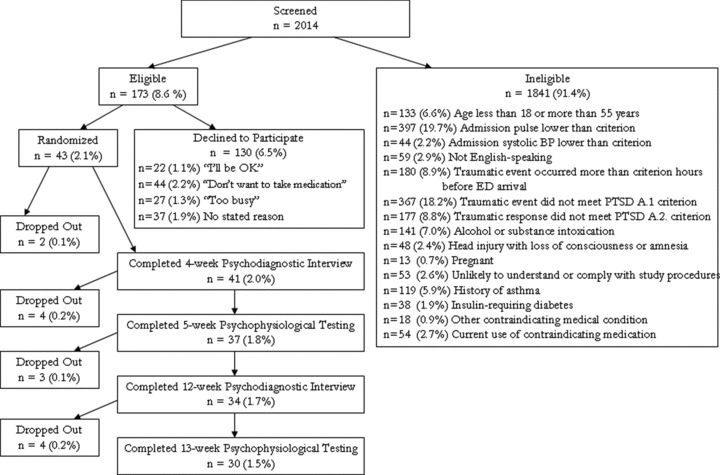
Consort chart.
Procedure
Study Medication
Following screening, each participant was randomized to receive an initial oral dose of either 40 mg short‐acting propranolol or placebo. One hour after this first dose, if systolic blood pressure had not fallen by 10 mmHg or more, or to below 100 mmHg, an additional oral dose of 60 mg long‐acting propranolol or placebo was given; all participants received both doses. Participants continued taking long‐acting propranolol (or placebo) at home over a 19‐day course, starting with 120 mg every morning and evening for 10 days, and then tapering to 120 mg in the morning and 60 mg in the evening for 3 days, then 60 mg in the morning and 60 mg the evening for 3 days, then 60 mg in the morning only ×3 days, after which the study medication was discontinued. Research nurses performed semi‐weekly telephone checks to assess participant's tolerance of study medication and general well‐being.
Medication Adherence
This was measured via (a) participant's log, (b) pill count by staff at the end of the study (by design a few more pills were added to the bottle than needed to be taken), and (c) the Medication Event Monitoring System [MEMS, 12], which electronically recorded when participants opened their pill bottles. Participants were classified as “high adherence” if these three sources of evidence indicated that they had taken 90% or more of their medication doses during the period of the study. These data appear in Figure 1.
Psychometric Assessments
The Peritraumatic Emotional Distress Inventory [13] was administered in the ED. At the 4‐ and 12‐week posttrauma psychodiagnostic assessments, an experienced doctoral‐level psychologist administered the Clinician‐Administered PTSD Scale (CAPS) to assess the presence or absence of the categorical PTSD diagnosis and the Structured Clinical Interview for DSM‐IV (SCID) to assess the presence or absence of all other Type I mental disorders. The CAPS was also used to score the frequency and intensity of each of the 17 DSM‐IV PTSD symptoms on 0–4 scales. The sum of frequency plus intensity scores for all 17 symptoms yielded a total CAPS score, which represented overall severity of PTSD symptomatology.
Psychophysiological assessments.
During the 5‐week and 13‐week visits, participants were tested using a previously published script‐driven imagery procedure [14], which has been shown significantly to discriminate groups of participants with and without PTSD [15]. In brief, two personal “scripts” that portrayed the traumatic event that had brought the participant to the ED, as well as other control scripts (data not presented) were developed. These scripts include descriptions of bodily sensations that accompanied the event. Later, the scripts were read to the participant one at a time, and after listening to each script the participant imagined the portrayed event, as if they were re‐experiencing it. Prior to (baseline) and during script‐driven imagery, psychophysiological measurements were taken, including skin conductance (SC), heart rate (HR), and electromyogram (EMG) of the left lateral frontalis facial muscle. Response scores for each physiological measure were calculated by subtracting the 30‐second baseline period mean from the 30‐second imagery period mean. Responses to the two traumatic scripts were averaged prior to analysis. An a priori discriminant function derived from the HR, SC, and EMG responses during personal traumatic imagery of previously studied individuals with and without current PTSD was used to calculate each participant's posterior probability of being classified as PTSD [14, 15]. This served as a univariate, composite measure of overall physiological responding, circumventing the need for multivariate analyses of physiological responses in the small samples studied. (In cases in which one of these three physiological measures was missing due to technical failure, the physiological probability was calculated on the basis of the remaining two.)
Data Analysis
Continuous outcome measures were compared between the completers in the two groups (propranolol vs. placebo) via analyses of variance (ANOVAs). Dichotomized outcome measures were compared using Fisher's exact test. Pearson correlation coefficients were used to summarize the relationship between physiological probability and total CAPS score. Multivariable analyses were deemed unnecessary due to the small sample size and the fact that none of the potentially confounding variables were found to be significantly correlated with any of the outcome measures. In a post hoc analysis, we explored results in the subgroup of high medication‐adherence participants. A two‐sided P‐value < 0.05 was considered statistically significant except for the three individual physiological variables where Bonferroni correction was applied and a P‐value < 0.017 was used.
Results
ED Data
Patient characteristics are summarized in Table 1. Twenty‐two patients were randomized to the propranolol group and 21 patients to the placebo group. One subject in each group dropped out prior to any assessment; these subjects are not included in the results. The remaining patients’ characteristics are summarized in Table 1. There were no significant differences in age, gender, peritraumatic emotional distress level, hours elapsed from traumatic event to first dose of study medication, heart rate prior to, or 90‐min. following first dose of study medication, or medication adherence between the two treatment groups.
Table 1.
Group means (standard deviations) and cohort demographics
| Emergency Department | All Subjects | |
|---|---|---|
| Placebo n = 20 | Propranolol n = 21 | |
| Emergency Department | ||
| Male/female | 7/13 | 11/10 |
| Age | 33.8 (9.4) | 33.3 (11.0) |
| Peritraumatic emotional distress | 41.6 (11.4) | 39.7 (12.4) |
| Hours elapsed trauma to drug | 4.9 (2.0) | 4.0 (1.3) |
| Heart rate prior to drug (BPM) | 82.8 (14.7) | 81.4 (13.4) |
| Heart rate 11/2 hr. after drug (BPM) | 77.4 (10.7) | 72.6 (8.6) |
| Causal traumatic events | n | n |
| Motor vehicle accident | 12 | 14 |
| Work injury | 1 | 3 |
| Burn/electrical shock | 1 | 3 |
| Falls | 3 | 0 |
| Physical assault | 2 | 0 |
| Hit by bicycle | 0 | 1 |
| Fire | 1 | 0 |
| Comorbid Mental Disordersa | ||
| Major depressive disorder | 3 | 3 |
| Social phobia | 2 | 1 |
| Generalized anxiety disorder | 2 | 2 |
| Simple phobia | 1 | 0 |
| Alcohol abuse/dependence | 1 | 2 |
| Substance abuse/dependence | 0 | 1 |
| Attention deficit disorder | 0 | 1 |
| Bipolar affective disorder type II | 1 | 0 |
| Completed one‐month assessment | 20 | 21 |
| Completed three‐month assessment | 18 | 16 |
| High drug compliance | 12 | 8 |
n, sample size; BPM, beats per minute.
aAssessed at 4 weeks posttrauma.
Study Medication Tolerance
Overall, there were minimal reported side effects of either the propranolol or placebo. There was one adverse event that was possibly related to propranolol, which consisted of a fall without serious injury.
Substance Use
Six participants were found to be taking one or more potentially confounding substances at the time of one or the other outcome assessments, as determined by questioning or urine testing. However, because substance use status was not significantly associated with any outcome measures of interest, the substance‐using participants were retained in the data analysis to avoid unnecessary sacrifice of power.
Psychophysiological, PTSD Frequency, and PTSD Symptom Severity
All participants. Outcome data for all participants appear in Table 2. There were no significant effects of Drug for any outcome measure. Correlations between physiological probability and total CAPS score were: 4‐week, r = 0.46, n = 37; P= 0.004; 12‐week, r = 0.36, n = 30; P= 0.04.
Table 2.
Group means (standard deviations) and statistical contrasts for outcome measures in all completers
| Placebo n = 20 | Propranolol n = 21 | df, t, P | Difference in Means | 95% Confidence Interval | |
|---|---|---|---|---|---|
| One‐month posttrauma assessment | |||||
| Physiological PTSD probability (%) | 40.7 (17.0) | 33.7 (10.2) | 35, 1.5, 0.15a | 7.0 | −2.7 to 16.7 |
| Heart rate response (BPM) | 1.7 (4.1) | 0.2 (4.5) | 35, 1.1, 0.30 | 1.5 | −1.4 to 4.4 |
| Skin conductance response (μS) | 0.55 (1.08) | 0.14 (0.57) | 35, 1.5, 0.17a | 0.41 | −0.19 to 1.01 |
| Frontalis EMG response (μV) | 0.5 (1.5) | 0.4 (0.8) | 35, 0.3, 0.77a | 0.1 | −0.7 to 1.0 |
| Clinician Admin PTSD Scale | 28.5 (27.1) | 28.5 (21.5) | 39, −0.0, 0.99 | 0.0 | −15.5 to 15.3 |
| PTSD diagnosis (Y/N) | 5/15 | 5/16 | 1.00b | ||
| Three‐month posttrauma assessment | |||||
| Physiological PTSD probability (%) | 34.9 (13.1) | 32.0 (5.8) | 28, 0.8, 0.44a | 2.9 | −4.8 to 10.7 |
| Heart rate response (BPM) | 1.8 (3.8) | 1.2 (3.4) | 27, 0.4, 0.68 | 0.6 | −2.2 to 3.3 |
| Skin conductance response (μS) | 0.14 (0.80) | −0.08 (0.29) | 26, 1.0, 0.33a | 0.22 | −0.26 to 0.70 |
| Frontalis EMG response (μV) | 0.2 (0.5) | 0.4 (0.7) | 28, −1.2, 0.23 | −0.2 | −0.7 to 0.2 |
| Clinician Admin PTSD Scale | 19.0 (25.8) | 21.2 (26.1) | 32, −0.2, 0.81 | −2.2 | −20.3 to 16.0 |
| PTSD diagnosis (Y/N) | 4/14 | 2/14 | 0.66b | ||
n, sample size; df, degrees of freedom (where lower than expected according to sample sizes, this is due to missing data); t, Student's t; P, statistical probability level; PTSD, posttraumatic stress disorder; Physiological PTSD probability, percent posterior probability of classification in PTSD reference group, which is an a priori measure of overall physiological responding during script‐driven traumatic imagery (see text for explanation); BPM, beats per minute; μS, microSiemens; μV, microVolts.
aSatterthwaite t‐test for unequal variances.
bFisher's exact test.
High‐Adherence Participants Only
In a post hoc analysis, we explored drug effects in the subgroup of high medication‐adherence participants. Outcome data for these participants appear in Table 3. One high‐adherence participant in the placebo subgroup, and one in the propranolol subgroup, were taking one or more potentially confounding substances at the time of the 4‐week assessment. The propranolol group had a significantly lower 5‐week mean physiological PTSD probability than the placebo group (P < 0.05), as well as a significantly lower mean heart rate response (–1.5 vs. 3.1, P= 0.009). There were no other significant group differences. Correlations between physiological probability and total CAPS score were: 4‐week r = 0.49, n = 20; P= 0.015; 12‐week r = 0.49, n = 19; P= 0.016.
Table 3.
Group means (standard deviations) and statistical contrasts for outcome measures in high drug compliance subjects
| Placebo n = 12 | Propranolol n = 8 | df, t, P | Difference in Means | 95% Confidence Interval | |
|---|---|---|---|---|---|
| One‐month posttrauma assessment | |||||
| Physiological PTSD probability (%) | 44.6 (17.5) | 32.6 (5.9) | 18, 2.2, <0.05a | 12.0 | 0.3 to 23.6 |
| Heart rate response (BPM) | 3.1 (3.6) | −1.5 (3.2) | 18, 3.0, 0.009 | 4.6 | 1.3 to 7.9 |
| Skin conductance response (μS) | 0.68 (1.22) | 0.17 (0.30) | 18, 1.4, 0.19a | 0.51 | −0.28 to 1.31 |
| Frontalis EMG response (μV) | 0.9 (1.6) | 0.6 (1.2) | 18, 0.4, 0.68 | 0.3 | −1.1 to 1.6 |
| Clinician Admin PTSD Scale | 33.3 (30.3) | 22.1 (21.3) | 18, 0.9 0.38 | 11.2 | −14.8 to 37.3 |
| PTSD diagnosis (Y/N) | 4/8 | 1/7 | 0.60b | ||
| Three‐month posttrauma assessment | |||||
| Physiological PTSD probability(%) | 35.8 (15.3) | 31.0 (3.9) | 17, 1.0, 0.34a | 4.8 | −5.7 to 15.4 |
| Heart rate response (BPM) | 1.9 (4.1) | −0.2 (2.3) | 17, 1.3, 0.22 | 2.1 | −1.4 to 5.4 |
| Skin conductance response (μS) | 0.22 (0.95) | −0.01 (0.34) | 15, 0.7, 0.50a | 0.23 | −0.48 to 0.93 |
| Frontalis EMG response (μV) | 0.0 (0.5) | 0.2 (0.3) | 17, −1.0, 0.35 | −0.2 | −0.6 to 0.2 |
| Clinician Admin PTSD Scale | 25.3 (28.4) | 18.5 (31.1) | 18, 0.5, 0.62 | 6.8 | −21.5 to 35.0 |
| PTSD diagnosis (Y/N) | 3/9 | 1/7 | 0.62b | ||
n, sample size; df, degrees of freedom (where lower than expected according to sample sizes, this is due to missing data); t, Student's t; P, statistical probability level; PTSD, posttraumatic stress disorder; Physiological PTSD probability, percent posterior probability of classification in PTSD reference group, which is an a priori measure of overall physiological responding during script‐driven traumatic imagery (see text for explanation); BPM, beats per minute; μS, microSiemens; μV, microVolts.
aSatterthwaite t‐test for unequal variances.
bFisher's exact test.
Discussion
The obstacles to conducting the present trial were formidable. As was found in previous investigations [7, 9], enrolling participants in the ED setting to take a medication to prevent a disorder that they do not yet have is a major challenge. This study succeeded in bringing only 2% of participant candidates screened, and 24% of those found eligible, to 4‐week completion, raising a question as to the feasibility of this approach, at least within the current confines of clinical research. The Health Insurance Portability and Accountability Act was passed into law between our earlier study [7] and the current one. We found that this Act substantially curtailed recruitment because under it, it was necessary that a clinical caregiver (e.g., a busy ED physician) obtain a patient's permission before the patient could be approached by the investigators about the study. Before this law, patients could be approached by investigators directly.
This study attempted to replicate earlier findings that propranolol given shortly following a psychologically traumatic event decreases physiological reactivity during subsequent mental imagery of that event. Physiological reactivity was measured using a composite score of three responses, namely heart rate, skin conductance, and left lateral frontalis facial EMG, during personal script‐driven imagery of the traumatic event that had brought the participant to the ED. Among all participants who completed the 5‐week assessment, those who had received propranolol did not show significantly lower physiological reactivity during script‐driven traumatic imagery than those who had received placebo. In an analysis of the subgroup of participants who took 90% or more of their study medication over the 19‐day medication period, 4‐week physiological responses during traumatic imagery in the propranolol group were significantly lower than in the placebo group. Inspection of the subgroup means indicates that the latter difference is accounted for more by higher arousal scores in the high‐adherence placebo subgroup compared to the full placebo group, than by lower arousal scores in the high‐adherence propranolol subgroup compared to the full propranolol group. This suggests that participants who were more emotionally affected by the traumatic event were more likely to adhere to the study medication prescription over the course of the study and to receive some preventive psychophysiological benefit from propranolol. These results in the high‐medication‐adherence subgroup, however, should be interpreted with caution due to their post hoc nature and small sample sizes. They keep alive but fail to prove the proposition that posttrauma propranolol reduces one objective measure of PTSD, namely increased “physiological reactivity on exposure to internal cues that symbolize or resemble an aspect of the traumatic event” (DSM‐IV PTSD Criterion B.5).
Whereas the psychophysiological results may be equivocal, the clinical results are not, in that there were no significant differences, or even trends, in total CAPS score or in the rate of the PTSD diagnosis between propranolol and placebo groups at either 4‐ or 12‐weeks, either in all subjects or in high adherence subjects. As such, these results offer no support for the efficacy of the acute propranolol intervention for preventing subsequent PTSD. However, the confidence intervals for the differences in the means shown in Tables 2 and 3 were sufficiently wide that Type II error cannot be ruled out.
Given the preclinical science that supports the hypothesis that acute posttrauma propranolol should reduce PTSD symptoms, and especially physiological responding during traumatic recollection, one might ask why this study did not yield more positive results. In animal work, the strongest memory consolidation‐reducing effects of β‐blockers have been found when they have been administered directly into the amygdala prior to learning. In proof‐of‐concept work in healthy participants in which propranolol was found to reduce memory consolidation, the drug was administered 1 h before participants viewed slides of emotional scenes [5]. Reduction of memory consolidation by systemically administered propranolol following learning has been difficult to demonstrate, although not impossible [16], in animals. Factors that may have militated against positive results in this human study include the requirement for systemic drug and the length of time elapsed between the traumatic event and the propranolol administration. A retrospective study found that administration of morphine, which also reduces noradrenergic activity in the brain, to injured military personnel was associated with lower rates of subsequent PTSD. However, in that study, patients were able to receive intravenous morphine within an hour of being transported from the point of injury in 71% of cases [17]. Even though in this study there was not a significant correlation between time elapsed since the traumatic event and subsequent PTSD symptoms, no participant received propranolol earlier than 2 h following their traumatic event; the mean was approximately 4 h. Adding to that an additional 2 h for the short‐acting oral propranolol to reach peak drug level [18], even the first dose of propranolol would not have been active for at least 4 h (usually more) after the traumatic event. A substantial amount of traumatic memory consolidation may already have occurred by that time, making the propranolol intervention too late to be effective.
Another possible explanation of the lack of a beneficial effect of propranolol in this study is that the traumatic events were not severe enough in this sample to allow the drug to exert an effect. In our experience, severely traumatized patients are especially difficult to recruit in the immediate aftermath of the event. Moreover, in the case of physical injury, there is the additional difficulty of recruiting patients with high severity trauma because the level of medical care required can make study participation difficult or impossible. Finally, it is possible that the drug, or the allowable dosage of the drug, simply is insufficiently potent to produce the desired effect. A search for more effective secondary preventive agents for PTSD appears to be indicated.
Author Contributions
R.K.P., S.P.O., J.T.N., and R.M.Z. developed the concept or design of this study; E.A.H., J.J.W., R.K.P., E.B.K., C.M.F., A.R.K., J.M.G., M.L.R., and N.B.L. studied the participants and collected the data; Y.C., R.K.P., and S.P.O. performed the data analysis; E.A.H. and R.K.P. drafted the manuscript; M.H.P., R.M.Z., J.T.N., and J.J.W. provided important criticisms of the manuscript draft.
Funding
This study was funded by NIMH grant #MH068603 to R.K.P.
Disclosures
Hoge: Research Funding: Sepracor; Worthington: Research Funding: Forest Pharmaceuticals, Sepracor; Nagurney: none; Chang: none; Kay: none; Feterowski: none; Katzman: none; Goetz: none; Rosasco: none; Zusman: Speakers bureau/consultant: Bristol‐Myers Squibb, Sanofi‐Aventis, Novartis, Pfizer, Forest, Daichii Sankyo, Eli Lilly, Astra Zeneca and Nicox pharmaceutical companies; Pollack: Advisory Boards and Consultation: Brain Cells, Eli Lilly, Medavante, Labopharm, Mindsite, Sepracor, Targia Pharmaceuticals. Pfizer; Research Grants: Bristol Myers Squibb, Forest Laboratories, GlaxoSmithKline, Eli Lilly, NCCAM, NIDA, NIMH, Sepracor; CME Supported Activities: Astra‐Zeneca, Sepracor, Pfizer; Equity: Medavante, Mensante Corporation, Mindsite, Targia Pharmaceuticals; Royalty/patent: SIGH‐A, SAFER interviews; Orr: none; Pitman: Recipient of two current U.S. Army grants: #W81XWH‐07‐1‐0440 and #W81XWH‐08‐2‐0126; recipient of a small annual royalty for a book chapter on legal issues in PTSD; part‐time private practice of forensic psychiatry.
Conflict of Interests
The authors have no conflict of interest.
Acknowledgments
Glenn Saxe (Chairman), Theodore Colton, and Rubin Davidoff served as members of the project's Data Safety and Monitoring Board. Blair Perry provided assistance with participant recruitment.
References
- 1. Bryant RA, Moulds ML, Nixon RVD. Cognitive behavior therapy of acute stress disorder: A four‐year follow‐up. Behav Res Ther 2003;41:489–494. [DOI] [PubMed] [Google Scholar]
- 2. Rose SC, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002:CD000560. [DOI] [PubMed] [Google Scholar]
- 3. Pitman RK. Post‐traumatic stress disorder, hormones, and memory. Biol Psychiatry 1989;26:221–223. [DOI] [PubMed] [Google Scholar]
- 4. Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 1999;46:1192–1204. [DOI] [PubMed] [Google Scholar]
- 5. Cahill L, Prins B, Weber M, McGaugh JL. β‐adrenergic activation and memory for emotional events. Nature 1994;371:702–704. [DOI] [PubMed] [Google Scholar]
- 6. Hurlemann R, Walter H, Rehme AK, et al Human amygdala reactivity is diminished by the β‐noradrenergic antagonist propranolol. Psychol Med 2010;40:1839–1848. [DOI] [PubMed] [Google Scholar]
- 7. Pitman RK, Sanders KM, Zusman RM, et al Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002;51:189–192. [DOI] [PubMed] [Google Scholar]
- 8. Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003;54:947–949. [DOI] [PubMed] [Google Scholar]
- 9. Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof‐of‐concept trial in physically injured patients. J Traum Stress 2007;20:923–932. [DOI] [PubMed] [Google Scholar]
- 10. Sharp S, Thomas C, Rosenberg L, Rosenberg M, Meyer W 3rd. Propranolol does not reduce risk for acute stress disorder in pediatric burn trauma. J Trauma 2010;68:193–197. [DOI] [PubMed] [Google Scholar]
- 11. McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH. The effect of propranolol on posttraumatic stress disorder in burned service members. J Burn Care Res 2009;30:92–97. [DOI] [PubMed] [Google Scholar]
- 12. Rozenfeld V, Pflomm J‐M, Singh KK, Bazil MK, Cheng JWM. Assessing the impact of medication consultations with a medication event monitoring system. Hosp Pharm 1999;34:539–549,559. [Google Scholar]
- 13. Brunet A, Weiss DS, Metzler TJ, et al The Peritraumatic Distress Inventory: A proposed measure of PTSD criterion A2. Am J Psychiatry 2001;158:1480–1485. [DOI] [PubMed] [Google Scholar]
- 14. Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry 1987;44:970–975. [DOI] [PubMed] [Google Scholar]
- 15. Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post‐traumatic stress disorder. Psychiatr Clin North Am 2002;25:271–293. [DOI] [PubMed] [Google Scholar]
- 16. Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta‐adrenergic blockade. Neurobiol Learn Mem 2000;74:259–266. [DOI] [PubMed] [Google Scholar]
- 17. Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post‐traumatic stress disorder. N Engl J Med 2010;362:110–117 [DOI] [PubMed] [Google Scholar]
- 18. Marino MR, Dey M, Garg DC, Jallad MS, Dorick DM, Martinez JJ, Weidler DJ. Pharmacokinetics and pharmacodynamics of long‐acting propranolol 60‐mg capsules: a comparative evaluation. J Clin Pharmacol 1987;27:885–891. [DOI] [PubMed] [Google Scholar]


